Abstract
Injection drug users constitute the largest group of persons infected with the hepatitis C virus (HCV) in the United States, and most new infections occur in drug users. Controlling hepatitis C in the U.S. population, therefore, will require developing, testing, and implementing effective prevention and treatment strategies for persons who inject drugs. Fortunately, a substantial body of research and clinical experience exists on the prevention and management of chronic viral diseases among injection drug users. The need to implement interventions to stop the spread of HCV among drug users is critical. The capacity of substance-use treatment programs need to be expanded to accommodate all who want and need treatment. Physicians and pharmacists should be educated in how to provide access to sterile syringes and to teach safe injection techniques, both of which are lifesaving interventions. The treatment of hepatitis C in drug users requires an interdisciplinary approach that brings together expertise in treating hepatitis and caring for drug users. Treatment decisions should be made individually by patients with their physicians, based on a balanced assessment of risks and benefits and the patient's personal values. Physicians should carefully assess, monitor, and support adherence and mental health in all patients, regardless of whether drug use is known or suspected. Research is needed to better understand how best to prevent and treat hepatitis C in substance users. In the meantime, substantial progress can be made if existing knowledge and resources are brought to bear.
Injection drug users constitute the largest group of persons infected with the hepatitis C virus (HCV) in the United States, and most new infections occur in drug users. The prevalence of antibodies to HCV in most studies of injection drug users is 80% to 90%,1-3 and incidence rates generally range from 10% to 20% per year.3-7 Controlling hepatitis C in the U.S. population, therefore, will require developing, testing, and implementing effective prevention and treatment strategies for persons who inject drugs.8 Fortunately, a substantial body of research and clinical experience exists in the prevention and management of chronic viral diseases among drug users. This report discusses the prevention and treatment of hepatitis C in injection drug users, with attention to the specific questions posed to the Consensus Panel.
Treatment of Hepatitis C in Drug Users
Decisions about the treatment of hepatitis C in patients who use illicit drugs, as in other patients, should be made by the patients together with their physicians based on individualized risk-benefit assessments.9 Risk-benefit considerations for drug users include those that apply to all patients with hepatitis C, including the limited likelihood of achieving a sustained virological response, particularly in patients with genotype 1 infection, African-American ethnicity, or both; the substantial side effects; and, if the disease is not advanced, the option of delaying therapy while better regimens are developed. Moreover, although the likelihood of achieving a sustained virological response has been well studied in various patient groups, little is known about the likelihood that patients will develop clinical endpoints— cirrhosis, liver cancer, end-stage liver disease, or death—and even less is known about how much or even whether treatment will reduce those risks. Before embarking on therapy, therefore, patients should understand that although one can estimate the likelihood that treatment will clear HCV infection (or achieve a histological benefit), it is not known whether treatment will reduce their chances of becoming sick or dying from hepatitis C. Patients should have access to treatment, but they should make their own decisions, with the aid of a balanced portrayal of the known risks and benefits. For patients with advanced hepatic fibrosis, in whom clinical progression is more imminent, treatment may be more compelling, although data are still needed on the effects of treatment on clinical endpoints such as decompensated cirrhosis and mortality in such patients. Liver biopsy examination can assist in making treatment decisions by identifying patients with advanced fibrosis, in addition to providing information to all patients about their disease status and prognosis.
For patients in stable, long-term recovery, including those receiving methadone maintenance therapy, there is no reason to withhold hepatitis C treatment because of a past history of illicit drug use. For active drug users, adherence, psychologic side effects, and the possibility of reinfection may present challenges to effective treatment. Each of these issues requires attention, but none warrants categorically excluding all active or recent drug users from therapy.9 Rather, these issues should be considered in each individual patient on a case-by-case basis. Patients who believe they can adhere to therapy can be allowed to try. Much less is lost by treating a patient who does not adhere to therapy than by letting a patient progress to cirrhosis or death without a trial of treatment because of a prior assumption that the patient would not adhere to the regimen.
Adherence. There is abundant evidence from diseases other than hepatitis C that drug users can adhere to medical treatments.10-28 When compared with nonusers in conventional clinical settings, drug users often, although not always, have lower levels of adherence (Table 1). But rates of adherence among drug users range from 30% to nearly 100%, a range that is similar to that in patients being treated for hypertension, diabetes, or asthma.29-31 Moreover, when programs are designed specifically for drug users by groups with experience working with substance abuse, adherence rates often exceed 80%.23-28 In addition, numerous studies have shown that most physicians are not able to predict patient adherence accurately.32-38 Thus, although there are many effective strategies for improving patient adherence, attempting to screen out patients who are predicted to have poor adherence is not effective. The extensive and rapidly growing literature on adherence has been summarized in the latest revision of the treatment guidelines for human immunodeficiency virus (HIV) infection.39 These guidelines recommend that readiness for treatment be assessed before therapy in all patients and that no patient be excluded automatically from treatment.
Table 1.
Adherence by Injection Drug Users to MedicalTreatments
| Study | N | Adherence | Regimen | Adherence Less in Drug Users Than Others? (Yes or No) |
|---|---|---|---|---|
| Tulsky, 200010 | 118 | 33% | TB PT | No |
| Pablos-Mendez, 199711 | 184 | 35% | TB Rx | Yes |
| Singh, 199612 | 46 | 38% | ART | Y/N* |
| Ferrando, 199613 | 57 | 47% | AZT | No |
| Haubrich, 199914 | 173 | 51% | HAART | Y/N* |
| Pilote, 199615 | 244 | 53% to 84% | TB appt | Y/N* |
| Eldred, 199816 | 244 | 60% | HIV Rx | No |
| Moatti, 200017 | 164 | 65% | HAART | Not assessed |
| Lucas, 200118 | 764 | 66% | HAART | Y/N* |
| Bangsberg, 200019 | 34 | 67% to 89% | HAART | Not assessed |
| Singh, 199920 | 123 | 76% | ART | No |
| Bamberger, 200021 | 68 | 76% | HAART | Not assessed |
| Chaisson, 200122 | 300 | 79% | TB PT | Not assessed |
| Broers, 199423 | 313 | 81% | AZT | Y/N* |
| Samet, 199224 | 83 | 83% | AZT | Y/N* |
| Mezzelani, 199125 | 79 | 85% | HBV vaccine | Not assessed |
| Marco, 199826 | 62 | 86% | TB Rx | No |
| Lorvick, 199927 | 27 | 96% | TB PT | Not assessed |
| Harrison, 199528 | 71 | 97% | HIV vaccine | No |
Abbreviations: TB PT, tuberculosis preventive therapy; TB Rx, tuberculosis therapy; ART, antiretroviral therapy; AZT, zidovudine; HAART, highly active antiretroviral therapy; HBV, hepatitis B virus.
Yes in some analyses and no in some analyses (in some studies, e.g., lower rates of adherence were found in all illicit drug users but not in injection drug users, or in current but not former drug users, or in univariate but not multivariate analysis). Adherence measures differed among studies.
Tolerance and Effectiveness. Few data are available on results of hepatitis C treatment in active injection drug users who are not receiving treatment for drug use. Several recent studies, however, have shown the safety and effectiveness of hepatitis C treatment in patients receiving drug use treatment, even when they were not completely abstinent from illicit drug use.40-43 In a study of 50 heroin injectors entering opiate detoxification in Munich, Germany, 34 patients were treated with interferon alfa monotherapy and 16 were treated with combination therapy of interferon and ribavirin for 24 to 48 weeks, depending on HCV genotype.40 The overall sustained virological response rate was 36% (Fig. 1), a rate comparable to that in other populations treated for hepatitis C, even though 80% of patients relapsed to drug use during the study. This response rate exceeded the 10% to 20% response rate for interferon alfa monotherapy that was recommended in the 1997 Consensus Development Conference44 and was similar to rates of response achieved with combination therapy in nonuser populations. In this study, all patients were managed by physicians who specialized in hepatology and in addiction medicine. Patients who relapsed to drug use were offered methadone maintenance therapy but were allowed to continue treatment for HCV even if they continued to inject illicit drugs. Sizeable proportions of patients had a sustained virological response, regardless of whether they relapsed to drug use or received methadone maintenance therapy (Fig. 1); indeed, sustained response rates were not significantly associated with either relapse to drug use or receipt of methadone maintenance therapy. The strongest predictor of virological response was adherence to their weekly clinic appointments. Of those who kept at least two thirds of appointments, 45% had a sustained virological response, compared with only 8% of those who did not. This study showed that drug users receiving treatment for substance use can be treated successfully for hepatitis C, despite ongoing drug use. The study also showed the importance of combining expertise in hepatology and substance use and maintaining strong relationships with patients that can continue even when patients relapse to drug use.
Fig. 1.
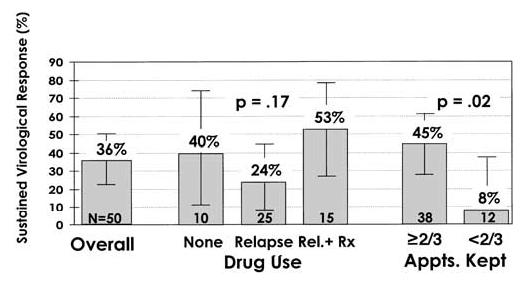
Sustained virological response rates to interferon-based treatment of hepatitis C in injection drug users entering opiate detoxification (N = 50). A total of 50 heroin injectors entering opiate detoxification in Munich, Germany, were simultaneously treated for chronic hepatitis C with either interferon alfa monotherapy (n = 34) or combination therapy with interferon alfa and ribavirin for 24 to 48 weeks depending on genotype. The sustained virological response rate was 36% overall, a rate comparable with that in other populations treated for hepatitis C, even though 80% of the patients relapsed to drug use during treatment. Sizeable proportions of patients had sustained virological responses, regardless of whether they relapsed to drug use or received methadone maintenance therapy. The strongest predictor of virological response was whether patients kept their weekly clinic appointments. Error bars show 95% confidence limits. Rel. + Rx, patients who relapsed to drug use and then received methadone maintenance therapy. Data from Backmund et al.40
Another ongoing study from Oakland, CA, reported a sustained virological response rate of 29% among 66 methadone maintenance patients treated with combination therapy of interferon alfa and ribavirin.41 This response rate was achieved despite the patients' relatively older age, longer duration of infection, more advanced liver disease, and predominance of genotype 1—all factors associated with reduced response rates. Treated patients were highly selected on the basis of demonstrated motivation to receive treatment for hepatitis C and attendance at weekly pretreatment educational sessions. Nevertheless, patients had substantial levels of psychiatric comorbidity and substance use: nearly two thirds of patients had a prior psychiatric diagnosis, mostly depression; more than 80% had received antidepressants by the time they competed treatment; 20% continued to drink alcohol during treatment, mostly in moderate quantities; and a third used illicit drugs during treatment. The patients discussed their medication experiences in weekly group support sessions. Careful attention was paid to managing side effects, and no serious psychologic side effects occurred. More than a third of patients required an increase in methadone dose. Response rates were not significantly associated with level of abstinence from illicit drugs before treatment, use of alcohol or illicit drugs (other than marijuana) during treatment, or pre-existing psychiatric diagnosis. Interestingly, patients who smoked marijuana were significantly more likely to respond to antiviral therapy than nonusers. This study showed that drug users receiving treatment for substance use can be treated successfully for hepatitis C despite ongoing drug use, moderate alcohol consumption, and significant psychiatric comorbidity.42 The study also showed the importance of distinguishing among different types of illicit drug use. Finally, it showed that with careful attention to managing side effects (including mental health assessment and monitoring, treatment of pre-existing or medication-related depression, and adjustment of methadone doses), that the psychologic side effects of interferon in drug users need not be excessive.
Reinfection. There have been few studies on the risk for reinfection in drug users treated successfully for hepatitis C. However, what data exist suggest that reinfection is rare in drug users who clear HCV with therapy even if they continue to inject drugs, as long as steps are taken to minimize the risk. Substance abuse is a chronic, relapsing condition. Acknowledging this fact, the investigators in the Munich study instructed all patients in safe injection practice so that they could avoid acquiring and transmitting blood-borne diseases in the event that they relapsed to drug use.40 Of the patients in the study who achieved an end-of-treatment response, 12 injected drugs during the 24 weeks after treatment, but only 2 redeveloped HCV RNA during the follow-up period. This viral relapse rate was no higher than would be expected in patients not using drugs. Both patients who became HCV-RNA positive had HCV genotype 3a, which was the same genotype they had before treatment. Another study from Scandinavia reported 5-year follow-up evaluations of 27 injection drug users who had cleared HCV RNA with interferon therapy. Nine patients (33%) relapsed to drug use, but only 1 became reinfected, despite a total of 40 person-years of observation.43 These data suggest that if steps are taken to help patients avoid high-risk injection practices, reinfection after successful therapy may be the exception rather than the rule.
In summary, few studies have reported results of treatment of chronic hepatitis C in active drug users, and more data are needed to determine optimal treatment strategies. The evidence to date does not bear out the concept that illicit drug use renders treatment futile. Although adherence, psychologic side effects, and the possibility of reinfection may limit the effectiveness of hepatitis C treatment in some drug users, treatment is successful in others despite ongoing drug use, moderate alcohol consumption, and significant psychiatric comorbidity. Treatment decisions should, therefore, be made by patients and their physicians on an individual basis.
Monitoring and Treating Injection Drug Users With Hepatitis C
Caring for drug users presents special challenges to the health care team requiring patience, experience, and tolerance. Fortunately, a substantial body of research and clinical experience exists on the prevention and management of chronic viral infections among injection drug users, especially HIV infection, and effective principles have been developed for engaging drug users in health care relationships (Table 2).45-48 Learning from this experience will be critical for efforts to control hepatitis C. Successful programs depend on a respectful approach to substance users, an understanding of the medical and behavioral sequelae of addiction, and an avoidance of moralistic judgments.
Table 2.
Principles for Managing Health Care Relationships With Substance-Using Patients
| Establish a climate of mutual respect |
| Maintain a professional approach that reflects the aim of enhancing patients’ well being; avoid creating an atmosphere of blame or judgment |
| Educate patients about their medical status, proposed treatments, and their side effects |
| Include patients in decision making |
| If possible, establish a multidisciplinary team consisting of primary care physicians, HIV specialists, psychiatrists, social workers, and nurses |
| Have a single primary care provider coordinate the care delivered by such a team to maximize consistency and continuity |
| Define and agree on the roles and responsibilities of both the health care team and the patient |
| Set appropriate limits and respond consistently to behavior that violates those limits |
| Minimize barriers to participation (penalties for missed visits, and so forth) |
| Recognize that patients must set their own goals for behavior change and work with patients to achieve commitment to realistic goals for healthier behaviors |
| Acknowledge that abstinence is not always a realistic goal; emphasize risk- reduction measures for patients who continue to use drugs |
| Acknowledge that sustaining abstinence is difficult and that success may require several attempts |
| Be familiar with local resources for the treatment of drug users |
| Pitfalls to avoid |
| Unrealistic expectations |
| Frustration |
| Anger |
| Moralizing |
| Blame |
| Withholding therapy |
Data from O’Connor et al.,45Batki and Sorensen,46Wartenberg,47and Selwyn and O’Connor.48
Harm reduction is the effort to help patients reduce high-risk behaviors without imposing unrealistic demands for global change.49-51 If patients are unlikely to discontinue injection drug use, interventions with limited but practical objectives can and should be taken to help reduce the harmful consequences of continued drug use. Harm reduction is an approach that recognizes that people must set their own agenda for change; that emphasizes the benefits of incremental changes; that recognizes that drug users are motivated to improve their health and well being; and that emphasizes the importance of removing barriers to healthier behaviors and helping people find ways to be healthier that will work for them.52,53
Medical care for drug users with hepatitis C should begin with strong linkages with prevention services, including community-based hepatitis C testing and counseling programs, so that drug users with hepatitis C can be identified and their entry into care facilitated. Success treating hepatitis C in injection drug users will require collaboration between experts in hepatitis and experts in substance use to create programs specifically designed for drug users. Collaboration between experts in HIV and experts in substance use has been stimulated by federal funding programs such as the Ryan White Care Act. Similar programs are needed for hepatitis C. Expertise working with drug users is available from a variety of sources, including public health and community workers with experience in HIV prevention and harm reduction, HIV treatment providers, substance use treatment providers, substance use researchers, and, probably most importantly, drug users themselves. A multidisciplinary team, with input from primary care physicians, hepatologists, nurses, psychiatrists, social workers, drug counselors, and psychologists, may be the optimal approach. A flexible attitude is necessary so that unrealistic expectations do not lead to frustration and resentment. The measure of success of this effort is how much patients are helped to be healthier, not whether a predetermined goal is achieved.
Caring for drug users also requires providing treatment for substance use. Proven effective treatments for substance use exist.54,55 Opiate agonist therapy (e.g., methadone maintenance therapy) has been shown to diminish and often eliminate opiate use and to reduce transmission of many infections, including HIV.56-61 All patients with hepatitis C, regardless of whether they are known to have injected drugs, should be asked about past or current drug and alcohol use. Treatment for substance use should be discussed with those who use drugs or alcohol and provisions made to provide treatment for those who want and need it. Alcohol treatment is particularly important because of the strong effect of heavy alcohol intake on the progression of hepatitis C. Hepatitis C and substance use can be treated simultaneously,40-42 but there are no data on whether it is better to treat one or the other first, or both together. Attention should also be paid to assessing and treating mental health conditions, which are associated with both hepatitis C and substance use and may be induced or exacerbated by hepatitis C treatment. Medical services and mental health care should be integrated with substance use treatment.62
Attention to ensuring optimal adherence is important for all patients, not just those who use drugs.29-31 Patient readiness should be assessed before embarking on therapy, and adherence should be assessed and monitored regularly during therapy.34 Effective strategies for improving adherence range from basic clinical practices—such as establishing a consistent, trusting physician-patient relationship, providing clear information about expected outcomes and side effects of medication, and paying careful attention to perceived side effects—to specialized tools such as electronic reminder systems, directly observed therapy, and cash incentives (Table 3).63,64 Simplifying complex treatment regimens, treating depression, or helping a homeless patient find housing can improve adherence. Patients also may benefit from counseling to help them incorporate the regimen into their daily lives.
Table 3.
Effective Strategies for Improving Adherence
| Information about intended effects and side effects of medication |
| Attention to perceived side effects |
| Counseling addressing barriers to and facilitators of adherence |
| Respectful and nurturing provider-patient relationship |
| Treatment of depression if patient is depressed |
| Directly observed therapy |
| Cash incentives |
| Devices (pager reminders, pill organizer boxes, and so forth) |
Adherence to hepatitis C treatment often can be complicated by side effects, including depression. Thus, the management of side effects is critical to maximize the effectiveness of treatment for hepatitis C. The psychologic side effects of interferon are of concern in all patients, not just those who use drugs or those with preexisting psychiatric diagnoses. Interferon may have severe psychologic side effects in patients without preexisting psychiatric disorders.65,66 To minimize these effects, all patients should be screened for depression and other mental health conditions before undergoing hepatitis C treatment, treated for these conditions if necessary, and monitored (and treated if necessary) for them during hepatitis C treatment. Antidepressant medication may be helpful in a sizeable proportion of patients.
Caring for injection drug users should always include education and support for safe injection practices.67,68 Education is particularly important for drug users receiving hepatitis C treatment to reduce the chances of reinfection. The possibility of reinfection should be discussed with patients before starting hepatitis C treatment. Those who inject drugs after successful treatment for HCV infection may be able to avoid reinfection by using a new sterile syringe for each injection and by not using injection equipment that has been used by other persons. Physicians should refer patients who inject drugs to syringe exchange programs or, if necessary, directly prescribe syringes.69-71 There are now more than 200 syringe exchange programs in more than 150 cities in 36 states in the United States, and these numbers are increasing yearly. For drug users without access to such programs, physicians in at least 46 states are allowed by law to prescribe syringes so that their patients can avoid acquiring and transmitting blood-borne infections.70 Several studies have shown that injection drug users are able to master safe injection practices.72-74 When given access to sterile syringes, drug users readily make use of them, reducing their high-risk behavior and rates of disease transmission.72-77 Unfortunately, HCV may be more readily transmitted than HIV through the sharing of injection equipment other than syringes, such as cookers (bottle caps, spoons, and other containers used to dissolve drugs) and cottons (filters used to draw up the drug solution into a syringe),6,7 and probably through minor instances of blood contact, such as may occur when one person gives an injection to another.78 During the HIV epidemic, injection drug users learned quickly about the risks of sharing syringes, and behavioral norms changed, which resulted in dramatic decreases in rates of syringe sharing.65-67 The sharing of other injection equipment is, unfortunately, still relatively common,79 as is the practice of giving and receiving injections.80 For these reasons, it is important for physicians to educate patients not just to avoid sharing syringes, but to avoid sharing any injection equipment, to wash hands before and after giving injections, and to avoid any contact with blood from other people (Table 4).67,68
Table 4.
Medical Advice For Persons Who Inject Illicit Drugs
| Do not use illegal drugs |
| Receive substance use treatment |
| Never use syringes previously used by another person |
| Never use other injection equipment previously used by another person |
| Use a new, sterile syringe to prepare and inject drugs |
| Use a new or disinfected container (cooker) and a new filter (cotton) to prepare drugs |
| Wash hands and clean the injection site before injection |
| Wash hands before and after giving injections |
| Safely dispose of syringes after one use |
NOTE. Handwashing added by author. Adapted from U.S. Public Health Service Medical Advice for Persons Who Inject Illicit Drugs.68
Prevention of Hepatitis C in Injection Drug Users
Preventing morbidity and mortality from hepatitis C in injection drug users requires (1) reducing exposure to HCV, (2) reducing infection among those exposed, and (3) reducing disease among those infected.8 Injection drug use would be greatly reduced if all those who needed treatment for substance use treatment could get it (prevention of exposure). HCV spread can be prevented if drug users have access to sterile syringes and education in how they can avoid acquiring and transmitting the virus (prevention of infection). Finally, barriers to medical treatment must be overcome so that drug users can benefit from advances in HCV treatment (prevention of disease).9 HCV treatment also may reduce transmission (prevention of infection) because HCV-infected drug users are the source for most HCV transmission in the United States (Table 5).
Table 5.
Strategies for HCV Prevention and Control in Injection Drug Users
| Reducing injection drug use (prevention of exposure) |
| Evidence-based substance abuse prevention |
| Expansion of substance abuse treatment |
| Reducing HCV transmission among injection drug users (prevention of infection) |
| Access to sterile syringes and other injection equipment |
| Repeal of paraphernalia and syringe prescription laws |
| Establishment of syringe exchange and distribution programs |
| Education of physicians and pharmacists to provide injection drug users access to sterile injection equipment |
| Community-based outreach to injection drug users |
| Education in safe injection |
| Client-centered HCV counseling and testing |
| Reducing liver disease in infected injection drug users (prevention of disease) |
| Integration of prevention and care |
| Medical treatment for HCV infection |
| Integration of medical, mental health, substance use, and social services |
| Provision of services to incarcerated populations |
Fully implementing measures to prevent hepatitis C among drug users will require changing public policies and instituting or expanding public health programs, but physicians and other health care providers can have a positive effect without waiting for these changes. Access to sterile syringes should be expanded through expansion of syringe exchange programs and the repeal of laws restricting syringe access.75,76 However, health care providers also can play a critical role by referring patients to syringe exchange programs, teaching them safe injection, and prescribing and dispensing syringes. Physicians and pharmacists should be educated to recognize that helping injection drug users gain access to sterile syringes and educating them in safe injection are potentially life-saving interventions,81-84 and ones that cost little or nothing. All patients with hepatitis C should be warned that their blood may be infectious, even through trivial contact, and should be instructed in how to avoid transmitting the infection to others. Those who inject drugs should be given biohazard sharps containers or instructed to safely dispose of injection equipment in puncture-resistant containers.85,86
The capacity for substance use treatment should be expanded.56,57 The current capacity for drug use treatment is sufficient for only 15% to 20% of the drug users in the United States. This lack of availability may be responsible for more blood-borne disease transmission in the United States than any other deficiency except the failure to adequately fund needle exchange programs and to allow drug users access to sterile syringes. The shortage may be relieved somewhat if physicians in medical practice prescribe opiate replacement therapy, either through office-based methadone programs or the use of the recently approved opiate agonist buprenorphine for the outpatient treatment of opiate addiction. With these changes in public policy on opiate agonists, however, also must come changes in attitudes of physicians who will need to gain training and experience with these modalities and prescribe them, if they are to help. An immediate and substantial expansion of the substance use treatment capacity, through a variety of approaches, must be the cornerstone of any approach to reducing the harmful health consequences of substance use.
Community-based hepatitis C prevention programs are needed, to provide outreach, HCV testing and counseling, and education in safe injection, and to link patients who are found to be positive to medical care. Members of groups at high risk for HCV infection, such as injection drug users and incarcerated persons, should be regularly screened for HCV infection. Efforts are particularly important to identify persons with new HCV infections because treatment may be more effective during the acute phase than later, and those with advanced hepatic fibrosis, in whom treatment may reduce the incidence of hepatocellular carcinoma and improve survival.
Finally, correctional facilities provide an enormous opportunity to safely and effectively treat a large number of persons with hepatitis C and provide prevention services to persons at risk for hepatitis C.87 Approximately one quarter of the nearly 2 million individuals incarcerated in state and federal correctional facilities in the United States have hepatitis C. Efforts are needed to ensure that therapy and education on prevention of hepatitis C are provided to prisoners. The medical issues involved in treating prisoners for hepatitis C are no different from those involved in treating any patient with this disease, and in many respects the logistical issues (such as adherence and interference of therapy with work and daily activity) are less problematic.87 The withholding of hepatitis C prevention and treatment from incarcerated persons, although widespread, is unethical.88 Federal, state, and local correctional departments must be given the resources to provide optimal therapy and means of prevention of hepatitis C to prisoners.
Conclusion
A sound policy for the control of hepatitis C will require implementing prevention and treatment programs designed for injection drug users, the group most severely affected by this infection.8,9 Controlling hepatitis C in the United States will require further research to develop and test effective strategies for prevention and treatment for persons who inject drugs. In the meantime, substantial progress can be made to control hepatitis C if existing knowledge and resources are brought to bear.
Future Research Needs
Research is needed to better understand the epidemiology and natural history of hepatitis C in injection drug users. The number of injection drug users in the United States who have hepatitis C is unknown, as is the number of those who become infected each year and the numbers who develop cirrhosis and die of hepatitis C annually. It has been estimated that 35,000 persons are newly infected with HCV each year in the United States, and that about 60% of infected persons are injection drug users.44 These estimates, however, are derived from data from a surveillance system in 4 U.S. counties89 and rely on estimates that 1 in 6 new infections in these counties manifest as acute hepatitis, and 42% of these cases are reported to the surveillance system.90 It is rare, however, for HCV infection in drug users to be associated with a clinical illness that comes to medical attention and clinical cases of acute hepatitis among injection drug users often are not reported.91 Thus, the current estimates may underestimate the true number of drug users becoming infected with HCV in the United States. Ongoing programs to assess the current rates as well as future changes in the incidence of HCV infection in injection drug users are needed to provide data on the size of this problem as well as the efficacy of control measures as they are introduced.
The natural history of hepatitis C among injection drug users needs to be better defined. There have been few longitudinal studies of hepatitis C in representative cohorts of injection drug users.92 The course and outcome of hepatitis C may be different in injection drug users than in other populations because of differences in size of the inoculum, the frequency of repeated exposure, concurrent nutritional status, coinfections (with known and unknown infectious agents), associated comorbidities, and other factors that affect the natural history of the disease. Community-based studies are needed because studies of clinical populations often overestimate the frequency of clinical disease.93 Ultimately, decisions on therapy need to be based on an accurate understanding of the risk for serious disease in patients who are not treated.
Research is needed on strategies for treating hepatitis C in substance users. Studies are needed to define the optimal approach to therapy in patients who use various substances (opiates, stimulants, marijuana, alcohol) and are at various stages in recovery or relapse. Studies are needed to determine whether substance use is best treated before, during, or after treatment of hepatitis C. Of great importance is research on how to assess and manage mental health conditions in drug users with hepatitis C, improve treatment readiness and rates of treatment initiation, optimize adherence, and manage the side effects, particularly the psychological side effects, of interferon. Studies are needed to determine the safety and effectiveness of various treatment strategies, the rates of adherence that can be achieved, the risk of serious side effects, and the risk of reinfection. The pharmacokinetic interactions between therapies for hepatitis C and opiate agonists as well as illicit drugs need to be elucidated. Strategies need to be developed for treating hepatitis C in correctional facilities while preserving confidentiality, maximizing continuity of care after release, and preventing reinfection in prison and after release. Perhaps most importantly, research is needed on the prevention of hepatitis C among injection drug users. Of particular importance is the development of methods of reaching new initiates to injection before they become infected with HCV, with effective strategies to help them stop injecting or avoid engaging in high-risk injection practices. Perhaps most importantly, research is needed on the prevention of hepatitis C among injection drug users. Of particular importance is the development of methods of reaching new initiates to injection before they become infected with HCV, with effective strategies to help them stop injecting or avoid engaging in high-risk injection practices.
Acknowledgments
Acknowledgment: The author gratefully acknowledges the help of all those who contributed to this report: Scott A. Allen, Frederick L. Altice, Tomas Aragon, Markus Backmund, Joshua D. Bamberger, David R. Bangsberg, Robert E. Booth, Scott Burris, Charles C. J. Carpenter, Margaret A. Chesney, Allan Clear, Nick Crofts, James W. Curran, Don C. Des Jarlais, Maria L. Ekstrand, Neil M. Flynn, Gerald H. Friedland, Samuel R. Friedman, Cynthia A. Gomez, Lawrence O. Gostin, Marc N. Gourevitch, Marilyn Hollinquest, Peter Hauser, Robert Heimer, James G. Kahn, Mitchell H. Katz, Susan M. Kegeles, Robert S. Klein, Stewart Leavitt, Bernard Lo, David S. Metzger, Stephen F. Morin, Nancy Moss, Phillip I. Nieburg, Thomas R. O'Brien, Kim Page-Shafer, Allan Rosenfield, Josiah D. Rich, Ellie E. Schoenbaum, Peter A. Selwyn, James L. Sorensen, Sharon L. Stancliff, Steffanie A. Strathdee, Diana L. Sylvestre, David L. Thomas, David Vlahov, Paul A. Volberding, Robert M. Weinrieb, Ian T. Williams, Alex D. Wodak, Teresa L. Wright, and Barry Zevin.
Footnotes
Supported by National Institutes of Health grants R01-DA-11860, R01-DA-12109, and R01-DA-13245.
- HCV
- hepatitis C virus
- HIV
- human immunodeficiency virus
References
- 1.Thomas DL, Vlahov D, Solomon L, Cohn S, Taylor E, Garfein R, Nelson KE. Correlates of hepatitis C virus infections among injection drug users. Medicine (Baltimore) 1995;74:212–220. doi: 10.1097/00005792-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Lorvick J, Kral AH, Seal KH, Gee L, Edlin BR. Prevalence and duration of hepatitis C among injection drug users in San Francisco. Calif. Am J Public Health. 2001;91:46–47. doi: 10.2105/ajph.91.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagan H, McGough JP, Thiede H, Weiss NS, Hopkins S, Alexander ER. Syringe exchange and risk of infection with hepatitis B and C viruses. Am J Epidemiol. 1999;149:203–213. doi: 10.1093/oxfordjournals.aje.a009792. [DOI] [PubMed] [Google Scholar]
- 4.Garfein RS, Doherty MC, Monterroso ER, Thomas DL, Nelson KE, Vlahov D. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(Suppl 1):S11–S19. doi: 10.1097/00042560-199802001-00004. [DOI] [PubMed] [Google Scholar]
- 5.Hahn JA, Page-Shafer K, Lum PJ, Ochoa K, Moss AR. Hepatitis C virus infection and needle exchange use among young injection drug users in San Francisco. Hepatology. 2001;34:180–187. doi: 10.1053/jhep.2001.25759. [DOI] [PubMed] [Google Scholar]
- 6.Hagan H, Thiede H, Weiss NS, Hopkins SG, Duchin JS, Alexander ER. Sharing of drug preparation equipment as a risk factor for hepatitis C. Am J Public Health. 2001;91:42–46. doi: 10.2105/ajph.91.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorpe LE, Ouellet LJ, Hershow R, Bailey SL, Williams IT, Williamson J, Monterroso ER, et al. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. Am J Epidemiol. 2002;155:645–653. doi: 10.1093/aje/155.7.645. [DOI] [PubMed] [Google Scholar]
- 8.Edlin BR. Hepatitis C prevention and treatment for substance users in the United States: acknowledging the elephant in the living room. Int J Drug Policy. (in press) [Google Scholar]
- 9.Edlin BR, Seal KH, Lorvick J, Kral AH, Ciccarone DH, Moore LD, Lo B. Is it justifiable to withhold treatment for hepatitis C from illicit-drug users? N Engl J Med. 2001;345:211–214. doi: 10.1056/NEJM200107193450311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tulsky JP, Pilote L, Hahn JA, Zolopa AJ, Burke M, Chesney M, Moss AR. Adherence to isoniazid prophylaxis in the homeless: a randomized controlled trial. Arch Intern Med. 2000;160:697–702. doi: 10.1001/archinte.160.5.697. [DOI] [PubMed] [Google Scholar]
- 11.Pablos-Mendez A, Knirsch CA, Barr RG, Lerner BH, Frieden TR. Non-adherence in tuberculosis treatment: predictors and consequences in New York City. Am J Med. 1997;102:164–170. doi: 10.1016/s0002-9343(96)00402-0. [DOI] [PubMed] [Google Scholar]
- 12.Singh N, Squier C, Sivek C, Wagener M, Nguyen MH, Yu VL. Determinants of compliance with antiretroviral therapy in patients with human immunodeficiency virus: prospective assessment with implications for enhancing compliance. AIDS Care. 1996;8:261–269. doi: 10.1080/09540129650125696. [DOI] [PubMed] [Google Scholar]
- 13.Ferrando SJ, Wall TL, Batki SL, Sorensen JL. Psychiatric morbidity, illicit drug use and adherence to zidovudine among injection drug users with HIV disease. Am J Drug Alcohol Abuse. 1996;22:475–487. doi: 10.3109/00952999609001674. [DOI] [PubMed] [Google Scholar]
- 14.Haubrich RH, Little SJ, Currier JS, Forthal DN, Kemper CA, Beall GN, Johnson D, et al. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response. California Collaborative Treatment Group. AIDS. 1999;13:1099–1107. doi: 10.1097/00002030-199906180-00014. [DOI] [PubMed] [Google Scholar]
- 15.Pilote L, Tulsky JP, Zolopa AR, Hahn JA, Schecter GF, Moss AR. Tuberculosis prophylaxis in the homeless. A trial to improve adherence to referral. Arch Intern Med. 1996;156:161–165. [PubMed] [Google Scholar]
- 16.Eldred LJ, Wu AW, Chaisson RE, Moore RD. Adherence to antiretroviral and pneumocystis prophylaxis in HIV disease. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:117–125. doi: 10.1097/00042560-199806010-00003. [DOI] [PubMed] [Google Scholar]
- 17.Moatti JP, Carrieri MP, Spire B, Gastaut JA, Cassuto JP, Moreau J. Adherence to HAART in French HIV-infected infecting drug users: the contribution of buprenorphine drug maintenance treatment. The Manif 2000 Study Group. AIDS. 2000;14:151–155. doi: 10.1097/00002030-200001280-00010. [DOI] [PubMed] [Google Scholar]
- 18.Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. J Acquir Immune Defic Syndr Hum Retrovirol. 2001;27:251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- 19.Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, Bamberger JD, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 20.Singh N, Berman SM, Swindells S, Justis JC, Mohr JA, Squier C, Wagener MM. Adherence of human immunodeficiency virus-infected patients to antiretroviral therapy. Clin Infect Dis. 1999;29:824–830. doi: 10.1086/520443. [DOI] [PubMed] [Google Scholar]
- 21.Bamberger JD, Unick J, Klein P, Fraser M, Chesney M, Katz MH. Helping the urban poor stay with antiretroviral HIV drug therapy. Am J Public Health. 2000;90:699–701. doi: 10.2105/ajph.90.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaisson RE, Barnes GL, Hackman J, Watkinson L, Kimbrough L, Metha S, Cavalcante S, et al. A randomized, controlled trial of interventions to improve adherence to isoniazid therapy to prevent tuberculosis in injection drug users. Am J Med. 2001;110:610–615. doi: 10.1016/s0002-9343(01)00695-7. [DOI] [PubMed] [Google Scholar]
- 23.Broers B, Morabia A, Hirschel B. A cohort study of drug users' compliance with zidovudine treatment. Arch Intern Med. 1994;154:1121–1127. [PubMed] [Google Scholar]
- 24.Samet JH, Libman H, Steger KA, Dhawan RK, Chen J, Shevitz AH, Dewees-Dunk R, et al. Compliance with zidovudine therapy in patients infected with human immunodeficiency virus, type 1: a cross-sectional study in a municipal hospital clinic. Am J Med. 1992;92:495–502. doi: 10.1016/0002-9343(92)90746-x. [DOI] [PubMed] [Google Scholar]
- 25.Mezzelani P, Venturini L, Turrina G, Lugoboni F, Des Jarlais DC. High compliance with a hepatitis B virus vaccination program among intravenous drug users. J Infect Dis. 1991;163:923. doi: 10.1093/infdis/163.4.923. [DOI] [PubMed] [Google Scholar]
- 26.Marco A, Cayla JA, Serra M, Pedro R, Sanrama C, Guerrero R, Ribot N. Predictors of adherence to tuberculosis treatment in a supervised therapy programme for prisoners before and after release. Study Group of Adherence to Tuberculosis Treatment of Prisoners. Eur Respir J. 1998;12:967–971. doi: 10.1183/09031936.98.12040967. [DOI] [PubMed] [Google Scholar]
- 27.Lorvick J, Thompson S, Edlin BR, Kral AH, Lifson AR, Watters JK. Incentives and accessibility: a pilot study to promote adherence to TB prophylaxis in a high-risk community. J Urban Health. 1999;76:461–467. doi: 10.1007/BF02351503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison K, Vlahov D, Jones K, Charron K, Clements ML. Medical eligibility, comprehension of the consent process, and retention of injection drug users recruited for an HIV vaccine trial. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:386–390. [PubMed] [Google Scholar]
- 29.Sackett DL, Snow JC. The magnitude of compliance and noncompliance. In: Haynes RB, Taylor DW, Sackett DL, editors. Compliance in Health Care. Johns Hopkins University Press; Baltimore: 1979. pp. 11–22. [Google Scholar]
- 30.Miller NH. Compliance with treatment regimens in chronic asymptomatic diseases. Am J Med. 1997;102:43–49. doi: 10.1016/s0002-9343(97)00467-1. [DOI] [PubMed] [Google Scholar]
- 31.Cochrane MG, Bala MV, Downs KE, Mauskopf J, Ben-Joseph RH. Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest. 2000;117:542–550. doi: 10.1378/chest.117.2.542. [DOI] [PubMed] [Google Scholar]
- 32.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Wagener MM, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 33.Bangsberg DR, Hecht FM, Clague H, Charlebois ED, Ciccarone D, Chesney M, Moss A. Provider assessment of adherence to HIV antiretroviral therapy. J Acquir Immune Defic Syndr Hum Retrovirol. 2001;26:435–442. doi: 10.1097/00126334-200104150-00005. [DOI] [PubMed] [Google Scholar]
- 34.Mushlin AI, Appel FA. Diagnosing potential noncompliance. Physicians' ability in a behavioral dimension of medical care. Arch Intern Med. 1977;137:318–321. doi: 10.1001/archinte.137.3.318. [DOI] [PubMed] [Google Scholar]
- 35.Roth HP, Caron HS. Accuracy of doctors' estimates and patients' statements on adherence to a drug regimen. Clin Pharmacol Ther. 1978;23:361–370. doi: 10.1002/cpt1978233361. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert JR, Evans CE, Haynes RB, Tugwell P. Predicting compliance with a regimen of digoxin therapy in family practice. Can Med Assoc J. 1980;123:119–122. [PMC free article] [PubMed] [Google Scholar]
- 37.Bosley CM, Fosbury JA, Cochrane GM. The psychological factors associated with poor compliance with treatment in asthma. Eur Respir J. 1995;8:899–904. [PubMed] [Google Scholar]
- 38.Blowey DL, Hebert D, Arbus GS, Pool R, Korus M, Koren G. Compliance with cyclosporine in adolescent renal transplant recipients. Pediatr Nephrol. 1997;11:547–551. doi: 10.1007/s004670050335. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Recommendations of the Panel on Clinical Practices for Treatment of HIV. MMWR Morb Mortal Wkly Rep. 2002;51(RR7):1–55. [PubMed] [Google Scholar]
- 40.Backmund M, Meyer K, Von Zielonka M, Eichenlaub D. Treatment of hepatitis C infection in injection drug users. Hepatology. 2001;34:188–193. doi: 10.1053/jhep.2001.25882. [DOI] [PubMed] [Google Scholar]
- 41.Sylvestre DL. Treating hepatitis C in methadone maintenance patients: an interim analysis. Drug Alcohol Depend. 2002;67:117–123. doi: 10.1016/s0376-8716(02)00010-8. [DOI] [PubMed] [Google Scholar]
- 42.Sylvestre DL. Treatment of HCV in the methadone patient; Co-Morbid Conditions Associated With Hepatitis C. Hepatitis Single Topic Conference, Chicago, Illinois, 26-28 April 2002; Alexandria, VA: American Association for the Study of Liver Diseases. 2002.pp. 103–107. [Google Scholar]
- 43.Dalgard O, Bjoro K, Hellum K, Myrvang B, Skaug K, Gutigard B, Bell H. Treatment of chronic hepatitis C in injecting drug users: 5 years' follow-up. Eur Addict Res. 2002;8:45–49. doi: 10.1159/000049487. [DOI] [PubMed] [Google Scholar]
- 44.National Institutes of Health Management of hepatitis C [On-line] NIH Consens Statement. 1997;15:1–41. Available. [PubMed] [Google Scholar]
- 45.O'Connor PG, Selwyn PA, Schottenfeld RS. Medical care for injection-drug users with human immunodeficiency virus infection. N Engl J Med. 1994;331:450–459. doi: 10.1056/NEJM199408183310707. [DOI] [PubMed] [Google Scholar]
- 46.Batki S, Sorensen JL. Care of injection drug users with HIV. In: Cohen PT, Sande MS, Volderding PA, editors. The AIDS Knowledge Base: A Textbook on HIV Disease. The University of California, San Francisco and San Francisco General Hospital. 3 rd ed. Lippincott, Williams and Wilkins; Philadelphia: 1999. [Google Scholar]
- 47.Wartenberg AA. HIV disease in the intravenous drug user: role of the primary care physician. J Gen Intern Med. 1991;6(Suppl 1):S35–S40. doi: 10.1007/BF02599256. [DOI] [PubMed] [Google Scholar]
- 48.Selwyn PA, O'Connor PG. Diagnosis and treatment of substance users with HIV infection. Prim Care. 1992;19:119–156. [PubMed] [Google Scholar]
- 49.Des Jarlais DC, Friedman SR, Ward TP. Harm reduction: a public health response to the AIDS epidemic among injecting drug users. Annu Rev Public Health. 1993;14:413–450. doi: 10.1146/annurev.pu.14.050193.002213. [DOI] [PubMed] [Google Scholar]
- 50.Marlatt GA, editor. Harm reduction: pragmatic strategies for managing high-risk behaviors. Guilford Press; New York: 1998. [Google Scholar]
- 51.Riley D, Sawka E, Conley P, Hewitt D, Mitic W, Poulin C, Room R, et al. Harm reduction: concepts and practice. A policy discussion paper. Subst Use Misuse. 1999;34:9–24. doi: 10.3109/10826089909035632. [DOI] [PubMed] [Google Scholar]
- 52.Gostin L. Waging a war on drug users: an alternative public health vision. Law Med Health Care. 1990;18:385–394. doi: 10.1111/j.1748-720x.1990.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 53.Robertson R, editor. Management of drug users in the community: a practical handbook. Arnold; London: 1998. [Google Scholar]
- 54.Lowinson JH. Substance abuse: a comprehensive textbook. 3 rd ed. Williams & Wilkins; Baltimore: 1997. [Google Scholar]
- 55.Strain EC, Stitzer ML, editors. Methadone treatment for opioid dependence. Johns Hopkins University Press; Baltimore: 1999. [Google Scholar]
- 56.Gerstein DR, Lewin LS. Treating drug problems. N Engl J Med. 1990;323:844–848. doi: 10.1056/NEJM199009203231230. [DOI] [PubMed] [Google Scholar]
- 57.National Institutes of Health Effective medical treatment of opiate addiction [On-line] NIH Consens Statement. 1997;15:1–38. Available. [Google Scholar]
- 58.Ball JC, Lange WR, Myers CP, Friedman SR. Reducing the risk of AIDS through methadone maintenance treatment. J Health Soc Behav. 1988;29 [PubMed] [Google Scholar]
- 59.Metzger DS, Navaline H, Woody GE. Drug abuse treatment as AIDS prevention. Public Health Rep. 1998;113(Suppl 1):97–106. [PMC free article] [PubMed] [Google Scholar]
- 60.Hartel DM, Schoenbaum EE. Methadone treatment protects against HIV infection: two decades of experience in the Bronx, New York City. Public Health Rep. 1998;113(Suppl 1):107–115. [PMC free article] [PubMed] [Google Scholar]
- 61.Sorensen JL, Copeland AL. Drug abuse treatment as an HIV prevention strategy: a review. Drug Alcohol Depend. 2000;59:17–31. doi: 10.1016/s0376-8716(99)00104-0. [DOI] [PubMed] [Google Scholar]
- 62.Weisner C, Mertens J, Parthasarathy S, Moore C, Lu Y. Integrating primary medical care with addiction treatment: a randomized controlled trial. JAMA. 2001;286:1715–1723. doi: 10.1001/jama.286.14.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Friedland GH, Williams A. Attaining higher goals in HIV treatment: the central importance of adherence. AIDS. 1999;13(Suppl 1):S61–S72. [PubMed] [Google Scholar]
- 64.Reiter GS, Stewart KE, Wojtusik L, Hewitt R, Segal-Maurer S, Johnson M, Fisher A, et al. Elements of success in HIV clinical care: multiple interventions that promote adherence [On-line] Top HIV Med. 2000;8:21–30. Available. [Google Scholar]
- 65.Dieperink E, Willenbring M, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: a review. Am J Psychiatry. 2000;157:867–876. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- 66.Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill JA, Gulati M, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 67.U.S. Preventive Services Task Force . Guide to Clinical Preventive Services. 2 nd ed. Williams & Wilkins; Baltimore: 1996. p. 591. [Google Scholar]
- 68.U.S. Public Health Service; Rockville, MD: HIV prevention bulletin: medical advice for persons who inject illicit drugs [On-line] 1997 Available http://www.cdc.gov/idu/pubs/hiv_prev.htm.
- 69.Rich JD, Macalino GE, McKenzie M, Taylor LE, Burris S. Syringe prescription to prevent HIV infection in Rhode Island: a case study. Am J Public Health. 2001;91:699–700. doi: 10.2105/ajph.91.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burris S, Lurie P, Abrahamson D, Rich JD. Physician prescribing of sterile injection equipment to prevent HIV infection: time for action. Ann Intern Med. 2000;133:218–226. doi: 10.7326/0003-4819-133-3-200008010-00015. [DOI] [PubMed] [Google Scholar]
- 71.Centers for Disease Control . Fact sheet: physician prescription of sterile syringes to injection drug users [On-line] Academy of Educational Development; Atlanta, GA: 2002. Available http://www.cdc.gov/idu/facts/Physician.htm. [Google Scholar]
- 72.Watters JK, Estilo MJ, Clark GL, Lorvick J. Syringe and needle exchange as HIV/AIDS prevention for injection drug users. JAMA. 1994;271:115–120. [PubMed] [Google Scholar]
- 73.Bluthenthal RN, Kral AH, Erringer EA, Edlin BR. Use of an illegal syringe exchange and injection-related risk behaviors among street-recruited injection drug users in Oakland, California, 1992-1995. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:505–511. doi: 10.1097/00042560-199808150-00013. [DOI] [PubMed] [Google Scholar]
- 74.Bluthenthal RN, Kral AH, Gee L, Erringer EA, Edlin BR. The effect of syringe exchange use on high-risk injection drug users: a cohort study. AIDS. 2000;14:605–611. doi: 10.1097/00002030-200003310-00015. [DOI] [PubMed] [Google Scholar]
- 75.Normand J, Vlahov D, Moses L, editors. National Research Council. Institute of Medicine. National Academy Press; Washington: 1995. Preventing HIV transmission: the role of sterile needles and bleach. [PubMed] [Google Scholar]
- 76.National Institutes of Health Interventions to prevent HIV risk behaviors [On-line] NIH Consens Statement. 1997;15:1–41. Available. [PubMed] [Google Scholar]
- 77.Edlin BR, Gee L, Kral AH, Seal KH, Lorvick L, Tobler LH, Andrews AA. Decline of hepatitis C virus transmission among injection drug users, San Francisco, 1977-1998 [abstract #615] Hepatology. 2000;32 (4 Pt 2):313A. American Association for the Study of Liver Diseases 51st Annual Meeting, Dallas, TX, October 27-31, 2000 (oral presentation) [Google Scholar]
- 78.Flynn NM, Anderson R, Clancy L, Britton J. Seeing is believing: videotaped high-risk injection behaviour; 7th International Conference on the Reduction of Drug Related Harm; Hobart, Australia. March 3-7, 1996. [Google Scholar]
- 79.McCoy CB, Metsch LR, Chitwood DD, Shapshak P, Comerford ST. Parenteral transmission of HIV among injection drug users: assessing the frequency of multiperson use of needles, syringes, cookers, cotton, and water. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(Suppl 1):25–29. doi: 10.1097/00042560-199802001-00006. [DOI] [PubMed] [Google Scholar]
- 80.Kral AH, Bluthenthal RH, Erringer EA, Lorvick J, Edlin BR. Risk factors among IDUs who give injections to or receive injections from other drug users. Addiction. 1999;94:675–683. doi: 10.1046/j.1360-0443.1999.9456755.x. [DOI] [PubMed] [Google Scholar]
- 81.Gostin LO, Lazzarini Z, Jones TS, Flaherty K. Prevention of HIV/AIDS and other blood-borne diseases among injection drug users: a national survey on the regulation of syringes and needles. JAMA. 1997;277:53–62. [PubMed] [Google Scholar]
- 82.Holtgrave DR, Pinkerton SD, Jones TS, Lurie P, Vlahov D. Cost and cost-effectiveness of increasing access to sterile syringes and needles as an HIV prevention intervention in the United States. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(Suppl 1):S133–S138. doi: 10.1097/00042560-199802001-00022. [DOI] [PubMed] [Google Scholar]
- 83.Fact sheet: Policy efforts to increase IDUs' access to sterile syringes [On-line] Atlanta, GA: Centers for Disease Control, Academy of Educational Development; [2002]. Available http://www.cdc.gov/idu/facts/aed_idu_pol.htm. [Google Scholar]
- 84.Gleghorn AA, Gee G, Vlahov D. Pharmacists' attitudes about pharmacy sale of needles/syringes and needle exchange programs in a city without needle/syringe prescription laws. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(Suppl 1):S89–S93. doi: 10.1097/00042560-199802001-00016. [DOI] [PubMed] [Google Scholar]
- 85.Macalino GE, Springer KW, Rahman ZS, Vlahov D, Jones TS. Community-based programs for safe disposal of used needles and syringes. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(Suppl 1):S111–S119. doi: 10.1097/00042560-199802001-00019. [DOI] [PubMed] [Google Scholar]
- 86.Centers for Disease Control . Fact sheet: Syringe disposal [On-line] Academy of Educational Development; Atlanta, GA: 2002. Available http://www.cdc.gov/idu/facts/aed_idu_dis.htm. [Google Scholar]
- 87.Allen SA, Spaulding AC, Osei AM, Taylor LE, Cabral AM, Rich JD. Treatment of chronic hepatitis C in a state correctional facility. Ann Intern Med. 2003;138:187–190. doi: 10.7326/0003-4819-138-3-200302040-00010. [DOI] [PubMed] [Google Scholar]
- 88. United Nations General Assembly Resolution 37/194. Principles of Medical Ethics relevant to the Role of Health Personnel, Particularly Physicians, in the Protection of Prisoners and Detainees Against Torture and Other Cruel, Inhuman or Degrading Treatment or Punishment. Adopted December 18, 1982.
- 89.Alter MJ, Margolis HS, Krawczynski K, Judson FN, Mares A, Alexander WJ, Hu PY, et al. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med. 1992;327:1899–1905. doi: 10.1056/NEJM199212313272702. [DOI] [PubMed] [Google Scholar]
- 90.CDC . Hepatitis C disease burden: estimation method. Centers for Disease Control and Prevention; Atlanta: 2002. http://www.cdc.gov/ncidod/diseases/hepatitis/resource/dz_burden02. htm Available from. [Google Scholar]
- 91.Hagan H, Snyder N, Hough E, Yu T, McKiernan S, Boase J, Duchin J. Case-reporting of acute hepatitis B and C among injection drug users. J Urban Health. 2002;79(4):579–585. doi: 10.1093/jurban/79.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, Nolt K, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 93.Freeman AJ, Dore GJ, Law MG, Thorpe M, Von Overbeck J, Lloyd AR, Marinos G, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809–816. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]


