Abstract
A key characteristic of highly social animals is collective group response to important stimuli such as invasion by enemies. The marine societies of social snapping shrimp share many convergences with terrestrial eusocial animals, including aggressive reaction to strangers, but no group actions have yet been observed in shrimp. Here we describe ‘coordinated snapping’, during which a sentinel shrimp reacts to danger by recruiting other colony members to snap in concert for several to tens of seconds. This distinctive behaviour is a specific response to intrusion by strange shrimp into the colony's sponge and is highly successful at repelling these intruders. Although coordinated snapping apparently functions analogously to alarm responses in other social animals, colony members in social shrimp do not rush to the site of the attack. Coordinated snapping appears instead to be a warning signal to would-be intruders that the sponge is occupied by a cooperative colony ready to defend it. This is the first evidence for coordinated communication in social shrimp and represents yet another remarkable convergence between social shrimp, insects and vertebrates.
Keywords: communication, defence, shrimp, social evolution, Synalpheus
1. Introduction
In eusocial animal colonies, most individuals forego reproduction to focus on raising the offspring of a few (Wilson 1971; Crespi 1994). Many aspects of colony performance depend on close coordination of individual behaviours because natural selection often acts on the colony as a whole (e.g. Moritz & Southwick 1992). In both eusocial insects and naked mole-rats, such collective actions are initiated by specific signals, including recruitment signals that lead many nest mates to new nest sites or rich food sources, and alarm signals, whereby individuals alert others to danger and recruit them to the place of attack (Wilson 1971; Pepper et al. 1991; Aoki 2003).
Social shrimp (Synalpheus) are tiny (∼5–10 mm) obligate inhabitants of tropical sponges that live and feed within their host's internal canals. Despite their specialized life style and distinct marine habitat, social shrimp exhibit several striking parallels (Duffy 1996, 2003) with terrestrial eusocial insects and vertebrates (Wilson 1971; Sherman et al. 1991; Choe & Crespi 1997; O'Riain et al. 2000). Most importantly, shrimp colonies contain one to a few reproductive females and tens to hundreds of genetically related, non-breeding colony members. These colonies consist of several cohabiting generations, with the one or few reproductive females typically being the largest individuals (Duffy & Macdonald 1999), qualifying them as eusocial (‘truly social’). Moreover, colony cohesion is enhanced in social shrimp by elevated aggression toward strangers (Duffy 1996; Duffy et al. 2002), just as in many terrestrial social animals (Wilson 1971; Sherman et al. 1991; Choe & Crespi 1997). Yet, despite the importance of coordinated group behaviour to colony fitness in other eusocial animals (Wilson 1971; Pepper et al. 1991; Aoki 2003), no such behaviour has been observed previously in eusocial shrimp.
A major impetus to cooperation in snapping shrimp appears to be competition for territories (Duffy et al. 2000, 2002). All snapping shrimp (Alpheidae) are territorial and equipped with a powerful weapon, the fighting claw (major chela), used in communication and combat (Nolan & Salmon 1970; Duffy et al. 2002). Rapid closure of the claw directs a powerful water jet at a nearby opponent and generates a loud noise (Knowlton & Moulton 1963; Versluis et al. 2000). In eusocial shrimp specifically, competition for sponge hosts appears intense (Duffy 2003), and aggression against nest intruders is accordingly strong (Duffy 1996; Duffy et al. 2002). To date, however, only individual responses to intruders have been observed in shrimp. To investigate the possibility of coordinated cooperative action in social shrimp colonies, we studied colony defence in two related shrimp species under semi-natural conditions.
2. Material and methods
(a) Whole-sponge experiments
We explored responses of minimally disturbed Synalpheus rathbunae colonies to intruders by placing whole sponges (Xestospongia roasariensis, n=8) containing shrimp colonies in separate, running seawater aquaria at Bocas del Toro, Panama. After 12 h acclimation, we introduced four conspecific intruders simultaneously to the surface of a sponge, and counted single and coordinated snaps (see §3) for 30 min before, and 30 min after, introduction.
(b) Individual observations
We studied individual interactions between residents and intruders in seven partial colonies (each with queen and 60 males and juveniles) of Synalpheus regalis from Carrie Bow Cay, Belize. Colonies were placed into Plexiglass chambers (10×10×0.5 cm) containing a slice of sponge (Lissodendoryx colombiensis, see Duffy et al. (2002) for details). We observed behaviour of resident shrimp toward conspecific intruders (both experimentally introduced individuals, and individuals from the same sponge that elicited aggression), and videotaped all snapping events between 05.00 and 24.00 for 3–7 days. We recorded 96 coordinated snapping events from seven colonies and tallied event duration, and numbers of snappers and snaps. To illustrate a coordinated snapping event, we converted the digital audio recording (22 050 Hz, 16 bit resolution) to an amplitude plot after bandpass-filtering at 6600–6700 Hz using a Bessel filter implemented in LabVIEW 7.
(c) Statistics
All analyses treated individual colonies (n=6–8) as independent replicates. For a given response variable, the datum analysed was the colony-specific median value, calculated across all snapping events observed in that colony.
3. Results
Conspecific intruders introduced to whole sponges repeatedly tried to enter the sponge, each time eliciting vigorous snaps from the individual residents inside. In addition to these ‘single snaps’, attempted intrusions sometimes caused many residents to suddenly begin snapping in unison, producing a distinctive crackling noise lasting for several to tens of seconds (‘coordinated snapping’, figure 1a). Single snaps, which occurred occasionally before intruder introduction, increased sharply afterwards (figure 2a), whereas coordinated snapping, observed in five of the eight trials, occurred exclusively after introducing intruders (figure 2b). Although all attacked intruders eventually withdrew from sponge entrances, sometimes swimming away, coordinated snapping usually occurred only after intruders ignored repeated single snaps. Specifically, repulsion of an intruder required an average (±1 s.e.m.) of 3.7±0.5 snaps when no coordinated event occurred, but 8.7±0.9 snaps prior to a coordinated snapping event (figure 2c,d). This difference suggests that coordinated snapping is an escalated response elicited after several individual warning snaps fail to repel intruders. Supporting this hypothesis, two intruders that became stuck in an entrance hole elicited not one but several coordinated snapping events, which ceased only after we removed these intruders.
Figure 1.
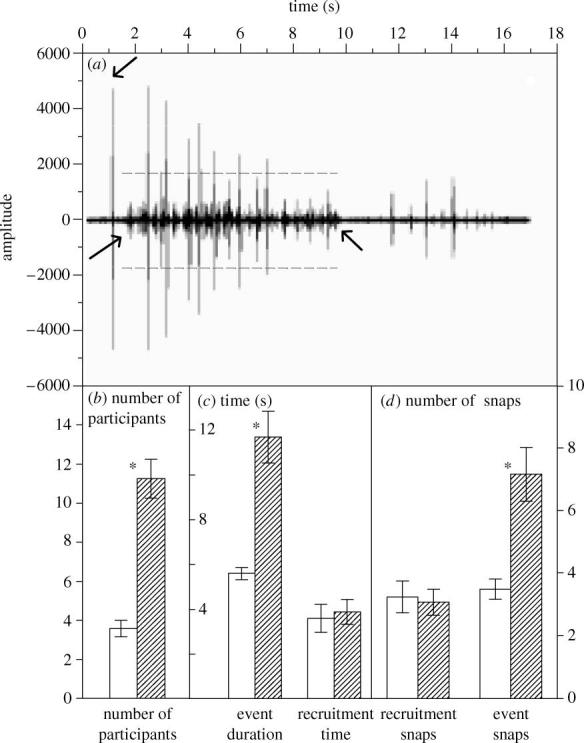
(a) Amplitude plot of a coordinated snapping event in Synalpheus regalis. The upper arrow indicates a recruiting snap by the defender at the intruder, and lower arrows indicate starting and ending points of the ensuing event. Dashed horizontal lines bound the coordinated snaps, which are much more frequent but lower amplitude, than defender's snaps. Snaps exceeding dashed lines are by the defender. (b–d) Characteristics of local (open bars) and general (hatched bars) coordinated snapping events. Bars show the mean (±s.e.m.), calculated across colonies (n=6), of colony-specific median values (see §2). (b) Participation is the number of individuals involved in the event (t=7.22, p=0.001). (c) Event duration is the time between beginning and end of coordinated snapping (t=5.19, p=0.003). Recruitment time is the interval between a defender's first snap at an intruder and initiation of coordinated snapping (t=0.26, p=0.81). (d) Recruitment snaps is the number of snaps by a defender at an intruder before coordinated snapping begins (t=0.26, p=0.805). Event snaps indicate the number of snaps a defender made at an intruder during the coordinated event (t=5.5, p=0.001). An asterisk indicates significant difference between local and general events (t-test).
Figure 2.
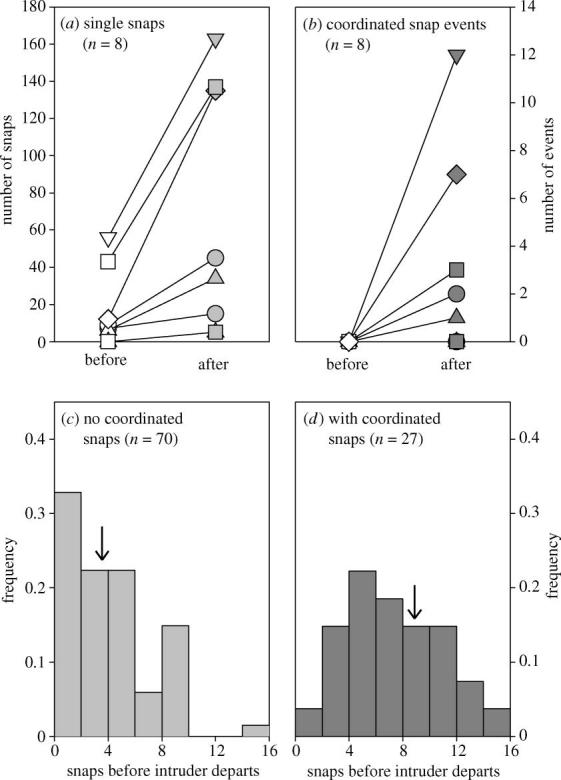
Responses of Synalpheus rathbunae to intruders in whole-sponge experiments. (a,b) Numbers of (a) single snaps, and (b) coordinated snap events, for 30 min before (open symbols) and 30 min after (filled symbols) introduction of intruders in each of eight colonies. Both single ( p=0.012, n=8 colonies, Wilcoxon paired-sample test) and coordinated snaps increased in frequency after introduction of intruders. (c,d) Frequency distributions of numbers of snaps required to repel an intruder when the intrusion (c) failed, versus (d) succeeded, in eliciting a coordinated snapping event. Arrows above histograms show the mean across colonies (n=5) of the median value per colony, which differ in absence versus presence of coordinated snapping ( p=0.010, paired-sample t-test).
As in whole-sponge experiments, coordinated snapping occurred in observation chambers after a defender forcefully snapped at an intruder several times in short succession. After these recruitment snaps, up to 60% of the visible colony members began snapping rhythmically, on average twice per second. We distinguished two classes of coordinated snapping events based on bimodal distributions of both the number of participants (figure 1b) and duration of events (figure 1c): more common ‘local events’ (n=65, from seven colonies) involved less than seven residents within 2 cm of the intruder, whereas ‘general events’ (n=31 from six colonies) included many individuals throughout the sponge. The two classes of coordinated snapping events did not differ in either number of snaps by the defender before an event began (i.e. recruiting snaps), nor in time between the first attacking snaps and beginning of the event (recruitment time, figure 1c,d ). However, in general events, the defender produced more snaps (event snaps, figure 1d ) than during local events. Its colony mates responded by prolonging the coordinated snapping; thus, the difference in scale of events appeared to be controlled by the defender. Moreover, the defender's snaps against the intruder were considerably louder than the ensuing snaps of colony members responding in a coordinated event, and also appeared louder than single snaps that did not elicit a coordinated snapping event (figure 1a).
While defenders often maintained antennal contact with the intruder, their simultaneously snapping colony mates generally remained stationary. Although intruders in the experimental chambers could not flee as far from defenders as they could in nature, most attempted to do so and eventually ended up on the sponge exterior where they were less prone to attacks. Intruders were sometimes injured by defenders. We also witnessed the killing of three intruders after they became trapped in a sponge canal, and could not escape.
4. Discussion
Our experiments suggest that coordinated snapping in social shrimp is a specific and effective group warning signal to nest intruders, produced when individual defenders are unable to chase intruders away. The proposition that individual defenders elicit coordinated snapping and act as pacemakers for the group response is supported by the higher amplitude of the defender's snaps, compared with the ensuing coordinated event, and by the correlation between number of defender's (event) snaps and duration of the coordinated event. The function of coordinated snapping as a specific warning to intruders is supported by its occurrence only after introductions of intruders, and its effectiveness at repelling them even after single snaps fail to do so. Finally, coordinated snapping is an honest warning signal because the few intruders unable to flee were subsequently killed.
Coordinated snapping in social shrimp shows remarkable parallels to coordinated communication and defence in other highly social animals. Defenders that encounter intruders send a specific signal to other colony members, which is followed by a coordinated response. Unlike many social animals, however, social shrimp do not react by attacking the intruder en masse. There are several potential explanations for this lack of movement toward intruders. First, avoiding fights probably reduces accidental injury as residents close to the intruder often received snaps from defenders, probably due to recognition error. Second, residents may have difficulty approaching an intruder through the narrow sponge canals. Finally, residents converging on one location might block the intruder's escape route, causing it to fight and potentially injure defenders.
Thus, the function of coordinated snapping in social shrimp is not to draft defenders to attack intruders directly. Nor is its purpose to warn vulnerable colony members to hide, since neither juveniles nor queen usually moved during or after coordinated snapping events. Because only defenders in close proximity fought with intruders, and the latter usually retreated, we conclude that coordinated snapping is probably a warning signal directed at intruders. The synchrony of the signal probably indicates that the sponge harbours a social colony whose members are prepared to fight to defend their territory.
Coordinated snapping in social shrimp thus represents a mass communication among colony members, a fundamental characteristic of highly social insects and vertebrates. Coordinated group communication in social shrimp constitutes another close parallel with eusocial insects and vertebrates, along with strong reproductive skew, polymorphism, and behavioural specialization. These parallels suggest that fundamental ecological challenges are often resolved by similar adaptations in disparate lineages.
Acknowledgments
We thank K. S. Macdonald and A. Castillo for field assistance, M. R. Patterson for help with the sound analysis, and D. C. Queller for comments on the manuscript. This work was supported by the Smithsonian Tropical Research Institute, the Smithsonian's Caribbean Coral Reef Ecosystem Program (contribution number 708), and the NSF (IBN 0131931 to J.E.D.).
References
- Aoki S. Soldiers, altruistic dispersal and its consequences for aphid societies. In: Kikuchi T, Azuma N, Higashi S, editors. Genes, behavior and evolution in social insects. Hokkaido University Press; Sapporo: 2003. pp. 201–215. [Google Scholar]
- Choe J.C, Crespi B.J. Cambridge University Press; Cambridge: 1997. Social behavior in insects and arachnids. [Google Scholar]
- Crespi B.J. Three conditions for the evolution of eusociality: are they sufficient? Insect Soc. 1994;41:395–400. [Google Scholar]
- Duffy J.E. Eusociality in coral reef shrimp. Nature. 1996;381:512–514. [Google Scholar]
- Duffy J.E. The ecology and evolution of eusociality in sponge-dwelling shrimp. In: Kikuchi T, Azuma N, Higashi S, editors. Genes, behavior and evolution in social insects. Hokkaido University Press; Sapporo: 2003. pp. 217–252. [Google Scholar]
- Duffy J.E, Macdonald K.S. Colony structure of the social snapping shrimp Synalpheus filidigitus in Belize. J. Crust. Biol. 1999;19:283–292. [Google Scholar]
- Duffy J.E, Morrison C.L, Ríos R. Multiple origins of eusociality among sponge-dwelling shrimps (Synalpheus) Evolution. 2000;54:503–516. doi: 10.1111/j.0014-3820.2000.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Duffy J.E, Morrison C.L, Macdonald K.S. Colony defense and behavioral differentiation in the eusocial shrimp Synalpheus regalis. Behav. Ecol. Sociobiol. 2002;51:488–495. [Google Scholar]
- Knowlton R.E, Moulton J.M. Sound production in the snapping shrimps Alpheus (Crangon) and Synalpheus. Biol. Bull. 1963;125:311–331. [Google Scholar]
- Moritz R.F.E, Southwick E.E. Springer Verlag; New York: 1992. Bees as superorganisms. An evolutionary reality. [Google Scholar]
- Nolan B.A, Salmon M. The behavior and ecology of snapping shrimp (Crustacea: Alpheus heterochaelis and Alpheus normanni) Forma Functio. 1970;2:289–335. [Google Scholar]
- O'Riain M.J, Jarvis J.U.M, Alexander R, Buffenstein R, Peeters C. Morphological castes in a vertebrate. Proc. Natl Acad. Sci. USA. 2000;97:13194–13197. doi: 10.1073/pnas.97.24.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper J.W, Braude S.H, Lacey E.A, Sherman P.W. Vocalizations of the naked mole-rat. In: Sherman P.W, Jarvis J.U.M, Alexander R.D, editors. The biology of the naked mole-rat. Princeton University Press; Princeton: 1991. pp. 243–274. [Google Scholar]
- Sherman P.W, Jarvis J.U.M, Alexander R.D. Princeton University Press; Princeton: 1991. The biology of the naked mole-rat. [Google Scholar]
- Versluis M, Schmitz B, von der Heydt A, Lohse D. How snapping shrimp snap: through cavitation bubbles. Science. 2000;289:2114–2117. doi: 10.1126/science.289.5487.2114. [DOI] [PubMed] [Google Scholar]
- Wilson E.O. Harvard University Press; Cambridge: 1971. The insect societies. [Google Scholar]


