Abstract
Some prey or predator organisms exhibit striking rapid morphological plastic changes with distinct morphology under the condition of predator or prey presence. Remote chemicals propagating from the inducing agents are the prevalent induction cues for most examples of induction of distinct morphs. Sonic and visual cues, as well as chemical cues, are known as triggers for induction of behavioural plasticity. Here we show that hydraulic vibration originating from flapping tails of anuran tadpoles is a key cue in relation to induction of a distinct carnivorous morphology, a broad-headed morph, in larval salamander Hynobius retardatus, which is able to efficiently capture and handle prey. This result was further supported by the fact that simple mechanical vibrations of tail-like vinyl fins were able to induce the morph without any biological cues. Induction of the morph triggered by hydraulic vibration provides a novel concept for understanding the proximate mechanisms of induction of morphological changes.
Keywords: broad-headed morph, chemical cue, hydraulic vibration, phenotypic plasticity, sonic cue
1. Introduction
Recent studies have shown that the environmental context plays a significant role in the development of almost all species, and that animal and plant genomes have evolved to respond to environmental conditions (West-Eberhard 2003). Phenotypic plasticity that has been designed by natural selection is one form of organic adaptation. Conspicuous plastic morphological changes induced by biological agents are known to be inducible morphological defences, triggered by remote chemical cues of the inducing agents in several prey species (Stemberger & Gilbert 1984; Lively 1986; Dodson 1989; Kusch 1993; Bronmark & Pettersson 1994; Tollrian 1995; Trussell 1996; McCollum & Leimberger 1997; Dahl & Peckarsky 2002; Laurila & Kujasalo 1999; Van Buskirk & Arioli 2002; Kishida & Nishimura 2004). Inducible predator morphs (carnivorous morphs) have also been reported in some organisms such as rotifers, anuran tadpoles and larval salamanders (Gilbert 1973; Pfennig 1990; Hoffman & Pfennig 1999; Michimae & Wakahara 2002). Differing from most inducible morphological defences, the carnivorous morphs are commonly triggered by crowding (Gilbert 1973; Hoffman & Pfennig 1999; Michimae & Wakahara 2002), but in spadefoot toad tadpoles are triggered by anostracan shrimp ingestion (Pfennig 1990).
Larvae of the salamander Hynobius retardatus show a broad-headed morph that facilitates carnivorous resource acquisition by preying on conspecific larvae and anuran larvae when salamander larvae are crowded with conspecifics or, especially, anuran tadpoles (Rana pirica), which co-occur with H. retardatus larvae in many natural ponds (Michimae & Wakahara 2002). Even though the induction process needs the proximate cause of crowding, we previously did not identify the nature of the causal factors. In an aquatic environment, an individual in a crowded situation with many signal senders would receive not only visual, tactile and/or close chemical cues but also hydraulic ones from the signal senders. Among these cues, reliable information that specifies the circumstance in which induction of the carnivorous morph is suitable would be included.
Sonic cues, as well as chemical cues, are known as triggers for induction of behavioural plasticity (Montgomery et al. 1988; Janssen et al. 1990). Here, we have advanced the novel hypothesis that the broad-headed carnivorous morph of larval salamander H. retardatus is induced by the flapping tails of tadpoles that create a specific hydraulic vibration. To demonstrate the hypothesis, we conducted the following two experiments. In experiment 1 where live tadpoles' tails were manipulated, we reared larvae of the salamander under four distinct experimental conditions: with two anuran species (factor: species), i.e. R. pirica, which coexists with H. retardatus larvae in many natural ponds, and the heterogeneric species Xenopus laevis, with which salamanders have never coexisted in their evolutionary history, and with different tadpole conditions (factor: tail), i.e. either normal intact (flapping tails) or shortened tails (no flapping tails). In experiment 2, salamander larvae were individually reared in their plastic cups under the condition where tadpoles' tail-like vinyl fins were mechanically flapping.
2. Materials and methods
We collected fertilized eggs of the salamander H. retardatus and the brown frog R. pirica in 2003 and only fertilized eggs of H. retardatus in 2004 in the vicinity of Sapporo, Japan, during the breeding season (from early April to late May). Fertilized eggs of African clawed frogs (X. laevis) were obtained through induced mating in a laboratory. Eggs of each species were separately placed in stock tanks filled with 1.6 l of dechlorinated tap water at room temperature (20–21°C) until hatching.
In all experiments, to prevent differences in quality and quantity of feeding by the larval salamander, the salamander larvae were fed with sufficient frozen Chironomidae from 09.00 to 12.00 every other day. Any food remaining in their tanks (experiment 1) and cups (experiment 2) was removed after the feeding period. The rearing water was exchanged every other day during the experiments (two weeks). All experiments were conducted at room temperature in the laboratory with a natural light/dark schedule. The experimental salamander larvae were monitored, and scored every morning as being either typical or broad-headed morphotypes using criteria given by Michimae & Wakahara (2002). Experiment 1 and experiment 2 were conducted in 2003 and 2004, respectively.
(a) Experiment 1
We randomly assigned nine newly hatched larval H. retardatus (mean (±s.d.) body length 19.12±0.875 mm, n=30) to one of four treatment tanks (with six replicates per treatment), each of which contained 18 R. pirica (mean (±s.d.) body length 15.540±0.528 mm, n=30) or 18 X. laevis (mean (±s.d.) body length 15.570±0.336 mm, n=30) tadpoles with normal intact tails or with shortened tails from which the posterior two-thirds of total tail length had been amputated. These amputated tadpoles did not die. Body sizes of R. pirica and X. laevis tadpoles were smaller than those of the salamander larvae during the experiment. The experimental tank (22 cm × 15 cm × 12.5 cm) contained 1.6 l of dechlorinated tap water.
Induction of broad-headed morphs was frequently observed during the first week after hatching. Cannibalism did not occur frequently during this period. Since we counted the number of broad-headed morphs in each tank every day, the numbers of morphs in the tanks could be accurately determined even if the larvae died by cannibalism. The frequency of occurrence of the broad-headed morph was expressed as the ratio of the number of broad-headed morphs to the initial number of larvae in the tank. Angular transformation of the data was performed.
The numbers of X. laevis and R. pirica tadpoles in each tank were counted every morning because unexpected feeding by H. retardatus larvae occurred during the experimental period. Then, these tadpoles were added to each tank as necessary to replace those that had been eaten by the H. retardatus larvae since the previous morning so that each tank always contained 18 tadpoles.
(b) Experiment 2
One approach to test directly the role of tadpoles' flapping tails excluding all confounding factors would be to manipulate the flapping tails mechanically, and examine how such manipulation affects the induction of the broad-headed morph. To achieve this goal, we developed a rearing system in which newly hatched salamander larvae were individually allowed to develop in their plastic cups under the condition where four tadpoles' tail-like vinyl fins (2 mm × 10 mm × 0.04 mm, transparent) were flapping, generated by a constant reciprocating motion of a stepping motor (figure 1). The artificial flapping tails were scheduled with 10 Hz flapping over 1.5 and 20 s resting intervals between the consecutive flapping periods.
Figure 1.
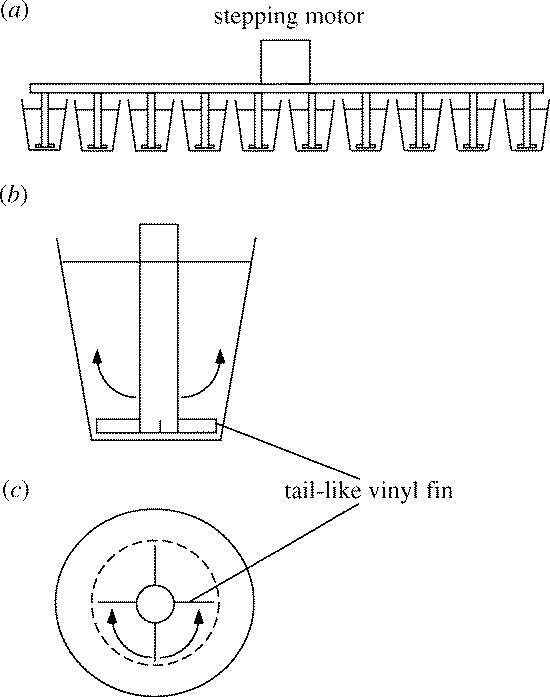
Rearing system with artificial tadpoles' flapping tails. (a) Schematic representation of the rearing system. A stepping motor simultaneously operates 10 pivots with four tadpole tail-like vinyl fins. (b) More detailed side view of the plastic cup. (c) More detailed top view of the plastic cup.
Twenty newly hatched larvae were allotted, 10 larvae each, either to the treatment group where artificial tadpoles' tail were flapping or to control group. The 10 larvae of each group were placed individually in the experimental plastic cups (top diameter 6.5 cm × bottom diameter 4.5 cm × height 9.0 cm, semi-transparent, thus each larva could not see others) each containing 150 ml of dechlorinated tap water.
3. Results
The salamander larvae preyed on tadpoles regardless of whether the tails were intact or shortened (two-way ANOVA: species by tail, F1,20=0.054, p=0.8187; tail, F1,20=0.195, p=0.6633), indicating that they perceived tadpoles with shortened tails as typical prey items. The number of tadpoles preyed during the experiments was much larger in the condition in which the salamander larvae were co-reared with X. laevis tadpoles (mean±s.d., 173.500±5.753/tank, n=12) than in the condition in which the salamander larvae were co-reared with R. pirica tadpoles (mean±s.d., 145.583±8.990/tank, n=12) (species, F1,20=6.296, p=0.0208).
Species and tail factors significantly affected the rate of occurrence of the broad-headed morph in the larvae of H. retardatus (two-way ANOVA: tail, F1,20=19.533, p=0.0003; species, F1,20=7.051, p=0.0152), but the interaction of species and tail factors was not significant (species by tail, F1,20=0.016, p=0.9003). The salamander larvae co-reared with tadpoles without flapping tails (shortened tails) showed significantly less production of the broad-headed morph than did those co-reared with tadpoles with flapping tails (intact tails) regardless of species (figure 2). The larvae co-reared with the R. pirica tadpoles more frequently expressed the broad-headed morph than did those co-reared with X. laevis tadpoles regardless of tail type (figure 2).
Figure 2.
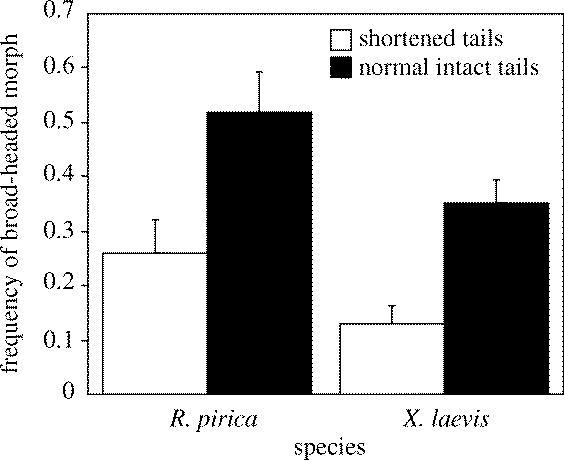
Effects of tails and species on the rate of occurrence of the broad-headed morph. Columns indicate differences in the frequency of occurrence of the broad-headed morph (mean±s.d.) in four treatments; shortened tails and R. pirica (0.259±0.062), normal intact tails and R. pirica (0.518±0.074), shortened tails and X. laevis (0.130±0.034) and normal intact tails and X. laevis (0.351±0.045).
The broad-headed morphs were induced under our experimental system (figure 1) where artificial tadpoles' tails were flapping. However, no broad-headed morphs were induced in the 10 control individuals. The number of the broad-headed morphs was six individuals per 10 individuals in the experimental group (Fisher's exact test: p=0.0108).
4. Discussion
Our experimental results revealed that flapping tails of tadpoles stimulated production of the broad-headed morph in the salamander H. retardatus larvae. Among the several possible cues related to the crowding condition inducing the broad-headed morph, the possibility of a visual cue is excluded because salamander larvae in experiment 2 could not see whether others developed the morph. A less important role for chemical cues is suggested by the fact that tadpoles with intact tails more frequently induced the morph than did those with shortened tails, which must secrete more chemicals from amputated tails, and the fact that the morph was induced even in the solitary environment with mechanical vibration of artificial vinyl fins. The effect of actual predation itself was not involved in the induction of the morph, because the numbers of broad-headed morphs in the replicated tanks had no significant correlation with the numbers of tadpoles preyed on in the tanks (R. pirica, shortened tail, r=0.219, p=0.6997, n=6; R. pirica, normal intact, r=0.768, p=0.0786, n=6; X. laevis, shortened tail, r=0.379, p=0.4901, n=6; X. laevis, normal intact, r=0.216, p=0.7033, n=6), and because the morph was induced under conditions where the larvae in the experiment 2 were only fed with frozen Chironomidae.
Our results also indicate that the effect of tadpoles' flapping tails on production of the broad-headed morph is common to the heterogeneric anuran species X. laevis. R. pirica is an endemic anuran species coexisting with larvae of H. retardatus in a natural environment. X. laevis, on the contrary, have never coexisted with H. retardatus in their evolutionary history. One of the possible proximate mechanisms underlying the results of experiment 1 and 2 is specific hydraulic vibrations generated by the flapping tails of anuran tadpoles, or their mimic. When a hydraulic cue from a biotic agent has unique characteristics in its strength and temporal pattern, the cue provides reliable information for the receiver. Sonic cues that are high-frequency vibrations of hydraulic pressure are reliable for identifying the type of agent, and the signal receiver demonstrates a behavioural response to the signal (Montgomery et al. 1988; Janssen et al. 1990).
There is no previous report that a medium vibration (ca 1–10 Hz) by a biological agent promotes transformation of the morphology of the signal sender. The flapping tail pattern is shared among anuran tadpoles and it provides a similar hydraulic cue for induction in the larval salamander of the broad-headed carnivorous morph. At present, however, we do not know what hydraulic vibration was made by the tail flapping of anuran tadpoles or what hydraulic vibrations work to trigger the induction of the broad-headed carnivorous morph of the larval salamander. Our experimental system described here is a promising method for solving these questions.
Acknowledgments
This work was supported by the Grant-in-Aid for Scientific Research (no. 15009850) from the Japan Society for the Promotion of Science.
References
- Bronmark C, Pettersson L.B. Chemical cues from piscivores induce a change in morphology in crucian carp. Oikos. 1994;70:396–402. [Google Scholar]
- Dahl J, Peckarsky B.L. Induced morphological defenses in the wild: predator effects on a mayfly, Drunella coloradensis. Ecology. 2002;83:1620–1634. [Google Scholar]
- Dodson S.I. The ecological role of chemical stimuli for the zooplankton: predator-induced morphology in Daphnia. Oecologia. 1989;78:361–367. doi: 10.1007/BF00379110. [DOI] [PubMed] [Google Scholar]
- Gilbert J.J. Induction and ecological significance of gigantism in the rotifer Asplachna sieboldi. Science. 1973;181:63–66. doi: 10.1126/science.181.4094.63. [DOI] [PubMed] [Google Scholar]
- Hoffman E.A, Pfennig D.W. Proximate causes of cannibalistic polyphenism in larval tiger salamanders. Ecology. 1999;80:1076–1080. [Google Scholar]
- Janssen J, Coombs S, Pride S. Feeding and orientation of mottled sculpin, Cottus bairdi, to water jets. Environ. Biol. Fish. 1990;29:43–50. [Google Scholar]
- Kishida, O. & Nishimura, K. 2004 Bulgy tadpoles: inducible defense morph. Oecologia140, 414–421. [DOI] [PubMed]
- Kusch J. Induction of defensive morphological changes in ciliates. Oecologia. 1993;94:571–575. doi: 10.1007/BF00566974. [DOI] [PubMed] [Google Scholar]
- Laurila A, Kujasalo J. Habitat duration, predation risk and phenotypic plasticity in common frog (Rana temporaria) J. Anim. Ecol. 1999;68:1123–1132. [Google Scholar]
- Lively C.M. Predator-induced shell dimorphism in the acorn barnacle Chthamalus anisopoma. Evolution. 1986;40:232–242. doi: 10.1111/j.1558-5646.1986.tb00466.x. [DOI] [PubMed] [Google Scholar]
- McCollum S.A, Leimberger J.D. Predator-induced morphological changes in an amphibian: predation by dragonflies affects tadpole shape and color. Oecologia. 1997;109:615–621. doi: 10.1007/s004420050124. [DOI] [PubMed] [Google Scholar]
- Michimae H, Wakahara M. A tadpole-induced polyphenism in the salamander Hynobius retardatus. Evolution. 2002;56:2029–2038. doi: 10.1111/j.0014-3820.2002.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Montgomery J.C, Macdonald J.A, Housley G.D. Lateral line function in an Antarctic fish related to the signals produced by planktonic prey. J. Comput. Physiol. A. 1988;163:827–834. [Google Scholar]
- Pfennig D.W. The adaptive significance of an environmentally-cued developmental switch in an anuran tadpole. Oecologia. 1990;85:101–107. doi: 10.1007/BF00317349. [DOI] [PubMed] [Google Scholar]
- Stemberger R.S, Gilbert J.J. Spine development in the rotifer Keratella cochlearis: induction by cyclopoid copepods and Asplanchna. Freshwat. Biol. 1984;14:639–647. [Google Scholar]
- Tollrian R. Predator-induced morphological defenses: costs, life history shifts, and maternal effects in Daphnia pulex. Ecology. 1995;76:1691–1705. [Google Scholar]
- Trussell G.C. Phenotypic plasticity in an intertidal snail: the role of a common crab predator. Evolution. 1996;50:448–454. doi: 10.1111/j.1558-5646.1996.tb04507.x. [DOI] [PubMed] [Google Scholar]
- Van Buskirk J, Arioli M. Dosage response of an induced defense: how sensitive are tadpoles to predation risk? Ecology. 2002;83:1580–1585. [Google Scholar]
- West-Eberhard M.J. Oxford University Press; Oxford: 2003. Developmental plasticity and evolution. [Google Scholar]


