Abstract
Flight feather moult in small passerines is realized in several ways. Some species moult once after breeding or once on their wintering grounds; others even moult twice. The adaptive significance of this diversity is still largely unknown. We compared the resistance to mechanical fatigue of flight feathers from the chiffchaff Phylloscopus collybita, a migratory species moulting once on its breeding grounds, with feathers from the willow warbler Phylloscopus trochilus, a migratory species moulting in both its breeding and wintering grounds. We found that flight feathers of willow warblers, which have a shaft with a comparatively large diameter, become fatigued much faster than feathers of chiffchaffs under an artificial cyclic bending regime. We propose that willow warblers may strengthen their flight feathers by increasing the diameter of the shaft, which may lead to a more rapid accumulation of damage in willow warblers than in chiffchaffs.
Keywords: moult, migration, mechanical fatigue, biomechanics
1. Introduction
Unlike bones, feathers do not have the capacity for self-repair. Feathers are constantly exposed to degrading agents, such as UV-B radiation (Bergman 1982; Borgudd 2003), keratin-degrading bacteria (Burtt & Ichida 1999) and mechanical fatigue. Hence, moult has evolved into a major event in avian life histories. Moult is costly in terms of energy and time (Jenni & Winkler 1994) and may be in conflict with other activities (Nilsson & Svensson 1996). The number, timing and intensity of moults are thus likely to be under strong selection.
Small passerines moult all of their flight feathers at least once a year. This renewal is realized in several ways. Among Old World warblers, the majority of species moult once at their summer quarters shortly after breeding, whereas others moult in their winter quarters (Svensson & Hedenström 1999). The willow warbler even has a biannual flight feather moult (Underhill et al. 1992)—once after breeding and subsequently once more in its winter quarters. The adaptive significance of these different strategies is still largely unknown. An investigation of the mechanical properties of feathers might provide us with some insights into the proximate causes of such differences. Material properties of feathers and design constraints are only rarely explored (Bonser & Purslow 1995; Bonser 1996; Corning & Biewener 1998) and rarely associated with the life-history context of the species in question (but see Burtt 1986; Dawson et al. 2000).
Flight feathers have to be stiff, strong and lightweight to serve as efficient aerofoils. One important property of a flight feather, which may be regarded as a beam, is bending stiffness. The tip deflection d of a beam under load P is inversely proportional to EI, bending stiffness. The term E is Young's modulus (stress divided by strain), and I is the second moment of area (, where y is the distance of a cross-sectional element A from the bending axis). Altering E or I or both can thus change the stiffness of a structure. For a circular hollow tube of constant thickness, I is proportional to the cube of the diameter.
Feather quality may be impaired by environmental assaults and mechanical fatigue—small passerines migrating from northern Europe to southern Africa require approximately 40 million wing-beats to complete the journey. The extent to which feathers are damaged also depends on somatic investment. Different moult strategies may therefore be a consequence of (i) species-specific environmental factors and (ii) differences in structural properties of feathers.
Here, we present preliminary data suggesting that differences in the fatigue resistance of flight feather shafts may be one correlate of different moult strategies in two congeneric species.
2. Material and methods
(a) Feather samples
Feathers without visible fault bars were collected between 5 May and 22 May 2002 at Ottenby Bird Observatory (Öland, Sweden). Only one innermost primary was taken from each individual.
(b) Fatigue apparatus
The apparatus used for simulating the cyclic bending of feathers consisted of a 12 V motor. Attached to its axle was a circular aluminium disc with four round horizontally oriented plastic bars. Feathers were clamped to a ramp so that the moving bars deflected the feathers downwards (figure 1a) with a bending frequency of 6.1 Hz.
Figure 1.
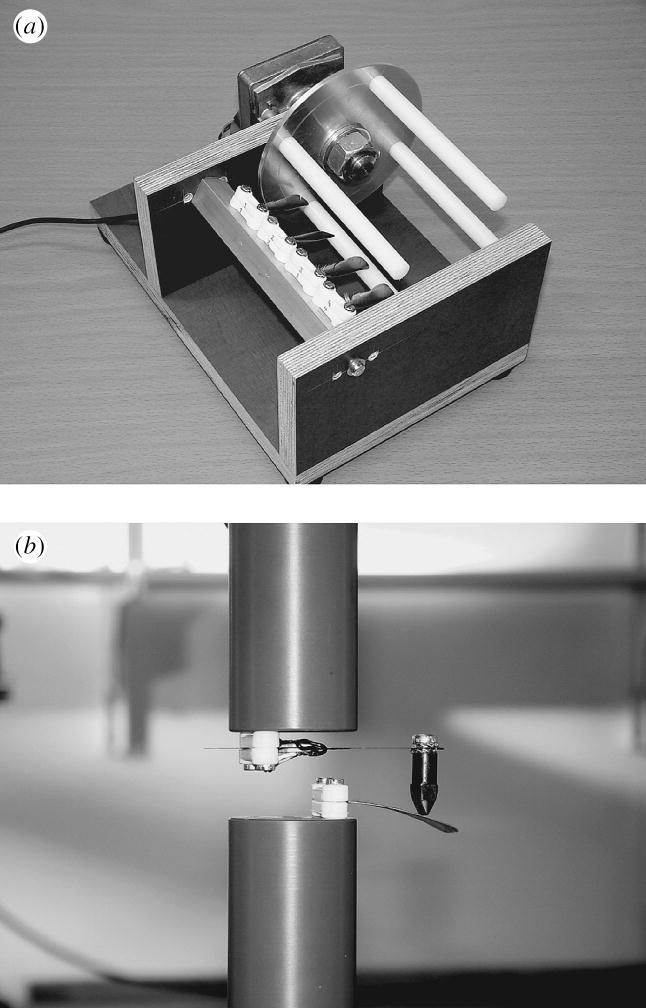
(a) The apparatus used for artificial cyclic bending of feathers; (b) the testing set-up.
(c) Mechanical testing equipment
We measured the feathers' bending stiffness in a two-point bending set-up in an MTS 810 testing machine (Eden Prairie, MN, USA; figure 1b). Force was measured from the strain of a 2 mm thick steel plate, which was attached to the upper fixed cylinder of the machine. Feathers were fixed between two cable-clamps so that they did not twist when loaded. To prevent damage, the clamps were filled with silicon. The feathers were clamped up to the calamus–rhachis border and fixed to the lower moving cylinder of the machine.
(d) Experimental protocol
(i) Fatigue protocol
Feather stiffness was measured before the start of the fatigue experiment, then after 5 h of fatigue and subsequently once after each of nine separate 4 h sessions of fatigue. The feathers were turned once halfway through each fatigue session.
(ii) Measurement of bending stiffness
The loading rate of the MTS testing machine was 2 mm min−1 and force was recorded every 1.5 s for 3 min in total. The point of bending was at 26 mm along the length of the rhachis. Stiffness was calculated from the slope of the force–displacement output line (see Electronic Appendix for more details).
3. Results
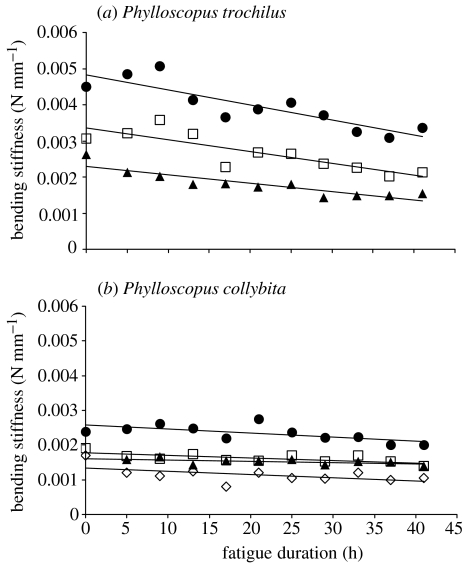
Figure 2a,b shows bending stiffness as a function of fatigue duration. Willow warbler feathers were stiffer (mean initial stiffness±s.d.=0.003 34±0.000 974 N mm−1) than chiffchaff feathers (mean initial stiffness±s.d.=0.001 82±0.000 47 N mm−1). This difference was significant: t=−2.88, d.f.=5, p=0.017. To test for differences between the two species, we regressed stiffness against fatigue duration for each feather and compared the slopes using t-tests. The mean rate of loss of stiffness for the willow warbler was 3.27×10−4 N mm−1 h−1 and it was 0.797×10−4 N mm−1 h−1 for the chiffchaff (t=5.141 6, d.f.=5, p=0.0036).
Figure 2.
Bending stiffness (P/d [N mm−1], i.e. external load divided by displacement) as a function of fatigue duration (h). (a) Willow warbler (Phylloscopus trochilus); (b) chiffchaff (Phylloscopus collybita). Different symbols represent individual feathers and lines are regression lines.
The feather rhachis of willow warblers was thicker than that of chiffchaffs. The mean dorsal–ventral diameter (±s.d.) at the point of clamping for willow warblers (n=3) was 0.83±0.058 mm and it was 0.69±0.063 mm for chiffchaffs (n=4). This difference was significant: t=−3.135, d.f.=5, p=0.025. The mean dorsal–ventral diameter (±s.d.) at the point of bending was 0.47±0.029 mm for willow warblers and 0.36±0.048 mm for chiffchaffs (t=−3.3, d.f.=5, p=0.021). This pattern was supported by data from a larger sample of feathers collected at the same time and site (see above) and used in different experiments (Borgudd 2003). In this larger sample, the mean diameter at the point of clamping (rhachis–calamus border) for willow warblers was 0.81±0.042 mm and at the point of testing, 0.43±0.032 mm (n=13). The respective values for chiffchaffs (n=15) were 0.67±0.082 mm and 0.37±0.041 mm. Each pair of mean values differed significantly between the species.
4. Discussion
Our results show that the innermost primaries of willow warblers are affected more by fatigue than the equivalent feathers of chiffchaffs. Thus, a suggestive pattern emerges: the species with feathers that fatigue faster moults twice annually and not once. This is consistent with two explanations: either (i) willow warblers have sufficient surplus energy and time, can easily fit two moults into their annual cycle and therefore do not have to invest much energy and time in fatigue-resistant feathers, or (ii) they suffer from time- and energy-stress during summer or winter, or both, and are simply unable to grow fatigue-resistant feathers. We believe the first scenario to be unlikely—for example, northerly breeding willow warblers seem to suffer from an increased likelihood of moult-migration overlap (Hedenström et al. 1995), which may compromise the quality of new feathers, and feathers that fatigue rapidly may ultimately increase flight costs in the course of long migratory flights. Although we do not have unequivocal evidence to rule out either of the two possible explanations, our data can still provide us with interesting glimpses into the mechanisms of feather fatigue and structure–performance relationships. Our reasoning depends only on the sensible assumption that birds need optimally stiff feathers for efficient flight.
There are some potential problems with our experiments, which need to be addressed. First, the sample size was small. However, the results are robust and the differences striking. Further support comes from the results of an experiment in which UV-B exposure and mechanical fatigue were alternated (Borgudd 2003). During mechanical fatigue sessions, chiffchaff feathers (n=4) lost on average 1.2×10−5 N mm−1 h−1 and willow warbler feathers (n=4) 4.7×10−5 N mm−1 h−1 of bending stiffness. These values are significantly different (t=1.966, d.f.=6, p=0.048). Second, neither the frequency nor the amplitude of the artificial bending matches the values expected during flight. However, as long as the experimental conditions are the same for both species, the results should mirror pertinent mechanical differences. Third, and potentially most seriously, the feathers were not of the same age. Because the samples were collected in spring, willow warbler feathers were relatively fresh, whereas chiffchaff feathers had undergone one extra migratory episode in the preceding autumn. This age difference, however, strengthens our case: although willow warbler feathers were younger, their stiffness decreased faster than the stiffness of the older chiffchaff feathers. The age difference may be reflected in the initial stiffness difference, not in the rate of stiffness loss.
The finding that rhachis diameter differs between the two species gives us some insight into possible structure–performance relationships. Assuming that the rate of keratin synthesis during moult is constant (Dawson 2003) and that feathers need to attain a certain length and stiffness to minimize flight costs, willow warblers may deposit less keratin per unit feather length than chiffchaffs—either because they do not need to do so or because they are not able to do so. The resulting loss in elastic modulus may be compensated for by increasing the second moment of area I through depositing the material further from the neutral line, which is neither in tension nor compression (so that the bending stiffness EI remains close to the optimal value). This, though, may result in increased damage accumulation in the outer layers of the feather, because strain during bending is proportional to distance from the neutral line and damage accumulation can be proportional to strain amplitude during cyclic bending (Degrieck & van Paepegem 2001). Fresh willow warbler feathers were twice as stiff as the older chiffchaff feathers. How can we support our claim that willow warblers may deposit keratin with a low elastic modulus? Two considerations support this assumption: (i) fresh chiffchaff feathers will most probably be stiffer than the half-year-old feathers used in the experiment and (ii) I is proportional to the third power of diameter. The rhachis of willow warblers has, on average, a 15% greater diameter than that of chiffchaffs, and this difference may compensate for a large difference in elastic modulus.
Investigations of the annual cycle and energy budgets of the two species and analyses of feather morphology and damage mechanisms in keratin are needed to test our provisional results. It will also be interesting to see whether birds can adjust rhachis diameter in response to variations in material properties of keratin, and what such a response may imply as regards fatigue properties—birds may have to trade-off stiff feathers against increased damage accumulation. Still to be investigated in detail are the effects of mechanical fatigue on flight performance. There are only a few experimental findings: Chai & Dudley (1999) and Williams & Swaddle (2003) showed that there are aerodynamic advantages of having fresh plumage compared with visibly undamaged, but old plumage.
Acknowledgements
T.P.W. and A.H. are supported by the Swedish Research Council. We thank the staff of Ottenby Bird Observatory for collecting the feathers and Thord Lundgren for help with the experiments. We thank two referees for comments on an earlier version of the manuscript. This is contribution 197 from Ottenby Bird Observatory.
Supplementary Material
References
- Bergman G. Why are the wings of Larus f. fuscus so dark? Ornis Fenn. 1982;59:77–83. [Google Scholar]
- Bonser R.H.C. The mechanical properties of feather keratin. J. Zool. Lond. 1996;239:477–484. [Google Scholar]
- Bonser R.H.C, Purslow P.P. The Young's modulus of feather keratin. J. Exp. Biol. 1995;198:1029–1033. doi: 10.1242/jeb.198.4.1029. [DOI] [PubMed] [Google Scholar]
- Borgudd, J. 2003 Mechanical properties of bird feathers—influence of UV-radiation and mechanical fatigue. Lund Technical University, Report TVSM-5121.
- Burtt E.H., Jr An analysis of physical, physiological and optical aspects of avian coloration with emphasis on wood-warblers. Ornithol. Monogr. 1986:38. [Google Scholar]
- Burtt E.H, Jr, Ichida J.M. Occurrence of feather-degrading bacilli in the plumage of birds. Auk. 1999;116:364–372. [Google Scholar]
- Chai P, Dudley R. Maximum flight performance of humming-birds: capacities, constraints, and trade-offs. Am. Nat. 1999;153:398–411. [Google Scholar]
- Corning W.R, Biewener A.A. In vivo strains in pigeon flight feather shafts: implications for structural design. J. Exp. Biol. 1998;201:3057–3065. doi: 10.1242/jeb.201.22.3057. [DOI] [PubMed] [Google Scholar]
- Dawson A. A detailed analysis of primary feather moult in the common starling Sturnus vulgaris—new feather mass increases at a constant rate. Ibis. 2003;145:E69–E76. [Google Scholar]
- Dawson A, Hinsley S.A, Ferns P.N, Bonser R.H.C, Eccleston L. Rate of moult affects feather quality: a mechanism linking current reproductive effort to future survival. Proc. R. Soc. B. 2000;267:2093–2098. doi: 10.1098/rspb.2000.1254. (doi:10.1098/rspb.2000.1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degrieck J, van Paepegem W. Fatigue damage modeling of fibre-reinforced composite materials: review. Appl. Mech. Rev. 2001;54:279–299. [Google Scholar]
- Hedenström A, Lindström Å, Pettersson J. Interrupted moult of adult Willow Warblers Phylloscopus trochilus during autumn migration through Sweden. Ornis Svecica. 1995;5:69–74. [Google Scholar]
- Jenni L, Winkler R. Academic; London: 1994. Moult and ageing in European passerines. [Google Scholar]
- Nilsson J.Å, Svensson E. The cost of reproduction: a new link between current reproductive effort and future reproductive success. Proc. R. Soc. B. 1996;263:711–714. [Google Scholar]
- Svensson E, Hedenström A. A phylogenetic analysis of the evolution of moult strategies in Western Palearctic warblers (Aves: Sylviidae) Biol. J. Linn. Soc. 1999;67:263–276. [Google Scholar]
- Underhill L.G, Prŷs-Jones R.P, Dowsett R.J, Herroelen P, Johnson D.N, Lawn M.R, Norman S.C, Pearson D.J, Tree A.J. The biannual primary moult of willow warblers Phylloscopus trochilus in Europe and Africa. Ibis. 1992;134:286–297. [Google Scholar]
- Williams E.V, Swaddle J.P. Moult, flight performance and wingbeat kinematics during take-off in European starlings Sturnus vulgaris. J. Avian Biol. 2003;34:371–378. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.