Abstract
To our knowledge, there is, so far, no evidence that incubation temperature can affect sex ratios in birds, although this is common in reptiles. Here, we show that incubation temperature does affect sex ratios in megapodes, which are exceptional among birds because they use environmental heat sources for incubation. In the Australian brush-turkey Alectura lathami, a mound-building megapode, more males hatch at low incubation temperatures and more females hatch at high temperatures, whereas the proportion is 1 : 1 at the average temperature found in natural mounds. Chicks from lower temperatures weigh less, which probably affects offspring survival, but are not smaller. Megapodes possess heteromorphic sex chromosomes like other birds, which eliminates temperature-dependent sex determination, as described for reptiles, as the mechanism behind the skewed sex ratios at high and low temperatures. Instead, our data suggest a sex-biased temperature-sensitive embryo mortality because mortality was greater at the lower and higher temperatures, and minimal at the middle temperature where the sex ratio was 1 : 1.
Keywords: sex ratio, environmental sex determination, megapode
1. Introduction
Temperature-dependent sex determination (TSD) during incubation is a well-known phenomenon in reptiles, whereas birds have genotypic sex determination (GSD) in which sex is determined at fertilization long before the incubation of eggs begins (Hardy 2002). There is, so far, no convincing evidence that incubation temperature can affect sex ratios of bird hatchlings (Pike & Petrie 2002). However, an Aboriginal elder told one of us (A.G.) that the number of male and female Australian brush-turkeys (Alectura lathami) differs after hot and cold nesting seasons (W. Candendo, personal communication). Based on this information, we tested the hypothesis that such a skewing of sex ratios could be caused by differences in incubation temperature.
The Australian brush-turkey belongs to the family Megapodiidae, which is the only bird family that uses external heat sources for incubation—a strategy similar to that of reptiles. Here, we report that incubation temperature does affect sex ratios of hatchling Australian brush-turkeys, megapodes that build incubation mounds of organic material in which incubation heat is produced by microbial decomposition. To further elucidate the ecological significance of the observed sex bias, we also report on how incubation temperature affects embryo mortality as well as the mass and size of hatchlings, both of which are likely to affect offspring fitness.
2. Material and methods
(a) Obtaining and sexing chicks
The average incubation temperature in natural Australian brush-turkey incubation mounds is ca. 34 °C, but can range from 30 to 38 °C (Booth & Jones 2002). Eggs were collected from mounds from the Central Coast (33°S, 151°E) of NSW, Australia. In 2002, they came from 12 mounds. In 2003, all except two of these mounds were still active, which enabled us to collect eggs with similar genetic material to the year before. In 2002, the eggs were artificially incubated at 34 °C; in 2003, 50% were incubated at 31 °C, the other half at 36 °C (randomly assigned). Incubation stages were determined by the degree of translucence of the eggshell using a Brinsea egg lume candling lamp. Incubators (Brinsea Octagon 250) were kept at a constant temperature of 31, 34 or 36 °C and humidity was kept constant at 80%. Only eggs that developed continuously during the first week of incubation after collection were included in our analysis.
Australian brush-turkeys breed from August to February. The season influences sex ratios in other birds (Pike & Petrie 2002), but it is unknown how important this is in megapodes, which produce one egg at a time, at intervals of several days and over several months. To limit potential seasonal effects, we restricted egg collection to October and November in each year.
Chicks were sexed three times between 1–21 days of age by examining a phallic structure on the ventral lip of the cloaca, which is larger in males (A. Göth, unpublished data). The accuracy of this method was confirmed by some chicks raising until sexual dimorphism became obvious.
(b) Statistical analysis
Analyses were conducted using SPSS (2000) and following Sokal & Rohlf (1995). Data exploration revealed high heterogeneity of variance. We used non-parametric χ2 tests to compare the frequencies of male and female hatchlings, and the number of hatched and unhatched eggs between different temperatures. Egg volume is correlated with hatchling size and mass (Göth & Evans 2004), so we used ANCOVAs with egg volume as a covariate, sex and temperature as fixed factors and mass, tarsus or head length as dependent variables to compare mass and size at different temperatures. Egg volume (cm3) was calculated as V=0.000 51×length×breadth2 (Hoyt 1979). Post hoc comparisons with Bonferroni adjustment followed significant ANCOVA results.
3. Results
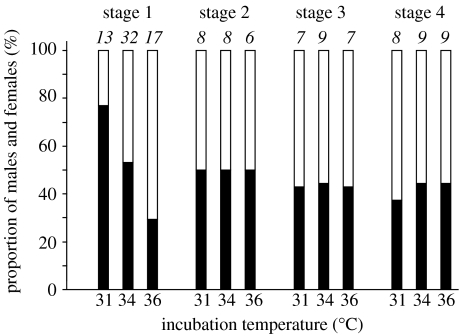
Temperature significantly affected the number of male and females hatching from young (stage 1) eggs (figure 1; p=0.035; χ2-test: χ2=6.72, d.f.=2). More males hatched at 31 °C, more females at 36 °C, and the ratio was almost 1 : 1 at 34 °C. No sex bias was evident for older eggs (stages 2–4; figure 1).
Figure 1.
Proportions of male (filled bars) to female (open bars) hatchlings when eggs were artificially incubated at three temperatures. Eggs were at different stages of development when collected: stage 1, eggs were transparent or contained a light-red yolk sac that covered up to half of the egg, but no dark parts that indicated the chorioallantois; stage 2, less than 50% of the egg was dark; stage 3, 50–75% dark; stage 4, 75–100% dark. Numbers in italics, n hatchlings. Temperature affected sex ratios if eggs were exposed to extreme temperatures within the first days after being laid, that is before the chorioallantois was visible by candling.
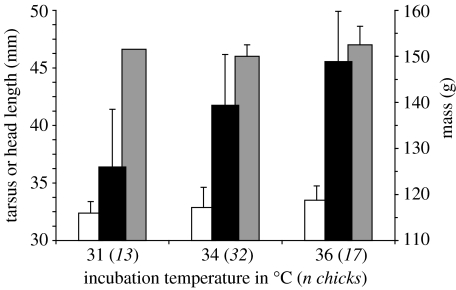
Incubation temperature also strongly affected the mass of hatchlings (p≪0.000 1, F2,58=23.88), but not their size, as described by tarsus length (p=0.41, F2,58=0.90) or head length (p=0.71, F2,58=0.34; all ANCOVAs, egg volume=covariate; figure 2). Hatchling mass increased with incubation temperature; chicks from 31 °C were significantly lighter than those from the 34 and 36 °C groups (post hoc comparisons, both p≪0.000 1). Differences in mass cannot be attributed to sex, because males and females incubated at 34 °C did not differ in mass (p=0.104, F1,29=2.82), tarsus length (p=0.064, F1,29=3.70) or head length (p=0.381, F1,29=0.79, all ANCOVAs; males n=15, females n=17; stage 1 egg only).
Figure 2.
Mass (filled bars) and size (open bars, tarsus; grey bars, head) for hatchlings from three incubation temperatures. Bars represent means+s.d. Only hatchlings from stage 1 eggs are included.
Incubation temperature also affected embryonic mortality rates. When stage 1 eggs were incubated at 34 °C, only 23.8% of the eggs failed to hatch (n=42), compared with 45.8% at 31 °C (n=24) and 34.6% at 36 °C (n=26). However, the interaction between temperature and mortality rates was not significant (p=0.179; χ2-test: χ2=3.44, d.f.=2).
4. Discussion
Our data show that incubation temperature affects sex ratios in megapodes, which is, to our knowledge, the first time that this has been reported in birds. It adds a new dimension to the mechanisms of sex ratio manipulation in birds (Hardy 2002; Pike & Petrie 2002). Previously reported mechanisms occur during egg production (e.g. Heinsohn et al. 1997; Komdeur & Pen 2002), or when parents differentially allocate resources to hatchlings (e.g. Nager et al. 2000; Hasselquist & Kempenaers 2002). To date, no reliable accounts exist for sex ratio manipulation during incubation (Pike & Petrie 2002). Based on our results, future studies should consider whether sex ratios could be affected by intermittent incubation of eggs, as well as other parental behaviours that affect cooling or heating of the eggs (Deeming 2002), or by the clutch size, which can be correlated with the temperature in a bird nest (Reid 2000).
Our results also suggest the possibility that megapode parents could manipulate sex ratios of hatchlings by altering the temperature of the mound, or by selecting thermally different sites within an individual mound. Male megapodes of several species regulate the temperature inside their incubation mound daily, and females have some influence on the exact site where eggs are laid within the mound (Jones et al. 1995).
The results on chick mass reported here could elucidate the adaptive significance of the observed sex bias. Harchlings incubated at 31 °C were lighter but not smaller than those incubated at 36 °C, which suggests that differences in residual yolk mass at the time of hatching were responsible for this effect. Megapodes eggs have large yolks, and embryos incubated at lower temperatures take longer to hatch (Booth 1987) and thus may use more yolk during incubation and hatch with a smaller residual yolk sac.
Yolk is important for megapode hatchlings for two reasons. First, because they dig themselves out of their underground nest, which takes ca. 40 h (Göth 2002) and hatchling mass is positively correlated with the digging speed (Göth & Evans 2004). Reaching the surface quickly ensures the retention of more internal yolk, a critical food reserve while chicks search for food, shelter and roosts without any parental assistance. Second, hatch mass predicts the ability to gain weight during the first month after hatching (Göth & Evans 2004). Overall, lighter chicks thus start their lives in less than optimal circumstances.
Future studies should investigate whether males and females obtain different fitness advantages from the effects of incubation temperature on mass and yolk reserves. In some birds, female hatchlings have higher mortality rates than males under sub-optimal circumstances (Ewen et al. 2001), or lower fecundity as adults when lighter at hatching (Haywood & Perrins 1992). Sex-specific fitness advantages in a given environment may also explain the adaptive significance of TSD in reptiles (Janzen & Paukstis 1991).
Mechanisms for avian sex ratio manipulations are largely unexplored (Pike & Petrie 2002). Two of our results hint at a potential mechanism underlying the observed sex bias, namely preferential death of male embryos at 36 °C and female embryos at 31 °C. The sex ratio was 1 : 1 at the average temperature, and there was a trend for egg mortality to increase at the other two temperatures. Sex-biased mortality needs to be confirmed in future studies, by sexing dead embryos with molecular techniques. Such a mechanism has been demonstrated for snakes (Burger & Zappalorti 1988), but has only been postulated for birds, without convincing experimental evidence (Krackow 1995; Pike & Petrie 2002).
Another possible mechanism is TSD, as found in reptiles, but this is unlikely for two reasons. First, the present study shows that brush-turkey sex is determined before the middle third of incubation, which is when TSD occurs in reptiles (Lance 1997). Second, sex in birds is determined by allocation of sex chromosomes at fertilization (Hardy 2002). Megapodes possess heteromorphic Z and W sex chromosomes, like other carinate birds. This has been confirmed for the wattled brush-turkey Aepypodius arfakianus and Bruijn's brush-turkey Aepypodius bruijnii (Belterman & De Boer 1994), and the Australian brush-turkey (G. Baker, personal communication, June 2004). However, GSD and TSD may not be incompatible in megapodes, as recent studies have shown that incubation temperatures can override sex chromosome influence in mouthbrooding fish, salamanders and lizards, including species with heteromorphic sex chromosomes (Shine et al. 2002).
A third, but so far unexplored, mechanism is a temperature-dependent sex reversal during incubation (Pike & Petrie 2002). One study reported that this caused abnormal sex ratios in chickens (Gallus gallus domesticus), but empirical data are not available (Ferguson 1996). Future studies will need to disentangle all three possible mechanisms to further elucidate the temperature-dependent sex ratio in megapode birds.
Acknowledgements
We thank Warren Canendo for triggering this study. We also thank C. Evans for logistical support, A. Taylor for help with statistical analyses and G. Baker for identifying karyotypes in Australian brush-turkeys. G. Baker, R. Dekker, K. Dial, J. Endler, A. Heiling, M. Herberstein, D. Jones, R. Peters and four anonymous referees made helpful comments on the manuscript. A.G. was supported by a Macquarie University postdoctoral fellowship.
References
- Belterman R.H.R, De Boer L.E.M. A karyological study of 55 species of birds, including karyotypes of 39 species new to cytology. Genetica. 1984;65:39–82. [Google Scholar]
- Booth D.T. Effect of temperature on development of Malle fowl Leipoa ocellata eggs. Physiol. Zool. 1987;60:437–445. [Google Scholar]
- Booth D.T, Jones D.N. Underground nesting in the megapodes. In: Deeming D.C, editor. Avian incubation: behaviour, environment, and evolution. Oxford University Press; Oxford: 2002. pp. 192–206. [Google Scholar]
- Burger J, Zappalorti R.T. Effects of incubation temperature on sex ratios in pine snakes: differential vulnerability of males and females. Am. Nat. 1988;132:492–505. [Google Scholar]
- Deeming D.C. Behaviour patterns during incubation. In: Deeming D.C, editor. Avian incubation: behaviour, environment and evolution. Oxford University Press; Oxford: 2002. pp. 63–87. [Google Scholar]
- Ewen J.G, Clarke R.H, Moysey E, Boulton R.L, Crozier R.H, Clarke M.F. Primary sex ratio bias in an endangered cooperatively breeding bird, the black-eared miner, and its implications for conservation. Biol. Conserv. 2001;101:137–145. [Google Scholar]
- Ferguson, M. W. J. 1996 Method of hatching avian eggs. Patent W094/13132.
- Göth A. Behaviour of Australian brush-turkey (Alectura lathami, Galliformes: Megapodiidae) hatchlings following underground hatching. J. Ornithol. 2002;143:477–488. [Google Scholar]
- Göth A, Evans C.S. Egg size in Australian brush-turkey Alectura lathami hatchlings predicts motor performance and postnatal weight gain. Can. J. Zool. 2004;82:972–979. [Google Scholar]
- Hardy C.W. Cambridge University Press; Cambridge: 2002. Sex ratios. Concepts and research methods. [Google Scholar]
- Hasselquist D, Kempenaers B. Parental care and adaptive brood sex ratio manipulation in birds. Phil. Trans. R. Soc. B. 2002;357:363–372. doi: 10.1098/rstb.2001.0924. doi:10.1098/rstb.2001.0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood S, Penins C.M. Is clutch size in birds affected by environmental conditions during growth? Proc. R. Soc. B. 1992;249:195–197. doi: 10.1098/rspb.1992.0103. [DOI] [PubMed] [Google Scholar]
- Heinsohn R, Legge S, Barry S. Extreme bias in sex allocation in Eclectus parrots. Proc. R. Soc. B. 1997;264:1325–1329. doi:10.1098/rspb.1997.0183 [Google Scholar]
- Hoyt D.F. Practical methods of estimating volume and fresh weight of bird eggs. Auk. 1979;96:73–77. [Google Scholar]
- Janzen F.J, Paukstis G.L. Environmental sex determination in reptiles: ecology, evolution, and experimental design. Q. Rev. Biol. 1991;66:149–179. doi: 10.1086/417143. [DOI] [PubMed] [Google Scholar]
- Jones D.N, Dekker R.W.R.J, Roselaar C.S. Oxford University Press; Oxford: 1995. The megapodes. [Google Scholar]
- Komdeur J, Pen I. Adaptive sex allocation in birds: the complexities of linking theory and practice. Phil. Trans. R. Soc. B. 2002;357:373–380. doi: 10.1098/rstb.2001.0927. doi:10.1098/rstb.2001.0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krackow S. Potential mechanisms for sex ratio adjustment in mammals and birds. Biol. Rev. 1995;70:225–241. doi: 10.1111/j.1469-185x.1995.tb01066.x. [DOI] [PubMed] [Google Scholar]
- Lance V.A. Sex determination in reptiles: an update. Am. Zool. 1997;37:504–513. [Google Scholar]
- Nager R.G, Monaghan P, Houston D.C, Genovart M. Parental condition, brood sex ratio and differential young survival: an experimental study in gulls (Larus fuscus) Behav. Ecol. Sociobiol. 2000;48:452–457. [Google Scholar]
- Pike T.W, Petrie M. Potential mechanisms of avian sex manipulation. Biol. Rev. 2002;78:533–574. doi: 10.1017/s1464793103006146. [DOI] [PubMed] [Google Scholar]
- Reid J.M. The consequences of clutch size for incubation conditions and hatching success in starlings. Funct. Ecol. 2000;14:560–565. [Google Scholar]
- Shine R, Elpick M.J, Donnellan S. Co-occurrence of multiple, supposedly incompatible modes of sex determination in a lizard population. Ecol. Lett. 2002;5:486–489. [Google Scholar]
- Sokal R.R, Rohlf F.J. 3rd edn. Freeman; New York: 1995. Biometry. [Google Scholar]
- SPSS. SPSS Inc; Chicago, IL: 2000. SPSS v. 11.5. 233 S. [Google Scholar]