Abstract
The first published record, from the early 1970s, of hibernation in sea turtles is based on the reports of the indigenous Indians and fishermen from Mexico, who hunted dormant green turtles (Chelonia mydas) in the Gulf of California. However, there were no successful attempts to investigate the biology of this particular behaviour further. Hence, data such as the exact duration and energetic requirements of dormant winter submergences are lacking. We used new satellite relay data loggers to obtain the first records of up to 7 h long dives of a loggerhead turtle (Caretta caretta) overwintering in Greek waters. These represent the longest dives ever reported for a diving marine vertebrate. There is strong evidence that the dives were aerobic, because the turtle surfaced only for short intervals and before the calculated oxygen stores were depleted. This evidence suggests that the common belief that sea turtles hibernate underwater, as some freshwater turtles do, is incorrect.
Keywords: hibernation, reptile, dive duration, aerobic dive limit, temperature effect
1. Introduction
Hibernating sea turtles were described as being lethargic, mud-covered and often partly buried in the bottom sediment (Felger et al. 1976; Ogren & McVea 1995). However, surfacing of dormant turtles has rarely, if at all, been observed. Felger's description gives the impression that hibernating sea turtles spend months on the sea floor without emerging. Direct underwater observations depend strongly on weather conditions and visibility and, hence, are often not applicable where hibernating turtles occur (Carr et al. 1980). Only some sea turtle populations hibernate and apparently only part of these populations choose this overwintering strategy, whereas others migrate into warmer regions (Ogren & McVea 1995). A temperature threshold for the entrance into dormancy is assumed to be just below 15 °C and it seems, at least in North American waters, that the locations of hibernacula are restricted to a narrow zone around the 29° N latitude (Ogren & McVea 1995).
Today, more than 30 years after the first publication on sea turtle hibernation, the true behaviour of these turtles remains unknown, as do the underlying physiological processes. Ogren & McVea (1995) reported that investigations of sea turtle hibernacula were hampered by the unpredictability of hibernation occurrences and inadequate sampling methods (Carr et al. 1980).
Modern satellite relay data loggers (SRDLs) allow us to monitor the diving behaviour of naturally behaving, unrestrained turtles over several months at least, thus providing an adequate method to study their overwintering behaviour. We deployed an SRDL on a subadult loggerhead turtle in the Mediterranean, which is located approximately between the 30° N and 46° N and represents a more temperate zone than those where former hibernacula were reported. In fact, water temperatures in the Mediterranean regularly fall below 15 °C in the winter, and a turtle would have to travel far into the eastern basin to remain in waters above this temperature (Brankart & Brasseur 1998). However, overwintering turtles have also been found in more western parts of the Mediterranean, such as southern Italy (Bentivegna et al. 2002), Croatia (Lazar et al. 2004), Greece (Bentivegna 2002) and Tunisia (Laurent & Lescure 1994). We were, therefore, confident that our turtle would adopt a state of hibernation.
2. Materials and methods
(a) Instrument attachment and description
The loggerhead turtle (body mass 52 kg) used in this study originated from the South Tyrrhenian Sea and had spent 10.5 months in the Stazione Zoologica Anton Dohrn (Naples, Italy) for rehabilitation. The turtle was released in the Gulf of Naples close to the island of Ischia (40°45′ N, 14°00′ E) on 23 July 2002 at 09.13 h local time. We attached an SRDL (Sea Mammal Research Unit, St Andrews, UK) to the turtle's carapace prior to the release using epoxy resin. The unit contained an integrated time-depth recorder which measured depth every 4 s with a resolution of 0.33 m. Dive data were processed and compressed onboard and transmitted via the ARGOS system, which also provided the location of the SRDL. Among the transmitted dive data were 24 h summary statistics including mean±s.d. dive duration, maximum dive duration, mean±s.d. depth, maximum depth, number of dives and proportion of time spent either diving or at the surface. Further information on single dives included surface duration, dive duration, time of end of dive and maximum dive depth. For some dives, profiles were also transmitted as the time and depth of the five most significant points of inflection together with the time of the end of the dive and the dive duration. A dive was recorded when the turtle descended below 3 m and ended when the turtle ascended above 3 m or when the saltwater switch was dry at any time.
(b) Reconstruction of migration route
Positions of the turtle were determined using the ARGOS system. Following widely used methods (Luschi et al. 1998), we reconstructed the migration route of the turtle using all locations but filtering those which required a high travel speed (>9 km h−1), which were located on land or which would have necessitated course reversals. This filter removed 102 from a total of 421 locations. The route was then plotted on the base of this filtered dataset using the Maptool program from www.seaturtle.org.
(c) Sea surface temperature
Sea surface temperatures (SSTs) for three putative hibernacula were obtained from the International Comprehensive Ocean-Atmosphere Data Set from http://www.cdc.noaa.gov/coads/products.html. The data included monthly summary statistics in a 1° grid resolution for the period between January 1970 and December 2002. The selected locations were the Gulf of Laconia, Greece (22° E 36° N; this study), and, for comparison, two hibernacula which were identified earlier in the Gulf of California (247° E 28° N) and at Cape Canaveral (279° E 28° N). The coordinates represent the left (western) corner longitude (E) and the lower (southern) corner latitude (N) of a 1° box. Additionally, we obtained a mean SST for each month of the tracking period for the respective locations. These were used later for the calculation of temperature-dependent oxygen consumption rates of loggerhead turtles.
(d) Calculated aerobic dive limits
Aerobic dive limits were calculated using published data on total oxygen store and oxygen consumption rates () calculated for a 52 kg loggerhead turtle. Since varies with temperature, monthly average SSTs (see above) were used to calculate the corresponding monthly average using the equation which best described the seasonal temperature effect on of loggerhead turtles (Hochscheid et al. 2004). Based on literature values, we assumed that the lung capacity of a loggerhead turtle was 8.9% of its weight (Lutz & Bentley 1985), that the oxygen content of the lungs at the start of a dive was 17.4% (Berkson 1966) and that the blood and muscle of a loggerhead turtle can hold 6.7 ml O2 kg−1 (Lutz & Bentley 1985). Summing up these values the calculated total oxygen store of our turtle was 22.2 ml O2 × 52 kg=1154.4 ml O2. Thus, an average calculated aerobic dive limits (cADLs) for each month was derived by dividing the total oxygen store by the corresponding average monthly .
3. Results
Upon release, the turtle travelled southeastwards and arrived in the Gulf of Laconia, South Peloponnese peninsula (Greece) after three and a half months. It remained there during the winter until the SRDL ceased transmitting in March 2003. Monthly average SSTs were between 14.7 and 14.9 °C in February and March.
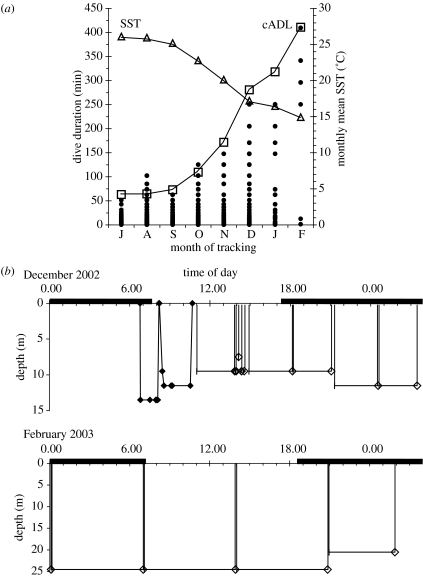
Duration and maximum depth were obtained for a total of 1952 dives and dive profiles were available for 229 of these. Median dive durations increased from a minimum of 5.5 min in July to a maximum of 341 min in February. The maximum recorded dive duration was 410 min. Surfacing intervals after such long dives lasted between 5 and 7 min. The increase in dive duration coincided with the decrease in SST and the change of season (figure, 1b and 2a).
Figure 1.
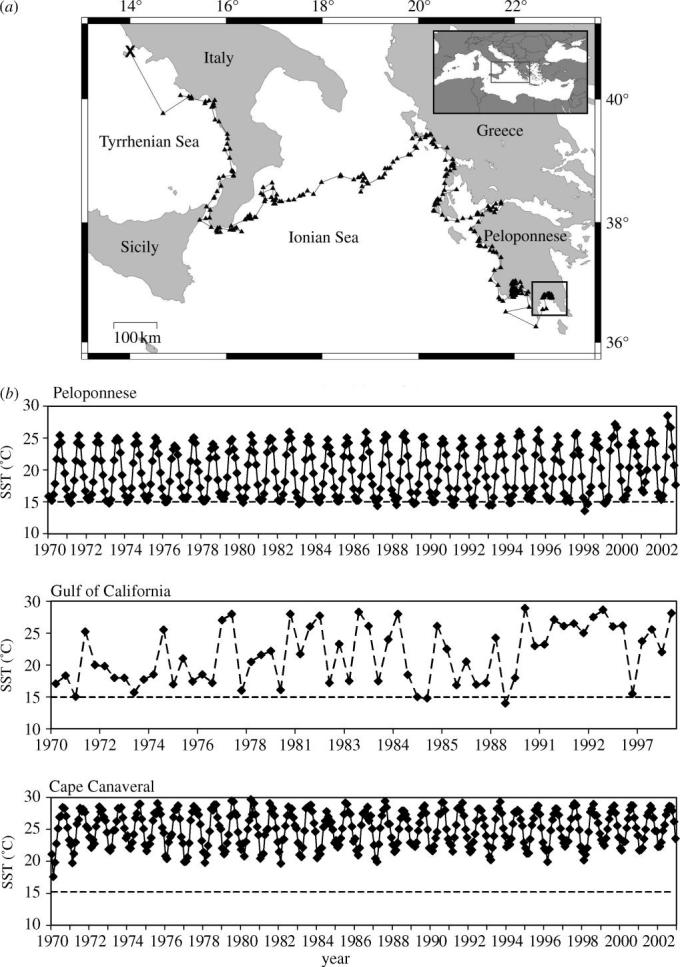
(a) Migration route of a subadult loggerhead turtle (X represents release site; filled triangles represent positions determined via the ARGOS system). The bottom right rectangle encloses the Gulf of Laconia where the turtle overwintered. (b) Monthly average SST from 1970 to December 2002 for the overwintering site of the turtle in this study (Peloponnese), and for two further putative overwintering sites: the Gulf of Calfornia and Cape Canaveral. Note: the dataset for the Gulf of California area is not complete and does not cover all months.
Figure 2.
(a) Dive durations (filled circles) of a loggerhead turtle recorded in different months during the tracking period. The monthly average aerobic dive limit (cADL; square) was calculated as detailed in §2. Open triangles represent the monthly average SST. (b) Dive profiles of an overwintering loggerhead turtle during December 2002 (top) and February 2003 (bottom). Two dive profiles were reconstructed from the five most significant points of inflection (filled diamonds). For the other dives only maximum dive depth (open diamonds), dive duration and surface duration were available. Black horizontal bars indicate hours of darkness and were derived from a sunrise/sunset table (Astronomical Applications Department, US Naval Observatory, http://aa.usno.navy.mil/data/docs/RS_OneYear.html) for the locations at which these dive data were received.
Also the cADL increased with decreasing water temperature (figure 2a). Maximum dive durations of our turtle appeared to follow closely the trend of the cADL increase. With the only exception of some dives in August and on one occasion in October, the turtle actually never exceeded its cADL.
Eighty-seven per cent of the dive profiles between December and March were typically U-shaped dives, i.e. upon surfacing the turtle descended to the maximum depth, stayed there for the major part of the dive until it ascended to breathe (cf. first two dive profiles in figure 2b). Such dives have formerly been identified as resting dives (van Dam & Diez 1996).
Whereas the turtle sometimes regained activity in December (e.g. frequent short dives around midday in figure 2b) it passed whole days resting during February (figure 2b).
4. Discussion
Temperature presumably plays the most important cue for the entrance into hibernation, as reported for freshwater turtles (Ultsch 1989). Over the last 30 years monthly mean SST in South Peloponnese waters almost always fell below 15 °C in the winter, whereas average SST of the hibernacula sites in the Gulf of California only rarely did so and at Cape Canaveral average SST actually never decreased below this presumable temperature threshold (figure 1b). Thus, it may be argued that turtles in the Cape Canaveral area experience cold water temperatures only very infrequently and probably only for short periods, since even in the aforementioned severe winter of 1978 water temperatures rose from 11 to 19 °C in only one month (15 February–15 March; Carr et al. 1980). If this temperature regime accounts for the unpredictability of hibernation occurrences then it could be concluded that hibernation in great parts of the Mediterranean Sea are a standard feature of overwintering in sea turtles. A recent tracking study has already demonstrated long quiescent winter dives in green turtles overwintering in Libya (Godley et al. 2002). Unfortunately, maximum dive durations were given as any time above 90 min, which was the upper maximum transmission limit in the software specification of the satellite transmitters. Clearly, this information is not sufficient to investigate the diving capacity in dormant sea turtles.
The aerobic dive limit is a common benchmark against which dive durations of an animal are compared in order to assess its diving strategy (Boyd 1997). The similarity in the seasonal increase of maximum dive duration and cADL suggests that the turtle indeed tended to terminate a dive when oxygen resources became depleted (figure 2a). Thus, if the turtle relied exclusively on aerobic metabolism during the long winter dives it had to remain at least active enough to commute between the bottom and the surface for gas exchange. The dive data and profiles document clearly the shift in behaviour with progression of the winter and particularly demonstrate that hibernation occurs in a form of intermittent dormancy (figure 2b). The occurrence of repeated, long, resting dives indicates that the turtle entered a dormant state on the sea bottom, which was infrequently interrupted by quick excursions to the surface. Moreover, the short surface intervals (maximum 7 min) also indicate that the turtle was not recovering from anaerobic metabolism. Such behaviour explains why sea turtles are seldom seen to surface in the winter: the turtle may appear at the surface only once during daylight hours (figure 2b, bottom graph). Presuming that most of the turtle sightings are during daylight it is not surprising when such a rare event is missed.
Yet, we need to be cautious when comparing hibernating sea turtles with hibernating freshwater turtles. Loggerhead turtle brains can tolerate up to 3 h of anoxia and probably even longer periods (Lutz et al. 1980), but it still has to be shown if they are as tolerant to anoxia as some freshwater turtles, which actually spend months submerged in cold waters (Ultsch 1989). While the hibernation physiology of several freshwater species could be studied in simulated winter conditions in the laboratory (e.g. Ultsch & Cochran 1994), a study aiming to induce hibernation in captive sea turtles did not succeed (Moon et al. 1997). There is practically no evidence that hibernating sea turtles necessarily remain underwater for the duration of the winter, nor do we know whether they can survive for months using anaerobic metabolism. It was previously shown that turtles that predominantly rest can save great amounts of energy (Hays et al. 2000). Moreover, at low water temperatures metabolic rates of sea turtles are low per se (Hochscheid et al. 2004). We conclude that a sea turtle adopting a ‘sit-and-wait’ overwintering strategy with infrequent surfacing intervals has sufficiently reduced metabolic costs to pass the winter without the necessity to become anaerobic.
Acknowledgments
This study was financed partly by the Stazione Zoologica Anton Dohrn (SZN) and partly by the Natural Environment Research Council of the UK. We thank Phil Lovell (Sea Mammal Research Unit, St Andrews, UK), the Fisheries Service from the SZN and Gianfranco Mazza for their advice and assistance during the study. We further acknowledge use of the Maptool (www.seaturtle.org) program for analysis and graphics in this paper.
References
- Bentivegna F. Intra-Mediterranean migrations of loggerhead sea turtles (Caretta caretta) monitored by satellite telemetry. Mar. Biol. 2002;141:795–800. [Google Scholar]
- Bentivegna F, Breber P, Hochscheid S. Cold stunned loggerhead turtles in the South Adriatic Sea. Mar. Turtle Newslett. 2002;97:1–3. [Google Scholar]
- Berkson H. Physiological adjustments to prolonged diving in the Pacific green turtle (Chelonia mydas agassizii) Comp. Biochem. Physiol. 1966;18:101–119. doi: 10.1016/0010-406x(66)90335-5. [DOI] [PubMed] [Google Scholar]
- Boyd I.L. The behavioural and physiological ecology of diving. Trends Ecol. Evol. 1997;12:213–217. doi: 10.1016/s0169-5347(97)01054-9. [DOI] [PubMed] [Google Scholar]
- Brankart J.M, Brasseur P. The general circulation in the Mediterranean Sea: a climatological approach. J. Mar. Syst. 1998;18:41–70. [Google Scholar]
- Carr A, Ogren L, McVea C.J. Apparent hibernation by the Atlantic loggerhead turtle off Cape Canaveral, Florida. Biol. Conserv. 1980;19:7–14. [Google Scholar]
- Felger R.S, Cliffton K, Regal P.J. Winter dormancy in sea turtles: independent discovery and exploitation in the Gulf of California by two local cultures. Science. 1976;191:283–285. doi: 10.1126/science.191.4224.283. [DOI] [PubMed] [Google Scholar]
- Godley B.J, Richardson S, Broderick A.C, Coyne M.S, Glen F, Hays G.C. Long-term satellite telemetry of the movements and habitat utilisation by green turtles in the Mediterranean. Ecography. 2002;25:352–362. [Google Scholar]
- Hays G.C, Adams C.R, Broderick A.C, Godley B.J, Lucas D.J, Metcalfe J.D, Prior A.A. The diving behaviour of green turtles at Ascension Island. Anim. Behav. 2000;59:577–586. doi: 10.1006/anbe.1999.1326. [DOI] [PubMed] [Google Scholar]
- Hochscheid S, Bentivegna F, Speakman J.R. Long-term cold acclimation leads to high Q10 effects on oxygen consumption of loggerhead sea turtles, Caretta caretta. Physiol. Biochem. Zool. 2004;77:209–222. doi: 10.1086/381472. [DOI] [PubMed] [Google Scholar]
- Laurent L, Lescure J. L'Hivernage des tortues caouannes Caretta caretta (L.) dans le sud Tunisien. Rev. Ecol. (Terre Vie) 1994;49:63–86. [Google Scholar]
- Lazar B, Margartoulis D, Tvrtkovic N. Tag recoveries of the loggerhead sea turtle Caretta caretta in the eastern Adriatic Sea: implications for conservation. J. Mar. Biol. Assoc. UK. 2004;84:475–480. [Google Scholar]
- Luschi P, Hays G.C, Del Seppia C, Marsh R, Papi F. The navigational feats of green turtles migrating from Ascension Island investigated by satellite telemetry. Proc. R. Soc. B. 1998;265:2279–2284. doi: 10.1098/rspb.1998.0571. (doi:10.1098/rspb.1998.0571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz P.L, Bentley T.B. Respiratory physiology of diving in the sea turtle. Copeia. 1985;1985:671–679. [Google Scholar]
- Lutz P.L, LaManna J, Adams M, Rosenthal M. Cerebral resistance to anoxia in the marine turtle. Respir. Physiol. 1980;41:241–251. doi: 10.1016/0034-5687(80)90074-2. [DOI] [PubMed] [Google Scholar]
- Moon D.-Y, MacKenzie D.S, Owens D.W. Simulated hibernation of sea turtles in the laboratory. I. Feeding, breathing frequency, blood pH, and blood gases. J. Exp. Zool. 1997;278:372–380. [PubMed] [Google Scholar]
- Ogren L, McVea C.J. Apparent hibernation by sea turtles in North American Waters. In: Bjorndal K.A, editor. Biology and conservation of sea turtles. Smithsonian Institution Press; Washington: 1995. pp. 127–132. [Google Scholar]
- Ultsch G.R. Ecology and physiology of hibernation and overwintering among freshwater fishes, turtles, and snakes. Biol. Rev. 1989;64:435–516. [Google Scholar]
- Ultsch G.R, Cochran B.M. Physiology of northern and southern musk turtles (Sternotherus odoratus) during simulated hibernation. Physiol. Zool. 1994;67:263–281. [Google Scholar]
- van Dam R.P, Diez C.E. Diving behaviour of immature hawksbills (Eretmochelys imbricata) in a Caribbean cliff-wall habitat. Mar. Biol. 1996;127:171–178. [Google Scholar]