Abstract
Mate attraction can be costly. Thus, individuals should modulate it according to its probable benefits. Specifically, individuals should modulate mate-attraction efforts based on their need for, the probability of attracting, and the reproductive competence of prospective mates. We tested these predictions by monitoring song output in laboratory-housed male Cassin's finches (Carpodacus cassinii) before, during and after brief female exposure following variable periods of isolation from females. We inferred individual reproductive competence from the product of season and reproductive schedule, the latter estimated from moult progress. Males produced little song in the presence of a female but robustly elevated song output in response to female loss. However, mere absence of a female did not elevate song output in males unaccustomed to female proximity. Furthermore, song output in response to female loss increased with her reproductive competence. We suggest that individuals modulate mate-attraction effort based on the benefits such efforts are likely to yield.
Keywords: bird song, courtship, reproductive decisions, moult, reproductive schedule, seasonal breeding
1. Introduction
Male songbirds attract mates by singing (McGregor 1991). Because song production may incur costs (Gil & Gahr 2002), males should modulate it according to its probable benefits. For instance, song effort should decline once a mate has been attracted (Ball 1999) but increase if the male loses the mate (Krebs et al. 1980; Cuthill & Hindmarsh 1985). When a male is unlikely to attract a mate because candidates are scarce, song effort should be low. Finally, song effort should increase with female reproductive competence, which, at any one time, can vary widely among females owing to individual variation in reproductive schedule (Perrins 1970).
Cassin's finches (Carpodacus cassinii ) breed in mid- to high-elevation coniferous forests in the western USA (Samson 1976; Mewaldt & King 1985). Most songs are relatively stereotyped across males of a population (Samson 1978) and consist of a rapid series of short, 2–6 kHz syllables (Hahn 1996; see Electronic Appendix for details on song and other aspects of their natural history). Using wild-caught laboratory-housed Cassin's finches, we manipulated the duration of isolation from females, which varied in reproductive competence, to test the prediction that males adjust song effort in response to female availability and reproductive competence. Reproductive competence is a function of the interaction between season and reproductive schedule, the latter inferred non-invasively by the progress of post-breeding feather moult. In photoperiodic species, onset of moult presages the termination of reproductive competence (Dawson et al. 2001). By comparing moult-stages between females, we inferred whether a female's reproductive schedule was advanced or delayed relative to that of the other females. We predicted, for example, greater late-season song output in males courting delayed females than in those courting advanced females, because delayed females are more likely to still be reproductively competent.
2. Material and methods
(a) Pre-experiment
On 31 October 2002, we captured 20 Cassin's finches in the Sierra Nevada, CA, USA (2300 m elevation; 37°50′ N, 119°10′ W). We held them at the University of California Davis on naturally changing photoperiods with ad libitum food and water and sexed them by laparotomy. On 14 January 2003, we transferred 10 males, determined by plumage to have hatched that spring or summer (Electronic Appendix), and 10 females of unknown age to single-sex sound-attenuation chambers, with three males and five females per chamber (except 1 male inadvertently housed with females; see §3a). We held them on an 8 h light:16 h dark photoperiod (8L:16D).
(b) Experiment procedure
On 23 August 2003, we changed the photoperiod to 15L:9D to stimulate reproductive physiology and behaviour, and initiated the experiment. We recorded each male's song during two trials. Each trial consisted of two consecutive days, one during which he was with the female randomly and uniquely assigned to him and the other during which he was alone. After three weeks, we completed trial 1 and began trial 2, recording males’ song in the same order and each with the same female but with the female-presentation sequence reversed. Therefore, for half of the males, female-presentation sequence was present–absent–absent–present; and, for the other half, it was absent–present–present–absent (hereafter P1A1–A2P2 and A1P1–P2A2, respectively; subscripts indicate trial and letters indicate female presence or absence for each of the two days of a trial).
Birds were always transferred to the recording chamber 2.0–3.5 h after lights-on and remained there until 2.0–3.5 h after lights-on the following day (females), or two days later (males). We placed the female in a separate cage immediately beside the male so that he could see and hear but not have physical contact with her.
(c) Quantification and analyses
Using a Radioshack Pro-Unidirectional Dynamic microphone (catalogue number 33-3001) suspended 5 cm above the centre of the male's cage and a Sony TCM-5000EV analogue tape recorder (recording frequency, 90–12 000 Hz), we recorded song during the first hour following the addition or removal of a female for both days of both trials, yielding four 1 h recordings for each male. Using Avisoft software (v. 4.1a), we digitized recordings at 8 bits and 22 050 kHz (time resolution, 5.8 ms; frequency resolution, 56 Hz).
On 17 November, three weeks after completion of trial 2, and again on 10 December 2003, we assessed moult progress of primary feathers as described previously (Hahn 1995), and separately ranked males and females from 1 to 10 (delayed–advanced schedule). None had begun moult during the experimental period but all were moulting on both assessment dates. The two dates' ranks were positively but not perfectly correlated (females: F1,8=16.75, p=0.003, r2=0.677; males: F1,8=13.40, p=0.006, r2=0.626), reflecting small changes in ranking during the three-week inter-assessment interval.
We analysed the number and total duration of songs (both log-transformed after adding 1 to homogenize variances; raw values graphically depicted) with respect to the presence or absence of a female, using repeated measures ANOVAs in which recording day was a within-subject factor and female-presentation sequence was a between-subjects factor. To investigate the effects of reproductive competence, we focused on the day immediately following female removal (A1 for P1A1–A2P2 males and A2 for A1P1–P2A2 males), when song output was generally high (see §3a). We used ANCOVAs in which the between-subjects variables trial and moult rank accounted for ‘season’ and reproductive schedule, respectively.
3. Results
(a) Effects of female presence on male song
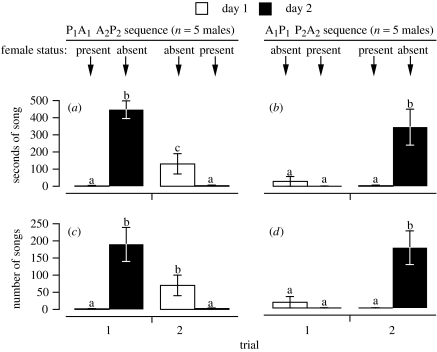
In the presence of a female, males sang very little (figure 1). Song output in their absence depended on recency of female exposure. In the 1 h immediately following removal of the female (A1 of P1A1–A2P2 and A2 of A1P1–P2A2), males sang prolifically. Males last exposed to females three weeks prior (A2 of P1A1–A2P2) sang less; and males deprived of female exposure for seven months (A1 of A1P1–P2A2) sang very little. The male inadvertently housed with females was tested in the P1A1–A2P2 sequence.
Figure 1.
Song output, measured as seconds of song (a, b) and number of songs (c, d) in male Cassin's finches in response to female presence or absence (mean±s.e.m.). Song was recorded for 1 h on each of two consecutive days over two trials separated by three weeks. Males were exposed to females in the order present–absent (trial 1) absent–present (trial 2; a, c) or absent–present (trial 1) present–absent (trial 2; b, d). Within a panel, bars without a letter in common were reliably different (p<0.05) based on Fisher's least-significant-difference post hoc comparisons.
Statistically reflected in the day×sequence interaction, this effect of recency of female presence was extremely reliable, regardless of whether our dependent measure was seconds or number of songs (each measure: F3,24>31.47, p<0.000 01).
(b) Effects of reproductive competence on male song
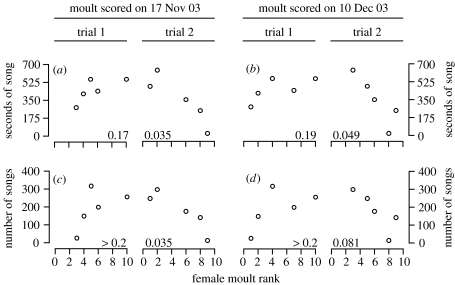
Reproductive competence is a function of the interaction between reproductive schedule and season. Therefore, we examined the effects of the moult rank×trial interaction on song output in response to female loss (A1 of P1A1–A2P2 and A2 of A1P1–P2A2). The relationship between female moult rank and song output during trial 1 contrasted markedly with that three weeks later during trial 2, regardless of moult assessment date or whether the measure was seconds (17 November moult×trial: F1,6=13.81, p=0.010; 10 December: F1,6=14.79, p=0.008) or number of songs (17 November: F1,6=7.43, p=0.034; 10 December: F1,6=6.70, p=0.041). Whereas the relationship between female moult and these measures of song output in trial 1 was positive, albeit unreliable (see post hoc linear regression results in figure 2), it was reliably negative three weeks later in trial 2. The effect of male reproductive competence on song output was unreliable (moult×trial effect for each model: F1,6<1.01, p>0.2).
Figure 2.
Song output, measured as seconds of song (a, b) and number of songs (c, d), in male Cassin's finches in response to the moult rank of the female just lost. Moult was scored on 17 November (a, c) and 10 December 2003 (b, d), several weeks after song was recorded, and females were ranked according to their moult progress on each date (1–10, delayed–advanced). Numbers at the bottom of each panel are p-values from post hoc linear regressions.
4. Discussion
The song behaviour of the male Cassin's finches in this laboratory study apparently functions primarily to attract females that are probably in the vicinity, as suggested by the strong propensity for males to sing in direct response to female loss, but not the mere absence or the presence of a female. Previous studies documenting a rise in male song output in response to mate loss (see §1) did not distinguish between loss of a mate and absence of a mate, or between the mate specifically and a nearby female. Here, we show that the longer males had been deprived of female exposure, the less they sang in their absence. Moreover, only one day of female visual and acoustic proximity was necessary to elicit a robust increase in song output in response to her loss.
Song output may depend on the reproductive competence of the female just lost. Early in the season, during trial 1, song output increased as the moult rank of the female just lost increased, although the repeatability of this increase is far from reliable. Three weeks later, during trial 2, song output showed a reliable decrease as moult rank of the female increased. Moreover, the effects of the female moult×trial interaction indicate that the slope of the song–moult relationship consistently differs between trials and, therefore, that the seasonal change in the relationship between song output and the reproductive schedule of the female just lost is itself reliable, regardless of moult-assessment date.
Our inference that moult rank late in the season reflects the reproductive schedule hinges on the validity of three assumptions. First, moult onset must be associated with the termination of reproductive competence, which is marked by the onset of photorefractoriness in photoperiodic species (Dawson et al. 2001). Numerous studies have shown that onset of moult and photorefractoriness are tightly coupled and may be under the same physiological regulation (Dawson & Sharp 1998), confirming the validity of this assumption.
Second, inter-individual variation in rate of moult progress must be small, such that moult rankings reflect the order in which individuals lose reproductive competence. We did observe variation in moult rate, but the fact that moult scores on the first assessment date yielded the same relationship with song output as those three weeks later suggests that, despite modest variation, rankings largely reflect the order in which individuals lose reproductive competence.
Third, the duration of reproductive competence must be similar among individuals, such that the order in which individuals lose reproductive competence reflects the order in which they gain it. It is indeed possible that the window of reproductive competence varies among individuals, particularly if they differ in age (Sockman et al. 2004). Variation in duration of reproductive competence may explain the reduced ability of moult rank to predict song output early in the season. Alternatively, males may not rely on female reproductive competence to gauge song output early in the season.
We found that male Cassin's finches produce song in proportion to the reproductive competence of the female they are attempting to attract. This dynamic modulation of mate-attraction effort, whereby males adjust behavioural output according to its probable dividends, would seem beneficial in situations where attracting a mate is costly.
Acknowledgments
We thank Rebecca L. Wiley, Russell D. Fernald and Sabrina S. Burmeister for help with this study. Funding was provided by NIH/NICHD Individual NRSA 41854 to K.W.S., NIH/NINDS R01 35467 to G.F.B and NSF IBN-0310995 to T.P.H.
Footnotes
Current address: Department of Biology, University of North Carolina, Chapel Hill, NC 27599-3280, USA.
Supplementary Material
References
- Ball G.F. The neuroendocrine basis of seasonal changes in vocal behavior among songbirds. In: Hauser M.D, Konishi M, editors. The design of animal communication. Bradford/MIT Press; Cambridge, MA: 1999. pp. 213–253. [Google Scholar]
- Cuthill I, Hindmarsh A. Increase in starling song activity with removal of mate. Anim. Behav. 1985;33:326–335. [Google Scholar]
- Dawson A, Sharp P.J. The role of prolactin in the development of reproductive photorefractoriness and postnuptial molt in the European starling (Sturnus vulgaris) Endocrinology. 1998;139:485–490. doi: 10.1210/endo.139.2.5701. [DOI] [PubMed] [Google Scholar]
- Dawson A, King V.M, Bentley G.E, Ball G.F. Photoperiodic control of seasonality in birds. J. Biol. Rhythm. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- Gil D, Gahr M. The honesty of bird song: multiple constraints for multiple traits. Trends Ecol. Evol. 2002;17:133–141. [Google Scholar]
- Hahn T.P. Integration of photoperiodic and food cues to time changes in reproductive physiology by an opportunistic breeder, the red crossbill, Loxia curvirostra (Aves: Carduelinae) J. Exp. Zool. 1995;272:213–226. [Google Scholar]
- Hahn T.P. Cassin's finch (Carpodacus cassinii) In: Poole A, Gill F, editors. The birds of North America, No. 240. The Academy of Natural Sciences; Philadelphia, PA: 1996. [Google Scholar]
- Krebs J.R, Avery M, Cowie R.J. Effect of removal of mate on the singing behaviour of great tits. Anim. Behav. 1980;29:635–637. [Google Scholar]
- McGregor P.K. The singer and the song: on the receiving end of bird song. Biol. Rev. 1991;66:57–81. [Google Scholar]
- Mewaldt L.R, King J.R. Breeding site faithfulness, reproductive biology, and adult survivorship in an isolated population of Cassin's finches. Condor. 1985;87:494–510. [Google Scholar]
- Perrins C.M. The timing of birds' breeding seasons. Ibis. 1970;112:242–255. [Google Scholar]
- Samson F.B. Territory, breeding density, and fall departure in Cassin's finch. Auk. 1976;93:477–497. [Google Scholar]
- Samson F.B. Vocalizations of Cassin's finch in northern Utah. Condor. 1978;80:203–210. [Google Scholar]
- Sockman K.W, Williams T.D, Dawson A, Ball G.F. Prior experience with photostimulation enhances photo-induced reproductive development in female European starlings: a possible basis for the age-related increase in avian reproductive performance. Biol. Reprod. 2004;71:979–986. doi: 10.1095/biolreprod.104.029751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.