Abstract
Spatial and temporal heterogeneity in relative fitness of competing species is a key factor affecting the structure of communities. However, it is not intuitive why species that are ecologically similar should differ in their response to environmental changes. Here we show that two sympatric flycatchers differ in reproductive strategy and in sensitivity to harsh environment. The fitness of collared flycatchers (Ficedula albicollis), which are dominant in interference competition, is more sensitive than the fitness of pied flycatchers (Ficedula hypoleuca) to the seasonal decline in environmental conditions. In order to control for the possibility that this pattern arises solely from differences in microhabitat use (i.e. a local niche differentiation), we performed a partial cross-fostering experiment of young between the two species (i.e. resulting in nests containing young of both species). Our results show that the growth of nestling pied flycatchers is less influenced by the seasonal decline in environmental conditions. We suggest that a life-history trade-off between interference competitive ability and robustness to harsh environment promotes a regional coexistence of the two species.
Keywords: coexistence, interspecific competition, niche differentiation, life history, trade-off, environmental heterogeneity
1. Introduction
A central question in ecology and evolutionary biology is to what extent species need to differ in the resources they use to coexist. Interspecific competition over similar resources was traditionally thought to lead to either niche differentiation or extinction of the poorer competitor (Volterra 1926; Lotka 1932). More recent models suggest that similar species may coexist if fluctuations in environmental conditions favour different species at different times or places (e.g. Chesson & Werner 1981; Chesson & Huntley 1997; Amarasekare & Nisbet 2001). In the latter models, the concept of niche differentiation is shifted from a local to a regional scale; relative competitive abilities change between sites (or in time) and typically only one species is assumed to occupy a specific local patch at a specific time (but see Amarasekare & Nisbet 2001).
It is not intuitive that species that use similar resources should differ in their response to changes in the environment. In this study, we use two approaches to test the possibility that differences in resource allocation strategies between species may lead to changes in their fitness ranking across environments. First, we used long-term data from sympatric populations of collared and pied flycatchers to investigate the covariation between life-history traits (e.g. number and growth of offspring produced) and the timing of breeding, an environmental factor with strong effects on reproductive success in birds (e.g. Daan et al. 1989). Second, we used an experimental approach where we exchanged half the broods between the two species to control for potential differences in niche use by the parents.
Pied and collared flycatchers are closely related and ecologically similar species (Lundberg & Alatalo 1992). Their distributions overlap in central and eastern Europe and on the Baltic islands of Öland and Gotland. Collared flycatchers are dominant in competition over nest sites within the preferred microhabitat of deciduous woodland (Lundberg & Alatalo 1992). Therefore, there is a trend towards competitive exclusion of pied flycatchers in some areas where the species co-occur (Gustafsson & Pärt 1991; Sætre et al. 1999a,b). However, the density of collared flycatchers is relatively more sensitive to large-scale climate variation, which may counteract competitive exclusion of pied flycatchers (Sætre et al. 1999b). This pattern is correlative and may therefore arise as a consequence of a local niche differentiation. Male pied and collared flycatchers may defend territories in different microhabitat where fluctuations in food availability differ (Sætre et al. 1999a; Veen et al. 2001). Another possibility is that the two species use largely similar resources within any given environment, but that their offspring differ in their intrinsic ability to allocate these resources to growth, which in turn leads to different responses of the fitness of the two species to environmental change. These two hypotheses are not mutually exclusive, and in this study we perform an experiment in order to disentangle them.
2. Material and methods
Data on life-history traits were collected from mixed populations of collared and pied flycatchers breeding on southern Gotland (57°10′ N, 18°20′ E) between 1980 and 2003, and on northern Öland (57°10′ N, 16°58′ E) in 2002–2004. All breeding birds were caught, weighed (to nearest 0.1 g), measured and ringed. Records were kept of the date of the onset of egg laying, total number of eggs laid, number of hatched offspring and number of fledged offspring. Nestlings were ringed, measured and weighed when 13 days old. Annual variation in laying date and reproductive performance was controlled for by using the residuals from ANOVAs with year as a factor. This was done using the entire database while the analyses were restricted to pairs in which both members had been identified as phenotypically pure species (because hybrids experience reduced fitness). Therefore, the sum of the overall mean residuals for both species will not be equal to zero.
The cross-fostering experiment was performed on Öland in 2003 and 2004. We exchanged approximately half of each brood between nests of pied and collared flycatchers with coinciding hatching dates. This was done when all the nestlings were 3 days old (i.e. there is no asynchrony of hatching date). All adult birds were caught, measured and ringed. We measured and weighed (to nearest 0.1 g) the nestlings both when 3 days old (and marked them individually by clipping their claws) and 12 days old. The total sample size was 88 nests, 44 attended by collared flycatchers and 44 attended by pied flycatchers.
3. Results
We found that pied and collared flycatchers differed significantly in several life-history traits. Pied flycatchers bred relatively later, after controlling for annual variation in average laying date (ANOVA; F1,7385=82.88, p<0.0001, mean residual laying date=2.24±0.32, and −0.68±0.05 for pied and collared flycatchers, respectively). When this difference in laying date is taken into account, pied flycatchers produced larger clutches (ANOVA; F1,7362=57.30, p<0.001; mean residual clutch size=0.42±0.05, and 0.05±0.01 for pied and collared flycatchers, respectively), and more fledged offspring (table 1; mean residual number of fledged offspring=1.32±0.17, and 0.80±0.02 for pied and collared flycatchers, respectively). Furthermore, the number of fledged offspring (table 1, figure 1) of female pied flycatchers was less sensitive to the seasonal deterioration of the breeding environment. The life-history differences between the two species were similar when we examined the data collected at the two islands separately (unpublished data).
Table 1.
Analyses of variation in reproductive success (estimated as number of offspring fledged, standardized by year) of Ficedula flycatchers in relation to species identity (collared or pied flycatcher) and timing of breeding (laying date, standardized by year). (Model F3,6492=105.29, p<0.0001.) SS, sums of squares.
| source | d.f. | SS | F | p |
|---|---|---|---|---|
| species | 1 | 39.624 | 9.536 | 0.002 |
| laying date | 1 | 71.564 | 17.223 | <0.0001 |
| laying date×species | 1 | 36.712 | 8.836 | 0.003 |
Figure 1.
Reproductive success of collared and pied flycatchers in relation to the timing of breeding. Open squares represent collared flycatchers and filled squares represent pied flycatchers. Collared flycatchers experience a significantly steeper seasonal decline in reproductive success than pied flycatchers.
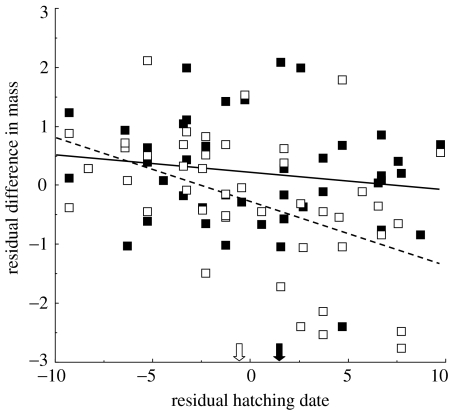
In a partial cross-foster experiment, we investigated whether timing of breeding and species identity of the parents attending the nest influence the relative fitness (relative intrinsic ability to gain weight) of nestling pied and collared flycatchers reared within the same nests. The weight of nestlings at day 3 (i.e. when they were cross-fostered) did not predict their weight at fledging (n=88, β=−0.11±0.11, p=0.30 and n=88, β=0.06±0.14, p=0.64 for nestling collared and pied flycatchers, respectively). In nests attended by collared flycatchers, there was a seasonal change in the relative fitness of nestlings of the two species. Early in the season, nestling collared flycatchers weighed relatively more at fledging, while the pattern was reversed late in the season (table 2, figure 2). In nests attended by pied flycatchers, nestling collared flycatchers on average experienced a relative fitness advantage (n=44, paired t-test; t=3.72, mean difference in weight=0.53±0.14, p=0.0006) throughout the breeding season (table 2, figure 2).
Table 2.
Analyses of variation in relative fitness (estimated as the difference in mean weight at fledging, standardized by year) of nestling collared and pied flycatchers being reared together in relation to species identity of the adult birds attending the nest, and timing of breeding (hatching date, standardized by year). (Model F3,84=5.96, p<0.001.) SS, sums of squares.
| source | d.f. | SS | F | p |
|---|---|---|---|---|
| species | 1 | 4.068 | 4.422 | 0.038 |
| hatching date | 1 | 9.537 | 10.366 | 0.002 |
| hatching date×species | 1 | 3.459 | 3.760 | 0.056 |
Figure 2.
Difference in mean weight (grams) at fledging between nestling collared and pied flycatchers being raised in the same nests in relation to timing of breeding. Open squares represent nests attended by adult collared flycatchers (n=44) and filled squares represent nests attended by pied flycatchers (n=44). Mean breeding dates are indicated by open and closed arrows for collared and pied flycatchers, respectively. Nestling collared flycatchers experience an advantage when reared early in the season and when reared by pied flycatchers.
4. Discussion
Our results demonstrate that pied and collared flycatchers differ in several life-history traits and in their response to environmental changes. Pied flycatchers produce larger broods, breed later, produce more fledged offspring and their reproductive success is less sensitive to the seasonal decline in environmental conditions (i.e. low availability of food). The last result appears to be caused both by a difference in the intrinsic ability of the young to allocate resources to growth during harsh conditions and by a difference in the behaviour of the adult birds. In experimental nests (i.e. consisting of young of both species) that were attended by collared flycatchers, there was a seasonal change in relative fitness of the young of the two species, such that nestling collared flycatchers gained more weight during favourable conditions (i.e. when reared early in the season), but nestling pied flycatchers gained more weight late in the season. However, in experimental nests attended by pied flycatchers, nestling collared flycatchers experienced a relative fitness advantage throughout the season suggesting that pied flycatchers provide a more stable nestling environment throughout the season.
The fact that pied flycatchers, on average, breed later must be interpreted with caution because collared flycatchers are socially and numerically dominant (Lundberg & Alatalo 1992). The later breeding could reflect a different life-history decision, but might also be enforced through competition or reflect a shortage of mates. The difference in the onset of breeding probably means that the nests we used in our experiment were not perfectly matched in terms of quality. However, this potential matching problem is unlikely to be large, especially because the experiment was performed on Öland where breeding densities are lower than on the neighbouring island of Gotland. This lower competition probably creates a smaller temporal variation in the quality of breeding flycatchers (i.e. the difference in mean breeding date is small in relation to the big overlap in breeding period; figure 2).
It has been shown that the adult birds of the two species bring slightly different types of food items to their offspring (Bures 1995). This has led to the suggestion that the two species of flycatchers differ in robustness to climatic changes because male pied and collared flycatchers defend territories in different microhabitats, where fluctuations in food availability differ (Sætre et al. 1999a; Veen et al. 2001). However, the results from our partial cross-fostering experiment indicate that such a local niche differentiation cannot provide the sole explanation for the species' difference in fitness sensitivity to the declining breeding date observed in our long-term study. We found a seasonal change in relative fitness of nestling collared and pied flycatchers reared in nests attended by collared flycatchers. Thus, there is a seasonal change in which type of nestlings have the intrinsic ability to allocate most resources to growth. As in life-history traits in general, the identified difference in growth potential probably depends on a number of underlying traits. That pied flycatcher young seem physiologically better adapted to poor environmental conditions could reflect a higher tolerance of stress in general or a better ability to digest certain types of food. We consider the first explanation most likely because nestling collared flycatchers experienced a fitness advantage throughout the season when reared by adult pied flycatchers (i.e. they appear able to digest the prey that are available late in the season). Thus, both adult and nestling pied flycatchers seem more able to cope with a declining availability of food but nestling collared flycatchers appear better to exploit good conditions, including the nest-environment provided by pied flycatchers. In general, offspring growth patterns are shaped by interactions between ecological (e.g. diet and climate) and developmental variables (O'Connor 1977), although social factors such as effects of sibling competition may play an additional role (Royle et al. 1999). Pied flycatchers breed throughout a larger part of Europe and are, therefore, exposed to a more northern climate and habitat compared with collared flycatchers. Thus, during their evolutionary history, pied flycatchers have probably been selected to grow under comparatively poorer conditions than collared flycatchers. Because a parent's optimal reproductive decisions depend on their offspring's ability to develop and grow within any given environment and vice versa (Roff 1992; Stearns 1992), the life-history traits of the parents and the growth patterns of their offspring have probably responded to selection in a correlated manner. The smaller clutch sizes produced by collared flycatchers may therefore partly result from their offspring being more sensitive to low food availability and possibly also from being more aggressive in sib–sib competition.
In regions of overlapping distribution of the two species, and where the density of collared flycatchers is high, pied flycatchers are mainly found in local pockets of poor habitat (Lundberg & Alatalo 1992; Sætre et al. 1999a). That subdominant competitors can persist in local sites that are unoccupied by the dominant competitor is well documented in studies on sessile organism such as plants (e.g. Conell 1978), and regional coexistence is often assumed to be maintained through a trade-off between dispersal and competitive ability of the seeds (Levins 1970). However, this mechanism does not apply to communities consisting of organisms with high dispersal abilities, such as birds. Trade-offs between competitive ability and longevity or fecundity may then play an important role, as demonstrated by a recent model on coexistence of parasitic wasps (Bonsall et al. 2004). Apart from life-history trade-offs, a key factor is spatial or temporal heterogeneity in fitness, which may lead to both regional and local coexistence of competing species through source–sink dynamics (Amarasekare & Nisbet 2001). In this study, we have experimentally demonstrated that species using similar resources may experience a reversal in relative fitness when environmental conditions change if their offspring differ in ability to gain mass under different conditions. We suggest that the overall distribution of the two species of flycatchers largely depends on a life-history trade-off between investment in interference competition and robustness to harsh environment. Collared flycatchers are dominant in interference competition, but the growth of their young is relatively more sensitive to harsh environmental conditions.
Acknowledgments
We thank F. Weissing and two anonymous reviewers for their constructive comments on the manuscript, M. Halvarsson, M. Olsson, A. Säfstren and J. Älvgren for help in the field, and the Swedish Research Council, the Royal Swedish Academy of Science and Knut and Alice Wallenberg's Foundation for financial support.
References
- Amarasekare P, Nisbet R.M. Spatial heterogeneity, source–sink dynamics, and the local coexistence of competing species. Am. Nat. 2001;158:572–584. doi: 10.1086/323586. [DOI] [PubMed] [Google Scholar]
- Bonsall M.B, Jansen V.A.A, Hassell P.M. Life history trade-offs assemble ecological guilds. Science. 2004;306:111–114. doi: 10.1126/science.1100680. [DOI] [PubMed] [Google Scholar]
- Bures S. Comparison of diet between collared flycatcher (Ficedula albicollis) and pied flycatcher (Ficedula hypoleuca) nestlings in a hybrid zone. Folia Zool. 1995;44:247–253. [Google Scholar]
- Chesson P.L, Huntley N. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. Am. Nat. 1997;150:519–553. doi: 10.1086/286080. [DOI] [PubMed] [Google Scholar]
- Chesson P.L, Werner R.R. Environmental variability promotes coexistence in lottery competitive systems. Am. Nat. 1981;117:923–943. [Google Scholar]
- Conell J.H. Diversity in tropical rainforests and coral reefs. Science. 1978;199:1302–1310. doi: 10.1126/science.199.4335.1302. [DOI] [PubMed] [Google Scholar]
- Daan S, Dijkstra C, Drent R.H, Meijer T. Food supply and the annual timing of avian reproduction. In: Ouellet H, editor. Acta XIX Congr. Int. Ornithol. University of Ottowa Press; 1989. pp. 392–407. [Google Scholar]
- Gustafsson L, Pärt P. Interspecific relations between collared and pied flycatchers. In: Bell B.D, Cossee R.O, Flux J.E.C, Heather B.D, Hitchmough R.A, Robertson C.J.R, Williams R.A, editors. Proc. XX Int. Ornithol. Congr. New Zealand Ornithological Congress Trust Board; Wellington: 1991. pp. 1425–1431. [Google Scholar]
- Levins R. Extinction. In: Gerstenhaber M, editor. Some mathematical problems in biology. American Mathematical Society; Providence, RI: 1970. pp. 75–107. [Google Scholar]
- Lotka A.J. The growth of mixed populations: two species competing for a common food supply. J. Wash. Acad. Sci. 1932;22:461–469. [Google Scholar]
- Lundberg A, Alatalo R.V. Poyser; London: 1992. The pied flycatcher. [Google Scholar]
- O'Connor R.J. Differential growth and body composition in altricial passerines. Ibis. 1977;119:147–166. [Google Scholar]
- Roff D. Chapman & Hall; NY: 1992. Evolution of life histories. [Google Scholar]
- Royle, N. J., Hartley, I. R., Owens, I. P. F. & Parker, G. A. 1999 Sibling competition and the evolution of growth rates in birds. Proc. R. Soc. B266, 923–932. (doi:10.1098/rspb.1999.0725)
- Sætre G.-P, Král M, Bures, Ims R.A. Dynamics of a clinal hybrid zone and a comparison with island hybrid zones of flycatchers. J. Zool. Lond. 1999;247:53–64. [Google Scholar]
- Sætre, G.-P., Post, E. & Král, M. 1999b Can environmental fluctuation prevent competitive exclusion in sympatric flycatchers? Proc. R. Soc. B266, 1247–1251. (doi:10.1098/rspb.1999.0770)
- Stearns S. Oxford University Press; Oxford, UK: 1992. Evolution of life histories. [Google Scholar]
- Veen T, et al. Hybridization and adaptive mate choice in flycatchers. Nature. 2001;411:45–50. doi: 10.1038/35075000. [DOI] [PubMed] [Google Scholar]
- Volterra V. 1926 Variations and fluctuations of the numbers of individuals in animal species living together. In Animal ecology (ed. R. N. Chapman). pp. 409–448. New York: McGraw Hill (Reprinted by McGraw Hill 1931).