Abstract
The numbers and sizes of eggs produced by adult females ultimately determine the viability of populations, as well as the evolutionary fitness of the females themselves. Despite an enormous amount of literature on the adaptive significance of fecundity variation within and among populations, simpler questions—such as the proximate mechanisms by which a female determines her clutch size—have attracted less attention. Our surgical manipulations show that the amount of space available to hold eggs within a female's abdomen influences her total reproductive allocation, enabling her to flexibly modify her reproductive output as she grows larger.
Keywords: clutch size, fecundity, life history, reptile
1. Introduction
By what mechanisms does a reproducing female determine the number and size of offspring she will produce? An extensive literature on life-history variation has explored the adaptive significance of variation in both offspring size and clutch size. Most authors treat reproductive output as a result of two separate processes at a proximate level. First, the female determines how much energy she will devote to the present clutch, and then, determines how this overall allocation will be divided (i.e. into many small offspring or fewer, larger progeny; see, for example, Winkler & Wallin 1987; Caley et al. 2001). These two components of reproductive output are likely to be under different kinds of control, in terms of selective forces as well as physiological control mechanisms. Thus, total allocation of resources to reproduction is usually interpreted in terms of optimization of reproductive effort, with female fitness enhanced by balancing energy allocation between the competing demands of reproduction, maintenance, growth and storage (Stearns 1976; Bonnet et al. 2001). In contrast, the evolutionary pressures on offspring size presumably relate to the viability of offspring of different sizes (King 1993; Olsson et al. 2002; Roff 2002).
As for the selective forces involved, the proximate mechanisms that control total resource allocation are likely to be quite different from those that control the division of this total pool into progeny of various sizes. We know considerably more about this second allocation issue than about the first. Experimental manipulations in lizards reveal a direct proximate trade-off between egg size and number; ablation of vitellogenic follicles prior to ovulation redirects the extra resources into the remaining follicles, so that the female produces fewer but larger eggs (Sinervo et al. 1992). The same cause-and-effect pathway can be demonstrated by increasing rather than reducing the number of vitellogenic follicles; hormonal stimulation to increase egg number reduces mean egg size (Sinervo & Licht 1991; Sinervo et al. 1992). However, we know much less about the factors controlling the other major component of reproductive output: how does a female determine the total amount of resources that she will invest in a clutch?
The obvious answer would appear to be rates of food intake, with energy allocation to reproduction directly reflecting rates of energy acquisition and thus, availability of ‘surplus’ energy. However, this answer must be wrong, at least for ectothermic animals such as reptiles. First, female reptiles typically accumulate resources over long periods prior to reproduction (‘capital breeding’; Bonnet et al. 1998) and thus, rates of energy gain are not the proximate determinant of clutch size. If the female's feeding rate is lowered, she will usually postpone clutch production rather than producing a smaller clutch (Saint Girons 1957; Shine & Madsen 1997). Second, neither can this investment process be based on any simple fixed rule (e.g. ‘produce three eggs’), because in most lizard species, clutch size is adjusted to the female's changing body size as she grows larger. Clutch sizes are constant in a few lizard lineages (notably, anoles and geckos) but these comprise only a small proportion of extant lizard taxa (Shine & Greer 1991). In most or all species with variable clutch sizes, larger females produce more eggs (Fitch 1970). Indeed, clutch sizes may increase by an order of magnitude from a female's first clutch post-maturation to that produced at her final maximum body size (Fitch 1970; Seigel & Ford 1987).
Although the situation has attracted little scientific attention, the facts that female ectotherms (i) span a wide range of body sizes during adult life and (ii) adjust their clutch sizes to their changing body sizes throughout this period, suggest that the proximate mechanisms determining total resource allocation to reproduction must somehow involve maternal body size. By what mechanism does a female adjust her reproductive output to match her body size? We envisage three alternative mechanisms:
based on body mass—the female could maintain a constant ratio of clutch mass to maternal body mass (relative clutch mass (RCM); Seigel & Fitch 1984);
based on body volume—she could produce enough eggs to fill the abdomen to some constant, optimal degree (Vitt & Congdon 1978; Shine 1992); or
based on some other body size parameter—she might link her reproductive output to some other indicator of her body size (e.g. body length, skeletal dimensions).
A simple manipulation allows us to test among these three ideas, because they make different predictions about the effects of surgically inserting objects into the female's abdomen. Such objects add to maternal mass but decrease ‘free’ abdominal space; thus, our three hypotheses above predict that subsequent clutch mass should be (i) increased (to maintain constant RCM); (ii) reduced (because less space is available); or (iii) unchanged, because the manipulation does not affect the critical ‘size’ indicator. Thus, experimentally reducing the amount of space inside the female's body cavity should provide a simple, robust way to test among the three hypotheses outlined above.
2. Materials and methods
Northern grass lizards (Takydromus septentrionalis) from northern China are elongate-bodied lacertids; females produce clutches of one to five eggs depending on maternal body size (Ji et al. 1998). We collected pre-reproductive females in late March, anaesthetized them, and inserted two egg-size (8.5 mm×5.8 mm, 0.39 g) pearls into the body cavity of each of 29 lizards. Another 28 females were sham-operated, and 32 others were kept as controls. Females were allocated randomly to treatment groups. Mean body sizes were similar among females in the three groups, ranging from 67.8 to 69.0 mm snout–vent length (F2,86=1.38, p=0.26) and 5.2 to 5.4 g mass (F2,86=0.77, p=0.46). Nonetheless, we retained maternal mass as a covariate in statistical analyses of clutch size, to control for minor size variation. Females were then kept in terraria with access to ad libitum food and water until oviposition, which occurred on an average of 26 days later (the time period was independent of experimental treatment: F2,86=0.22, p=0.80). Eggs were removed, measured (length and width) and weighed less than 2 h post-laying, to minimize water uptake or loss between the egg and the substrate. Because of maternal effects, it is not valid to treat eggs within the same clutch as independent for the purposes of statistical analysis; thus, our statistical analyses are based upon mean values for egg sizes per clutch.
3. Results
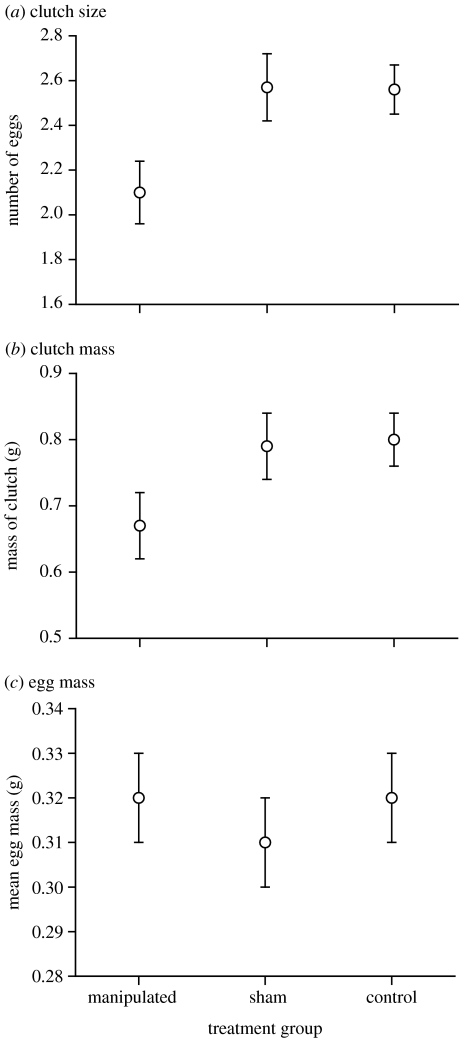
Maternal body mass was not significantly associated with egg size (body mass versus mean egg mass, n=89 clutches, r=0.10, p=0.34; versus mean egg length, r=0.04, p=0.70; versus mean egg width, r=0.11, p=0.33). However, larger females produced heavier clutches (body mass versus clutch mass, n=89 clutches, r=0.33, p<0.002). Egg sizes were not significantly affected by the experimental manipulation (ANOVA with treatment as the factor: for mean egg mass, F2,83=0.50, p=0.61; figure 1c; for mean egg length, F2,83=1.88, p=0.16; for mean egg width, F2,83=1.06, p=0.35). However, females with reduced abdominal volumes produced fewer eggs (ANCOVA, F2,83=3.99, p=0.022; figure 1a) and lower total clutch masses (F2,83=3.36, p<0.04; figure 1b).
Figure 1.
Effects of inserting egg-sized objects (pearls) into the abdomens of reproductive female lizards (Takydromus septentrionalis, Lacertidae). The consequent reduction in available body volume caused females to produce smaller clutches, but egg size was unaffected compared with sham-operated and control females. (a) Clutch size; (b) clutch mass; (c) egg mass.
4. Discussion
Our data support the hypothesis that female reptiles adjust their clutch sizes relative to the amount of space available within their abdomens. However, a major decrease in abdominal space generated only a minor decrease in clutch size. Thus, abdominal space does not constrain clutch mass simply by imposing a physical limit on reproductive output. Our data also enables us to reject predictions from the alternative hypotheses that maternal investment is functionally linked either to RCM per se or on some other indicator of maternal body size. As predicted by the idea that offspring size is determined separately from total resource allocation to reproduction, our experimental manipulations had no effect on egg sizes (figure 1c).
A proximate mechanism whereby clutch size is adjusted relative to maternal body volume fits well with previous correlational studies describing strong links between a reptile's body shape and its reproductive output (Vitt & Congdon 1978). For example, measures of body shapes of a wide range of snake and lizard species were significantly related to RCMs (Shine 1992). Likewise, experimental evidence that maternal body volume constrains the amount of water taken up by eggs in utero (Qualls & Andrews 1999) suggests a controlling role for maternal volume on litter mass. Nonetheless, it is also clear that not all female reptiles are ‘equally full’ of eggs when gravid. The evolution of viviparity (and thus, increased total litter mass owing to water uptake by developing embryos) was accompanied by increased RCM in a scincid lizard species with both oviparous and viviparous populations (Qualls & Shine 1995). However, although RCMs increased, they did so only about half as much as would be expected if there had been no compensation for clutch mass relative to abdominal volume (Qualls & Shine 1995). Similarly, in our own study, total clutch mass was reduced by 0.14 g in response to implantation of an additional 0.78 g (figure 1b). Thus, the compensation in terms of clutch volume was only partial, with the consequence that the experimental lizards were more severely distended by eggs after ovulation than were the sham-operated or control groups.
The relatively modest decrease in clutch sizes after experimental reduction of body volume indicates that reproductive output in T. septentrionalis cannot simply be attributed to a physical constraint imposed by available space. Clearly, the female lizards were able to physically fit a larger number of eggs than they usually carry. Nonetheless, the significant decrease in clutch sizes in response to our treatments suggests that abdominal volume is one of the factors involved in clutch-size determination. Although the mechanism linking clutch size to maternal body size involves more than a straightforward constraint imposed by abdominal body volume, our experiments suggest that space available to hold the eggs may be a significant influence on reptilian reproductive output.
Acknowledgements
The work was supported by grants from Hangzhou City, Zhejiang Provincial Nature Science Foundation, the Australian Research Council and the Paos' Foundation. Thanks also to H. Q. Shen, J. Q. Du and Y. Yang for their assistance.
References
- Bonnet X, Bradshaw D, Shine R. Capital versus income breeding: an ectothermic perspective. Oikos. 1998;83:333–342. [Google Scholar]
- Bonnet X, Naulleau G, Shine R, Lourdais O. Short-term versus long-term effects of food intake on reproductive output in a viviparous snake, Vipera aspis. Oikos. 2001;92:297–308. [Google Scholar]
- Caley M.J, Schwarzkopf L, Shine R. Does total reproductive effort evolve independently of offspring size? Evolution. 2001;55:1245–1248. doi: 10.1111/j.0014-3820.2001.tb00644.x. [DOI] [PubMed] [Google Scholar]
- Fitch H.S. Reproductive cycles in lizards and snakes. Univ. Kansas Mus. Nat. Hist. Misc. Publ. 1970;52:1–247. [Google Scholar]
- Ji X, Zhou W.H, Zhang X.D. Sexual dimorphism and reproduction in the northern grass lizard Takydromus septentrionalis. Russ. J. Herpetol. 1998;5:44–48. [Google Scholar]
- King R.B. Determinants of offspring number and size in the brown snake, Storeria dekayi. J. Herpetol. 1993;27:175–185. [Google Scholar]
- Olsson M, Wapstra E, Olofsson C. Offspring size-number strategies: experimental manipulation of offspring size in a viviparous lizard (Lacerta vivipara) Funct. Ecol. 2002;16:135–140. [Google Scholar]
- Qualls C.P, Andrews R.M. Maternal body volume constrains water uptake by lizard eggs in utero. Funct. Ecol. 1999;13:845–851. [Google Scholar]
- Qualls C.P, Shine R. Maternal body volume as a constraint on reproductive output in lizards: evidence from the evolution of viviparity. Oecologia. 1995;103:73–78. doi: 10.1007/BF00328427. [DOI] [PubMed] [Google Scholar]
- Roff D.A. Sinauer Associates; Sunderland, MA: 2002. Life history evolution. [Google Scholar]
- Saint Girons H. Le cycle sexual chez Vipera aspis (L) dans l'ouest de la France. Bull. Biologique. 1957;91:284–350. [Google Scholar]
- Seigel R.A, Fitch H.S. Ecological patterns of relative clutch mass in snakes. Oecologia. 1984;61:293–301. doi: 10.1007/BF00379625. [DOI] [PubMed] [Google Scholar]
- Seigel R.A, Ford N.B. Reproductive ecology. In: Seigel R.A, Collins J.T, Novak S.S, editors. Snakes: ecology and evolutionary biology. Macmillan Publishing; New York: 1987. pp. 210–252. [Google Scholar]
- Shine R. Relative clutch mass and body shape in lizards and snakes: is reproductive investment constrained or optimized? Evolution. 1992;46:828–833. doi: 10.1111/j.1558-5646.1992.tb02088.x. [DOI] [PubMed] [Google Scholar]
- Shine R, Greer A.E. Why are clutch sizes more variable in some species than in others? Evolution. 1991;45:1696–1706. doi: 10.1111/j.1558-5646.1991.tb02675.x. [DOI] [PubMed] [Google Scholar]
- Shine R, Madsen T. Prey abundance and predator reproduction: rats and pythons on a tropical Australian floodplain. Ecology. 1997;78:1078–1086. [Google Scholar]
- Sinervo B, Licht P. Hormonal and physiological control of clutch size, egg size, and egg shape in side-blotched lizards (Uta stansburiana)—constraints on the evolution of lizard life histories. J. Exp. Zool. 1991;257:252–264. [Google Scholar]
- Sinervo B, Doughty P, Huey R.B, Zamudio K. Allometric engineering: a causal analysis of natural selection on offspring size. Science. 1992;285:1927–1930. doi: 10.1126/science.258.5090.1927. [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Life-history tactics: a review of the ideas. Quart. Rev. Biol. 1976;51:3–47. doi: 10.1086/409052. [DOI] [PubMed] [Google Scholar]
- Vitt L.J, Congdon J.D. Body shape, reproductive effort, and relative clutch mass in lizards: resolution of a paradox. Am. Nat. 1978;112:595–608. [Google Scholar]
- Winkler D.W, Wallin K. Offspring size and number: a life history model linking effort per offspring and total effort. Am. Nat. 1987;129:708–720. [Google Scholar]