Abstract
The nutritional and social conditions that individuals experience during early development can have profound effects on their morphology, physiology, behaviour and life history. Experimental increases in brood size in birds can result in reduced offspring condition and survival, indicating that developmental deficits in enlarged broods have negative fitness consequences within the affected generation. To study long-term effects (i.e. transgenerational effects of developmental stress), we conducted a two-step breeding experiment in which we manipulated early developmental conditions in zebra finches Taeniopygia guttata. We raised zebra finches by manipulating brood sizes and controlled for maternal and genetic effects by cross-fostering. In a previous study, we showed that offspring condition and body size decreased with increasing brood size. Here we show that this effect was carried over to the next generation. Body size in nestlings and at nutritional independence was affected by the brood size in which the mothers were raised. Female offspring did significantly worse than male offspring when the mother had been raised in large broods, suggesting a sex-specific influence of maternal effects. These findings link early developmental stress in females with the phenotype of the next generation via maternal effects.
Keywords: cross-fostering, early developmental stress, life history, maternal effects, zebra finch
1. Introduction
Conditions experienced during early development have been shown to affect reproductive performance later in life (Lindström 1999; Metcalfe & Monaghan 2001). Experimental manipulations of brood size in birds have been a powerful tool to affect nestling condition through developmental stress because birds raised in enlarged broods have reduced growth, condition (Tinbergen & Boerlijst 1990; Brinkhof et al. 1999; Naguib et al. 2004), survival (de Kogel 1997) and recruitment rates after migration (Gustafsson & Sutherland 1988; Smith et al. 1989). However, in contrast to mammals (Huck et al. 1987) and insects (Fox & Mousseau 1998), in birds the extent to which these effects of developmental stress on offspring are projected into future generations is still not known. Given that effects of nutritional stress have been shown to be sex specific, with females being affected more than males (Kilner 1998; Martins 2004), we were also interested in determining whether these sex-specific effects would project into the next generation. Here we report a two-step breeding experiment in which we followed effects of brood size manipulation over two generations. Through cross-fostering, we imposed different degrees of developmental stress on nestlings by creating broods ranging in size from two to six nestlings. We followed the development of these nestlings into adulthood (Naguib et al. 2004) and allowed the females that were raised in these broods to breed to assess the long-term effects of the early developmental conditions of females on their own offspring.
2. Methods
(a) Material and initial breeding methods
We conducted the experiment on zebra finches of wild Australian origin at the University Bielefeld in 2003. Females that originated from a cross-fostering experiment in which we raised zebra finches in different brood sizes (Naguib et al. 2004) were used for breeding. Experimental broods were divided into three groups: small (two to three nestlings), medium (four nestlings) and large broods (five to six nestlings). The methods are described in detail in a previous paper (Naguib et al. 2004) in which we show that experimental manipulation affected nestling growth, testosterone and immunocompetence levels, as well as adult body size and body condition, irrespective of nestling sex.
(b) Breeding of the second generation
Female offspring from the above experiment were randomly assigned for breeding to unrelated males that had been raised in non-manipulated broods (12 females from small manipulated broods; 19 females from medium broods; 16 females from large broods). Pairs were supplied daily with dried and germinated seeds and fresh water (plus vitamins). Air temperature was 23 °C and the light/dark regime 16 h : 8 h. Offspring were kept with their parents until nutritional independence (day 40). Out of 47 females, 13 females did not reproduce (five females laid no egg; eight females produced no hatchling), and 13 did not produce surviving hatchlings. Although some females do not breed, partly owing to males failing to build a nest, the proportions of unsuccessful broods were higher than in previous experiments using the same conditions. However, the proportions of non-reproducing females were not affected by the brood size in which the females were raised (logistic regression, χ2<0.1, p>0.9), and thus do not bias the results reported here. The resulting sample sizes with respect to brood sizes in which the successfully breeding females were raised were: small brood (n=6), medium brood (n=8), and large brood (n=7). The sex ratio of offspring originating from these females was: small broods (5 males and 6 females in total), medium broods (12 males and 8 females in total), large broods (12 males and 8 females in total).
Biometric measurements were taken at hatching (day 1), 15 days post-hatching (shortly before fledging) and at 40 days (feeding independence).
(c) Statistical analysis
We analysed the data using mixed lineal models using Proc Mixed (Sas v. 8.1; Littell et al. 1996). Experimental brood size in which the breeding females were raised was declared as a fixed factor. To control for random effects of females sharing a common environment as nestlings or being genetically related, individual nestling data were first cross-classified by entering as random factors the original and experimental broods where females had been raised. Using this procedure, we controlled for the effect of females sharing a common rearing environment (i.e. when they were raised in the same nest). None of these factors explained a significant part of the variance and they were therefore removed from the models. Individual data were cross-classified within females, and the effect in this case was always highly statistically significant (all p<0.001). Therefore, we conserved female as a random factor. This method effectively removes the problem of non-independence of individual data by calculating their similarity and removing its effect in the model. Degrees of freedom were calculated using Satterthwaite's formula (Littell et al. 1996). We corrected p values for the expected ordered heterogeneity of the experimental groups but used two-tail prediction tables (Rice & Gaines 1994).
3. Results
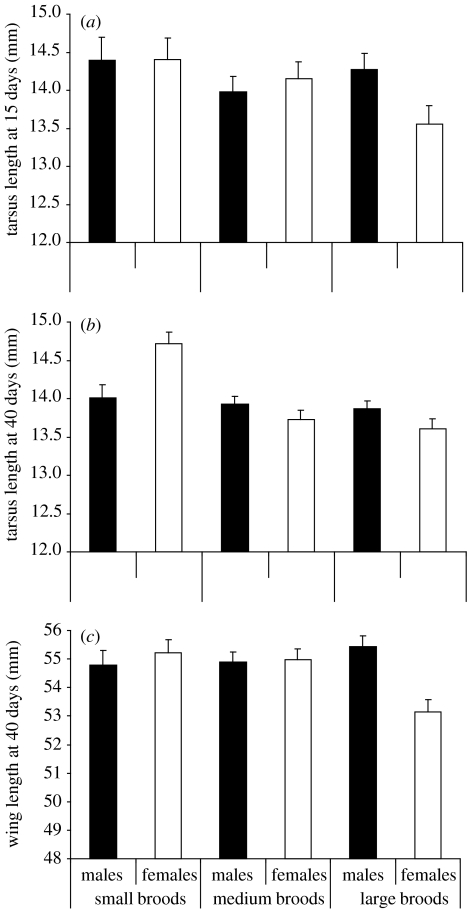
The experimental brood size in which a breeding female was raised had no effect on her latency to lay the first egg (F2,44=0.09, p=0.90) or on measures of quantity such as clutch size, brood size or number of fledglings (all F2,44<0.5, all p>0.6). Brood size was 3.3±0.3 (mean±s.e.) with 52 fledglings (29 males and 23 females). Tarsus length of breeding females had a positive effect on offspring weight at the day of hatching but not thereafter (i.e. once parental care had commenced; table 1). After hatching, the experimental brood size in which a female was raised had significant and sex-dependent effects on offspring biometry (table 1). Interactions between the brood size in which the mother was raised and offspring sex were significant in measures taken before fledging (day 15) and at nutritional independence (day 40), showing that females were specially negatively affected with increasing experimental brood size in which their mother was raised (table 1, figure 1). In other words, female offspring grew less than males when their mothers were raised in a large experimental brood, but the females grew more than males when their mother was raised in a small brood. Measures of offspring biometry at days 15 and 40, except wing length, correlated significantly with each other (tarsus length: r=0.51, n=68, p<0.001; weight: r=0.69, n=68, p<0.001; wing length: r=0.06, n=66).
Table 1.
Results from GLM analysis on factors affecting offspring measurements in first broods of females raised in different experimental brood sizes. (The Table shows F-values and significance values *p<0.05, **p<0.01, ***p<0.001; n.s. is given for variables that were not significant in the initial analysis and thus dropped for the final model; degrees of freedom were calculated by the Satterthwaite method (Littell et al. 1996) to correct for the random factor of brood.)
| body mass | tarsus length | wing length | |
|---|---|---|---|
| day 1 | |||
| mother's brood size | 0.52,19.2 | not measured | not measured |
| female tarsus length | 6.011,17* | ||
| day 15 (shortly before fledging) | |||
| mother's brood size | 0.092,17.9 | 1.132,17.8 | 0.982,15 |
| sex | n.s. | 1.381,41.7 | n.s. |
| mother's brood size×sex | n.s. | 3.972,41* | n.s. |
| day 40 (independence) | |||
| mother's brood size | 0.832,15 | 8.562,14.4** | 1.282,14.3 |
| sex | n.s. | 0.631,45 | 4.701,41.3* |
| mother's brood size×sex | n.s. | 7.732,44.6** | 10.532,40.2*** |
Figure 1.
Relation between brood size in which the mother was raised and biometric traits of her male and female offspring; (a) tarsus length at day 15 (shortly before fledging); (b) tarsus length at day 40 (nutritional independence); and (c) wing length at day 40. Shown are means+s.e. Only the females tend to do worse with increasing brood size.
4. Discussion
These experiments show that effects of early developmental stress experienced by females project into the subsequent generation, i.e. their offspring. Previous studies of birds showed effects of such early developmental stress on an individual's phenotype (de Kogel & Prijs 1996; Nowicki et al. 2000; Spencer et al. 2003; Naguib et al. 2004) and survival (de Kogel 1997), but it has remained unclear whether or not such effects have further consequences for the subsequent offspring. Experiments in collared flycatchers Ficedula albicollis (Gustafsson & Sutherland 1988) and great tits Parus major (Smith et al. 1989) have so far showed that individuals had lower return rates when being raised in enlarged broods, indicating that early developmental stress can affect reproductive potential. Our findings that offspring were increasingly smaller with increasing early developmental stress experienced by the mother expands on these previous findings and show transgenerational long-term consequences. Although females compensated for early developmental stress in several reproductive parameters such as time to egg laying, clutch size and brood size, the compensation was not complete once they started to provide parental care. Raising offspring with reduced body size may be advantageous for females coming from enlarged broods if this represents a reduction in the cost of parental care. Reduced body size, on the other hand, has been shown to be strongly selected against in field studies (Boag & Grant 1981; Alatalo et al. 1990) so that reduced parental effort may directly benefit the female, with costs being paid by the young. It remains to be seen whether these effects on offspring have sustained fitness consequences. Interestingly, effects on size were strongly sex biased, with females doing worse than males when their mothers were reared in large experimental broods. The findings are consistent with previous evidence that female nestlings are more vulnerable to nutritional stress (Bradbury & Blakey 1998; Kilner 1998; Martins 2004; Rutstein et al. 2004) and show that such effects can even last into the next generation (also see Gorman & Nager 2004). Since females raised in enlarged broods produce eggs with lower testosterone levels (Gil et al. 2004), it may be that our present findings were mediated through this mechanism, together with a possibly differential effect of yolk androgen in male and female embryos (Gil 2003). Future research needs to address whether the body size effects projecting into the generation of the offspring's offspring are adaptations by low quality females to invest more in the sex with larger reproductive potential.
Acknowledgments
We thank Edda Geissler for assistance. For their constructive comments on the manuscript, we thank Katharina Riebel and two referees. We also thank the Association for the Study of Animal Behaviour for funding.
References
- Alatalo R.V, Gustafsson L, Lundberg A. Phenotypic selection on heritable size traits: environmental variance and genetic response. Am. Nat. 1990;135:464–471. [Google Scholar]
- Boag P, Grant P.R. Intense natural selection in a population of Darwin's finches (Geospizinae) in the Galapagos. Science. 1981;214:82–85. doi: 10.1126/science.214.4516.82. [DOI] [PubMed] [Google Scholar]
- Bradbury, R. B. & Blakey, J. K. 1998 Diet, maternal condition, and offspring sex ratio in the zebra finch, Poephila guttata Proc. R. Soc. B265, 895–899. (doi: 10.1098/rspb.1998.0375)
- Brinkhof, M. G., Heeb, P., Kölliker, M. & Richner, H. 1999 Immunocompetence of nestling great tits in relation to rearing environment and parentage. Proc. R. Soc. B266, 2315–2322. (doi: 10.1098/rspb.1999.0925)
- de Kogel C.H. Long-term effects of brood size manipulation on morphological development and sex-specific mortality of offspring. J. Anim. Ecol. 1997;66:167–178. [Google Scholar]
- de Kogel C.H, Prijs H.J. Effects of brood size manipulations on sexual attractiveness of offspring in the zebra finch. Anim. Behav. 1996;51:699–708. [Google Scholar]
- Fox C.W, Mousseau T.A. Maternal effects as adaptations for transgenerational phenotypic plasiticity in insects. In: Mousseau T.A, Fox C.W, editors. Maternal effects as adaptations. Oxford University Press; New York: 1998. pp. 159–177. [Google Scholar]
- Gil D. Golden eggs: maternal manipulation of offspring phenotype by egg androgen in birds. Ardeola. 2003;50:281–294. [Google Scholar]
- Gil D, Heim C, Bulmer E, Rocha M, Puerta M, Naguib M. Negative effects of early developmental stress on yolk testosterone levels in a passerine bird. J. Exp. Biol. 2004;207:2215–2220. doi: 10.1242/jeb.01013. [DOI] [PubMed] [Google Scholar]
- Gorman, H. E. & Nager, R. G. 2004 Prenatal developmental conditions have long-term effects on offspring fecundity. Proc. R. Soc. B271, 1923–1928. (doi: 10.1098/rspb.2004.2799) [DOI] [PMC free article] [PubMed]
- Gustafsson L, Sutherland W.J. The costs of reproduction in the collared flycatcher Ficedula albicollis. Nature. 1988;335:813–815. [Google Scholar]
- Huck U.W, Laboy J.B, Lisk R.D. Food-restricting first generation juvenile female hamsters (Mesocricetus auratus) affects sex ratio and growth of third generation offspring. Biol. Reprod. 1987;37:612–617. doi: 10.1095/biolreprod37.3.612. [DOI] [PubMed] [Google Scholar]
- Kilner R. Primary and secondary sex ratio manipulation by zebra finches. Anim. Behav. 1998;56:155–164. doi: 10.1006/anbe.1998.0775. [DOI] [PubMed] [Google Scholar]
- Lindström J. Early development and fitness in birds and mammals. Trends Ecol. Evol. 1999;14:343–348. doi: 10.1016/s0169-5347(99)01639-0. [DOI] [PubMed] [Google Scholar]
- Littell R.C, Milliken G.A, Stroup W.W, Wolfinger R.D. SAS Institute; Cary, NC: 1996. SAS system for mixed models. [Google Scholar]
- Martins T.L.F. Sex-specific growth rates in zebra finch nestlings: a possible mechanism for sex ratio-adjustment. Behav. Ecol. 2004;15:174–180. [Google Scholar]
- Metcalfe N.B, Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- Naguib, M., Riebel, K., Marzal, A. & Gil, D. 2004 Nestling immunocompetence and testosterone covary with brood size in a songbird. Proc. R. Soc. B271, 833–838. (doi: 10.1098/rspb.2003.2673) [DOI] [PMC free article] [PubMed]
- Nowicki, S., Hasselquist, D., Bensch, S. & Peters, S. 2000 Nestling growth and song repertoire size in great reed warblers: evidence for song learning as an indicator mechanism in mate choice. Proc. R. Soc. B267, 2419–2424. (doi: 10.1098/rspb.2000.1300) [DOI] [PMC free article] [PubMed]
- Rice W.R, Gaines S.D. Extending nondirectional heterogeneity tests to evaluate simply ordered alternative hypothesis. Proc. Natl Acad. Sci. USA. 1994;91:225–226. doi: 10.1073/pnas.91.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutstein A.N, Slater P.J.B, Graves J.A. Diet quality and resource allocation in the zebra finch. Proc. R. Soc. B (Suppl. 5) 2004;271:S286–S289. doi: 10.1098/rsbl.2003.0154. (doi:10.1098/rsbl.2003.0154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H.G, Källander H, Nilsson J.-Å. The trade-off between offspring number and quality in the great tit Parus major. J. Anim. Ecol. 1989;58:383–401. [Google Scholar]
- Spencer K.A, Buchanan K.L, Goldsmith A.R, Catchpole C.K. Song as honest signal of developmental stress in the zebra finch (Taeniopygia guttata) Horm. Behav. 2003;44:132–139. doi: 10.1016/s0018-506x(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Tinbergen J.M, Boerlijst M.C. Nestling weight and survival in individual great tits (Parus major) J. Anim. Ecol. 1990;59:1113–1127. [Google Scholar]