Abstract
Type IV pili have been shown to play a role in the early stages of bacterial biofilm formation, but not in initial bacterial attachment. Here, using the surface analytical technique, surface plasmon resonance (SPR), we follow the attachment of the bacterium Pseudomonas aeruginosa in real time. In contrast to previous studies, we show that type IV pili mutants are defective in attachment. Both mutants lacking pili (pilA), and those possessing an overabundance of pili (pilT), showed reduced SPR measured attachment compared with the wild-type PAO1 strain. Both pil mutants also showed reduced pathogenicity in a model insect host, as measured by percentage mortality after 24 h. SPR revealed differences in the kinetics of attachment between pilA and pilT, differences obscured by endpoint assays using crystal violet stain. These results highlight the power of SPR in monitoring bacterial attachment in real time and also demonstrate an additional role for type IV pili beyond bacterial aggregation and micro-colony formation.
Keywords: Pseudomonas aeruginosa, bacterial attachment, pili, surface plasmon resonance, biofilm formation
1. Introduction
Bacterial attachment is a critical first step in both tissue colonization and biofilm formation (Stoodley et al. 2002). Current techniques to monitor colonization rely on the optical detection of bacteria, either in closed or flow-cell systems, or on methods such as crystal violet staining to detect the final presence of bacterial biofilms on artificial substrates (Mohamed et al. 2000; Stoodley et al. 2001, 2002). Staining techniques obscure the critical early stages of attachment as they rely upon the detection of the final numbers of attached bacteria; whereas the use of digital video within a flow-cell facilitates continuous real-time monitoring of bacterial attachment (Stoodley et al. 2001, 2002). To introduce physical attachment parameters into studies of these early stages of attachment we are using the optical technique surface plasmon resonance (SPR) to monitor the colonization of gold treated surfaces by the Gram-negative pathogen Pseudomonas aeruginosa in real-time (Jenkins et al. 2004). This bacterium is a potent pathogen which often infects patients with compromised immune systems or the lungs of patients with genetic diseases, such as cystic fibrosis. In the lungs of cystic fibrosis patients, P. aeruginosa grows in antibiotic-resistant biofilms which are therefore difficult to clear (Hoiby 1993; Singh et al. 2000).
Type IV pili are retractable hair-like structures on the surface of P. aeruginosa that have been shown to be important in biofilm development via their role in bacterial ‘twitching’ motility (O'Toole & Kolter 1998; Mattick 2002; Klausen et al. 2003). Current models of biofilm development in P. aeruginosa and other bacteria propose four stages of biofilm formation (O'Toole & Kolter 1998; Stoodley et al. 2002; Klausen et al. 2003; Ramsey & Whiteley 2004). (i) First there is the initial, reversible, attachment of the bacteria to the substrate. This process is governed by the net product of electrostatic forces, Brownian motion and flagella-mediated locomotion. (ii) This reversible attachment becomes irreversible when the bacteria spread via twitching motility and start to produce extracellular polysaccharides (EPS), a process which can start within 15 min of initial attachment. (iii) This stage is followed by the formation of microcolonies via both bacterial reproduction and further twitching-mediated motility. (iv) Finally, the mature structure of the biofilm is formed via further secretion of the EPS matrix and the adoption of a complex structural architecture. Despite the demonstrated role of type IV pili in the latter stages of biofilm development, presumably via their involvement in twitching motility (O'Toole & Kolter 1998; Klausen et al. 2003), they have not been previously shown to play a role in initial surface attachment.
In order to examine the possible importance of type IV pili in the early attachment of P. aeruginosa, we have been monitoring the attachment of bacteria with normal and defective type IV pili to gold surfaces using SPR. This optical technique is widely used to study protein–protein, or small molecule, interactions on surfaces (Berger et al. 1998) and we have recently adapted this technique to look at larger moieties, specifically the attachment of live bacteria (Jenkins et al. 2004). SPR itself is an evanescent wave phenomenon in which visible light is coupled to the electron field (plasmon wave) in a thin metal film (usually gold or silver) to create an energy wave (surface plasmon resonance). This wave is not confined purely within the metal film, but extends out into the dielectric material on the metal film where it decays exponentially. In commercial SPR instruments, such as the Ecochemie instrument used here, a glass prism couples laser light into the metal film. At a certain angle of incidence of incident light within the metal-prism (the angle of resonance), maximum coupling of the light with the surface plasmon occurs. The angle of resonance depends on the dielectric constant of the dielectric on the prism. Hence, binding of a film on the metal surface causes a change in dielectric constant, which in turn changes the angle of resonance. This change in resonance angle can be followed in real time, thus providing kinetic information on film formation or, in this case, the attachment of bacteria.
Here, we show that SPR can be readily adapted for monitoring the attachment of live bacteria to artificial substrates in real time. Moreover, SPR can be used to examine the differences in attachment kinetics shown by bacterial pil mutants carrying aberrant numbers of type IV pili. These differences are obscured by classical endpoint techniques for assessing net biofilm formation, such as crystal violet staining. Further, the differences in the ability of the pil mutants to attach to an artificial substrate directly correlates with differences in their pathogenicity to a model insect.
2. Material and methods
2.1 Bacterial strains
The wild-type P. aeruginosa strain used was PAO-1. Two transposon mutants, both affecting pillus density, were also generated from this same strain and termed pilA (from Andrew Spiers, Plant Sciences, University of Oxford, UK) and pilT (D'Argenio et al. 2001). The pilA mutant cannot produce pili and the pilT mutant is hyperpilated and cannot retract its pili (Mattick 2002). All bacterial strains were cultured at 37 °C in 30 ml glass universal tubes with 6 ml of King's medium B (KB) with shaking for 18 h. Subsequently, 1 ml portions were centrifuged for 1 min and the supernatant removed from the pellet. The pellet was washed twice and resuspended in phosphate-buffered saline (PBS) pH 7.4. Optical density (OD600) was measured for each experiment. Bacterial concentrations were adjusted for each experiment to give a final concentration of 109 ml−1.
2.2 Light microscopy and staining
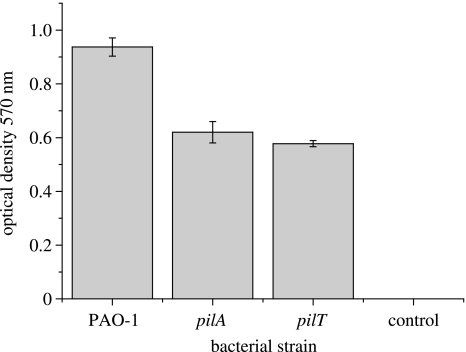
Two hundred microlitres of an overnight culture of the strains PAO1, pilA and pilT were added within a rubber O-ring on to a gold substrate disk and incubated for 1 h at room temperature. The substrate was lightly rinsed with distilled water and left to dry at room temperature. One hundred microlitres of 1% crystal violet dye was added within the O-ring and left for 5 min. The substrate was rinsed thoroughly with distilled water and left to dry at room temperature. The gold substrate was then observed under the microscope and photographed. Crystal violet staining for biofilm formation was performed in a 96 micro titre well plate (O'Toole & Kolter 1998). Briefly, 100 μl of PAO1, pilA and pilT were added to the eight wells per strain. The plate was then incubated for 30 min at room temperature, the supernatant removed, and 200 μl of 1% crystal violet dye added to each well. The plate was left for a further 10 min at room temperature and then washed thoroughly with distilled water. Then, 200 μl of an acetone (20% by volume) and ethanol (80% by volume) mixture was added to the wells to resuspend the attached bacteria. The resulting resuspension was removed and added to 800 μl of distilled water and the optical density at 570 nm recorded.
2.3 Surface plasmon resonance
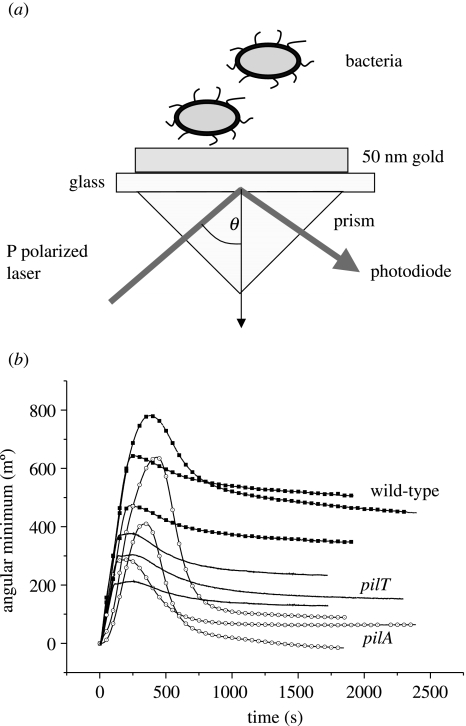
For SPR an Ecochemie E-SPR instrument was used, to facilitate continual monitoring of the angle of plasmon resonance. In this device (figure 1a), a 200 μl polypropylene cuvette is clamped on a prefabricated gold-coated glass sensor disk (Ecochemie). A semiautomatic pipette system is then used to transfer bacteria into the monitoring cuvette. For each experiment, the angle of resonance was recorded for a minimum of 600 s prior to the addition of the bacteria, and each experiment was repeated at least three times. After each run, the system was washed through with 60% ethanol, followed by PBS. For the experiment, 50 μl of bacterial culture was dispensed on to the substrate surface through channel 1, and 50 μl of PBS was dispensed though channel 2. The needles were kept in the ‘inject’ position during measurement to avoid temperature changes, and the programme was run for 30 min. Average values of angular resonance and standard errors were calculated for all the bacterial strains. The angle of resonance is proportional to the mass density of the adsorbed surface film.
Figure 1.
(a) Schematic showing the SPR setup used to measure bacterial attachment to gold. In this diagram, P polarized laser light is totally internally reflected, forming an energy wave at the surface. The angle θ of incident light which forms maximum excitation is sensitive to changes in the dielectric properties of thin films forming on the upper-side of the gold film. (b) Attachment of wild-type P. aeruginosa compared with pilA and pilT mutants, as measured by SPR. The rapid initial attachment of bacteria is reflected by the rapid increase in the angular minimum and is followed by a steady decline to an equilibrium (see text). Note that both pilA and pilT mutants show altered binding, but that the pattern of attachment over time differs between the two mutants.
2.4 Insect bioassays
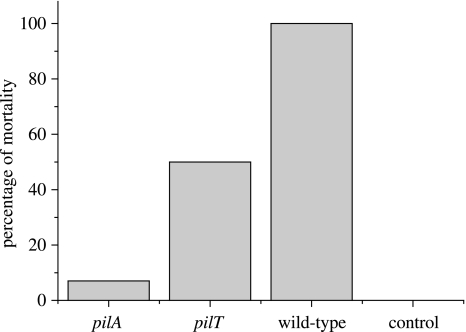
Larvae of the wax moth, Galleria mellonella (Livefood Company, UK), were used to assay the virulence of the strains used against insects (Miyata et al. 2003). Prior to injection of the bacteria the caterpillars were chilled on ice for 10 min and surfaced sterilized with ethanol. Ten microlitres of each of the three bacterial strains (102 cells) were then injected into the penultimate segment of the caterpillar abdomen. Twenty caterpillars were injected for every test strain, including a control sample containing bacterial broth alone. The caterpillars were then incubated at 37 °C for 24 h and the percentage mortality recorded.
2.5 Competition assays
To investigate whether variation in the virulence of strains was a function of their growth rate, all strains were independently competed against a methionine auxotroph of PAO-1, strain PA6049 (Meyer et al. 1996). Strains were grown overnight in KB media with aeration, as above, and 106 cells of both the test and competitor stains inoculated into KB media. Each of the three strains was competed in six independent replicates. Both starting cultures and cultures after 24 h growth (shaken at 200 r.p.m. at 37 °C with aeration), were then plated on to both KB agar and minimal media; only the test strains will form sizeable colonies on KB agar. Relative fitness (W) was calculated from the ratio of the Malthusian parameters (m) of the test and competitor strains, where m = ln(Nf/N0), and N0 and Nf are starting and final densities, respectively (Lenski et al. 1991).
3. Results and discussion
Here, we have shown that SPR can be used as a reliable and rapid assay for the attachment of the important bacterium P. aeruginosa. This physical surface analytical method complements the use of high speed video in monitoring the attachment of bacteria in real-time (Stoodley et al. 2001, 2002) as it is very sensitive to small mass changes at the surface being studied. We studied the attachment of bacteria in a closed, non-flowing system, however, most SPR machines can accommodate flow, and SPR analysis is therefore not restricted to static systems. In the present study, SPR was used to detect differences in the early attachment kinetics of mutant bacteria not previously detected by other techniques (Klausen et al. 2003). Thus Klausen et al. state that type IV pili were not necessary for P. aeruginosa initial attachment in their measurements using confocal microscopy and comstat image analysis in flow chambers (Klausen et al. 2003). In contrast, P. aeruginosa mutants, either lacking pili (pilA) or carrying an excess of non-retractable pili (pilT), both showed altered SPR-derived binding characteristics against the wild-type. These subtle differences in early stage attachment were also not detectable in endpoint assays using crystal violet stain, which showed no final difference between the mutant and wild-type strains in the apparent density of the resulting biofilms. The differences detected by SPR were, however, paralleled in the reduced virulence of both pil mutants over the wild-type following injection into the insect model G. mellonella. Thus, addition of a physical measuring technique to a field largely dominated by optical video data may show subtle differences not previously detectable by optical techniques alone.
Measurement of the attachment of the different bacterial strains by SPR gives a characteristic series of curves that all possess the same basic shape (figure 1b). First, there is an initial, and rapid (0–500 s), increase and attachment on injection of the bacterial samples into the SPR cell. From the peak of the SPR curve this subsequently falls away, suggesting that some bacteria are initially undergoing reversible adhesion. The wild-type strain PAO1 has a higher initial attachment peak at approximately 600 m° than both pil mutants, which then drops and levels off at approximately 460 m°. The pilA mutant, which lacks pili, has the next highest attachment peak (approximately 420 m°), but after the drop it levels off at a lower angular minimum of approximately 50 m°. Finally, pilT, with its non-retractable pili, peaks at approximately 300 m° and levels of at approximately 150 m°. These data show not only that pili are important in influencing initial attachment but also that their functionality is critical. Thus, it is not simply the presence of pili that is required for wild-type binding, but also the presence of retractable pili, as the pilT mutant has non-retractable pili.
After SPR analysis, the colonized gold surfaces were taken and examined by brightfield light microscopy, without further modification or fixation (figure 2). This shows visible differences in the colonization of the gold surface by wild-type versus mutant bacteria. However, it is not possible to distinguish between the pil mutants using optical microscopy. Similarly, endpoint assays of biofilm formation in PVC microtitre plates (figure 3) also showed a significant difference between wild-type and pil mutant biofilms (one-way ANOVA: F2,21=46.66, p<0.001) but again failed to distinguish between the pilA and pilT mutants themselves (two-sample t-test: p>0.5). We note that direct comparison of these visual assays with the SPR protocol is not strictly possible as the visual protocol involves additional passages through an air–water interface, which can substantially re-arrange biofilm structure (Gomez-Suarez et al. 2001). However, we do not feel that this invalidates the comparisons between the behaviour of the wild-type and mutant bacteria. Indeed the ability of SPR to monitor bacterial attachment in situ is one of the main strengths of the technique.
Figure 2.
Light microscopy of bacteria attached to the gold SPR substrate. Bacteria were visualized under brightfield microscopy after being taken directly from the SPR chamber and stained with crystal violet. Note the qualitative difference between the colonies formed by the wild-type (a) and mutant (b,c) bacteria. Scale bar 100 μm.
Figure 3.
Endpoint assay of biofilm formation by the wild-type P. aeruginosa strain (PAO-1) and the two pil mutants (pilA and pilT) using crystal violet stain. Note that the final apparent density of the biofilm is greater in the wild-type strain than in the two mutants.
Although no differences between the mutant strains could be detected in endpoint assays of biofilm formation, differences in virulence against insects were detectable. Thus, the mortality of G. melonella was significantly greater after inoculation with wild-type PAO1 compared with the pilT mutant (figure 4; Fisher's exact test: p<0.01), and the mortality caused by the pilT mutant was greater than that caused by pilA (figure 4; Fisher's exact test: p<0.01). These studies are consistent with virulence assays of pil mutants in mouse (Comolli et al. 1999) and Drosophila (D'Argenio et al. 2001) models, and also in assays of cell cytotoxicity (Comolli et al. 1999; Zolfaghar et al. 2003). As it is possible that differences in our insect virulence assay could simply be explained by differences in the intrinsic rate of bacterial replication, we also examined the relative growth rate of the strains. No difference in the relative growth rate of any of the strains was observed, when competed against a standard competitor in vitro (one-way ANOVA: p>0.2). These data, therefore, demonstrate a clear correlation between bacterial attachment and virulence that is independent of growth rate. Further, they demonstrate that type IV pili are important in bacterial attachment itself, a role not predicted from data showing their involvement in the later stages of biofilm formation.
Figure 4.
Percentage mortality of wax moth larvae injected with the wild-type (PAO-1) and mutant (pilA and pilT) strains of P. aeruginosa. Note the relative order of virulence: PAO-1>pilT>pilA (see text, §3).
In conclusion, although SPR has primarily been used to study protein–protein interactions, here we have shown that SPR can also be used to monitor the attachment of larger structures such as bacterial cells. This measurement of early bacterial adhesion using SPR will add physical parameters to studies involving the use of high speed video to monitor bacterial biofilm formation (Stoodley et al. 2001, 2002). These SPR-detectable differences in surface attachment, although subtle, are also reflected in the final virulence of these strains to insects. This shows that the ability to attach to artificial substrates, as measured by SPR, is correlated with the ability of the bacteria to colonize a living host, and suggests that SPR may be a powerful and rapid first assay of attachment and, correspondingly, virulence.
Acknowledgments
This work was funded by a Royal Society merit award to R.ff-C., a Royal Society university fellowship to A.B. and a Royal Society grant to A.T.A.J.
References
- Berger C.E.H, Beumer T.A.M, Kooyman R.P.H, Greve J. Surface plasmon resonance multisensing. Anal. Chem. 1998;70:703–706. [Google Scholar]
- Comolli J.C, Hauser A.R, Waite L, Whitchurch C.B, Mattick J.S, Engel J.N. Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect. Immun. 1999;67:3625–3630. doi: 10.1128/iai.67.7.3625-3630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argenio D.A, Gallagher L.A, Berg C.A, Manoil C. Drosophila as a model host for Pseudomonas aeruginosa infection. J. Bacteriol. 2001;183:1466–1471. doi: 10.1128/JB.183.4.1466-1471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Suarez C, Busscher H.J, van der Mei H.C. Analysis of bacterial detachment from substratum surfaces by the passage of air-liquid interfaces. Appl. Environ. Microbiol. 2001;67:2531–2537. doi: 10.1128/AEM.67.6.2531-2537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiby N. Antibiotic therapy for chronic infection of pseudomonas in the lung. Annu. Rev. Med. 1993;44:1–10. doi: 10.1146/annurev.me.44.020193.000245. [DOI] [PubMed] [Google Scholar]
- Jenkins A.T.A, ffrench-Constant R.H, Buckling A, Clarke D.J, Jarvis K. Study of the attachment of Pseudomonas aeruginosa on gold and modified gold surfaces using surface plasmon resonance. Biotechnol. Prog. 2004;20:1233–1236. doi: 10.1021/bp034367u. [DOI] [PubMed] [Google Scholar]
- Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-Jorgensen A, Molin S, Tolker-Nielsen T. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 2003;48:1511–1524. doi: 10.1046/j.1365-2958.2003.03525.x. [DOI] [PubMed] [Google Scholar]
- Lenski R.E, Rose M.R, Simpson S.C, Tadler S.C. Long-term experimental evolution in Escherichia coli. 1. Adaptation and divergence during 2000 generations. Am. Nat. 1991;138:1315–1341. [Google Scholar]
- Mattick J.S. Type IV pili and twitching motility. Annu. Rev. Microbiol. 2002;56:289–314. doi: 10.1146/annurev.micro.56.012302.160938. [DOI] [PubMed] [Google Scholar]
- Meyer J.M, Neely A, Stintzi A, Georges C, Holder I.A. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect. Immun. 1996;64:518–523. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Casey M, Frank D.W, Ausubel F.M, Drenkard E. Use of the Galleria mellonella caterpillar as a model host to study the role of the type III secretion system in Pseudomonas aeruginosa pathogenesis. Infect. Immun. 2003;71:2404–2413. doi: 10.1128/IAI.71.5.2404-2413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed N, Rainier T.R, Jr, Ross J.M. Novel experimental study of receptor-mediated bacterial adhesion under the influence of fluid shear. Biotechnol. Bioeng. 2000;68:628–636. [PubMed] [Google Scholar]
- O'Toole G.A, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- Ramsey M.M, Whiteley M. Pseudomonas aeruginosa attachment and biofilm development in dynamic environments. Mol. Microbiol. 2004;53:1075–1087. doi: 10.1111/j.1365-2958.2004.04181.x. [DOI] [PubMed] [Google Scholar]
- Singh P.K, Schaefer A.L, Parsek M.R, Moninger T.O, Welsh M.J, Greenberg E.P. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- Stoodley P, Hall-Stoodley L, Lappin-Scott H.M. Detachment, surface migration, and other dynamic behavior in bacterial biofilms revealed by digital time-lapse imaging. Methods Enzymol. 2001;337:306–319. doi: 10.1016/s0076-6879(01)37023-4. [DOI] [PubMed] [Google Scholar]
- Stoodley P, Sauer K, Davies D.G, Costerton J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- Zolfaghar I, Evans D.J, Fleiszig S.M. Twitching motility contributes to the role of pili in corneal infection caused by Pseudomonas aeruginosa. Infect. Immun. 2003;71:5389–5393. doi: 10.1128/IAI.71.9.5389-5393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]