Abstract
Kainic acid (KA)-induced status epilepticus in adult rats leads to delayed, selective death of pyramidal neurons in the hippocampal CA1 and CA3. Death is preceded by down-regulation of glutamate receptor 2 (GluR2) mRNA and protein [the subunit that limits Ca2+ permeability of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors] in CA1 and CA3, as indicated by in situ hybridization, immunolabeling, and quantitative Western blotting. GluR1 mRNA and protein are unchanged or slightly increased before cell death. These changes could lead to formation of GluR2-lacking, Ca2+-permeable AMPA receptors and increased toxicity of endogenous glutamate. GluR2 immunolabeling is unchanged in granule cells of the dentate gyrus, which are resistant to seizure-induced death. Thus, formation of Ca2+-permeable AMPA receptors may be a critical mediator of delayed neurodegeneration after status epilepticus.
Keywords: epilepsy, seizures, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, neurodegeneration, kainate
Systemic administration of kainic acid (KA) to adult rats induces a syndrome of neuronal hyperexcitation and sustained seizure activity termed status epilepticus, which leads to delayed, selective death of distinct neuronal populations, including hippocampal pyramidal neurons. KA-induced status epilepticus is widely regarded as an experimental model of temporal lobe epilepsy, as it reproduces the pattern of neurodegeneration seen in patients (1–3). Whereas pyramidal neurons of the hippocampal CA1 and CA3 are particularly vulnerable, GABAergic interneurons in CA1 and CA3 and granule cells of the dentate gyrus (DG) are highly resistant to seizure-induced death (1, 3). Although KA activates α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and can be toxic when applied to neurons, the degeneration after KA-induced status epilepticus appears to result from seizures rather than a direct action of kainate on the vulnerable neurons. There is still disagreement about the mechanisms whereby KA induces seizures (4–6).
Considerable evidence implicates a role for down-regulation of the AMPA receptor subunit glutamate receptor 2 (GluR2) in the neurodegeneration associated with severe limbic seizures (7–9). AMPA receptors containing GluR2 are relatively Ca2+ impermeable (10), and down-regulation of this subunit may lead to formation of Ca2+-permeable receptors and influx of toxic amounts of Ca2+ in response to endogenous glutamate. Kainate-induced status epilepticus is followed by down-regulation of GluR2 mRNA in CA3 pyramidal neurons before the onset of neuronal death, as indicated by in situ hybridization (11–13). In contrast, status epilepticus increases GluR2 mRNA expression in DG.
The present study was undertaken to examine seizure-induced changes in AMPA receptor subunit expression. Status epilepticus caused down-regulation of GluR2 mRNA (but not of GluR1 mRNA) in hippocampal CA1 and CA3, as indicated by in situ hybridization, at times preceding significant neuronal loss. About 8 h later but still before cell death, GluR2 protein was down-regulated in CA3, as indicated by immunocytochemistry and quantitative Western analysis. Double immunolabeling revealed a marked reduction in GluR2 protein, with no change in GluR1 protein, in individual pyramidal neurons of CA1 and CA3 after seizures. Seizures had little effect on levels of GluR2 and GluR1 proteins in granule cells of the DG, which are resistant to degeneration. These results support a role for Ca2+-permeable AMPA receptors as critical mediators of the delayed neurodegeneration after KA-induced status epilepticus.
Materials and Methods
KA-Induced Status Epilepticus.
Animals were maintained in a temperature- and light-controlled environment in accord with the principles and procedures of the National Institutes of Health Guidelines for Care and Use of Laboratory Animals. Seizures were induced in adult, male Sprague–Dawley rats (260–300 g) by injection of KA (15 mg/kg, i.p.). Animals were monitored behaviorally for seizures for 6 h after injection. Only rats displaying status epilepticus, defined as continual seizures for at least 1 h, were used in this study.
Histological Analysis.
Neuronal damage was assessed by examination of brain sections at the level of the hippocampus of animals sacrificed at 1 week after injection of KA (n = 7) or saline (n = 7). Additional animals were examined at 16, 20, or 24 h after KA (n = 5, 3, and 4 per time point, respectively). Animals were transcardially perfused with 4% paraformaldehyde in 10 mM PBS; brains were removed and immersed in fixative (4°C, overnight). Coronal sections (30 μm; 3.3–4.0 mm from bregma; ref. 14) were cut and stained with toluidine blue.
In Situ Hybridization.
GluR mRNA expression was assessed by in situ hybridization as described (9, 15). Animals were sacrificed at 6, 12, 16, 20, and 24 h after injection of KA (n = 3 per time point) or 24 h after injection of saline (n = 4). Brains were rapidly removed, frozen, sectioned (18 μm) with a cryostat, and labeled as described. In brief, frozen brain sections from experimental and control animals were hybridized (overnight, 49°C) with 35S-UTP-labeled GluR1 or GluR2 RNA probes (106 cpm/section, 1 ng/μl). After hybridization, sections were treated with RNase A (20 μg/ml), dehydrated in ethanol, and apposed to Kodak XAR-5 film (24–72 h).
For quantitation of mRNA levels, autoradiograms were analyzed with a Molecular Dynamics 300A Computing Densitometer using National Institutes of Health image 1.61 software. OD values (background subtracted) were determined for the CA1 and CA3 pyramidal cell layer and DG granule cell layer. Values from two sections per animal were averaged. Final OD values were expressed as grand means (± SEM) of individual means from each group and statistically analyzed by the Student's unpaired t test. Percent change in OD values was expressed relative to values of corresponding regions of control brains cut in the same experimental session, incubated with the same probe solution, and apposed to the same film.
Immunocytochemistry, Double Labeling of GluR1 and GluR2.
GluR1 and GluR2 protein expression was assessed in individual neurons by double immunolabeling of brain sections at the level of the dorsal hippocampus (3.3–4.0 mm from bregma, ref. 14). Animals were deeply anesthetized with pentobarbital (50 mg/kg, i.p.) and perfused transcardially with ice-cold 4% paraformaldehyde in PBS at 22 h after injection of KA (n = 4) or saline (n = 3). Brains were removed, cut into 5-mm blocks, postfixed for 6 h, and sectioned at 50 μm by vibratome. Tissue sections were processed for immunohistochemistry with subunit-specific antibodies as follows: GluR1, rabbit polyclonal antibody directed to a sequence in the C-terminal domain of the GluR1 subunit (16, 17) (Upstate Biotechnology, Lake Placid, NY); GluR2, mouse mAb (6C4) raised against residues 175–430 in the N-terminal domain of the GluR2 subunit (18) (gift of John H. Morrison, Mount Sinai, School of Medicine, New York). 6C4 is highly specific for GluR2 and does not crossreact with any other AMPA receptor subunit by Western blot analysis.
Sections were incubated with GluR1 and GluR2 primary antibodies at a concentration of 10 and 2 μg/ml, respectively, for 48 h at 4°C, washed, and incubated with secondary antibodies (GluR1: biotinylated goat anti-rabbit IgG; GluR2: Texas Red-conjugated horse anti-mouse IgG; Vector Laboratories) followed by fluorescein avidin. Sections then were mounted with Vectashield to reduce fluorescent quenching, and cover-slipped. Images were collected with a Bio-Rad MRC 600 Kr/Ar laser scanning confocal microscope. To assess specificity of GluR1 and GluR2 antibodies, separate control sections were processed with nonimmune rabbit and mouse IgG in place of the primary antibodies. Additional control sections were processed as above, but with the omission of either primary or secondary antibody. These control sections showed no labeling.
Quantitative Immunocytochemistry of GluR2.
Sections were labeled for GluR2 17–17.7 h after KA-induced status epilepticus (n = 7) or saline injection (n = 8) using the mouse GluR2 mAb as above in conjunction with biotinylated horse anti-mouse IgG and fluorescein avidin. GluR2 immunolabeling was assessed quantitatively with a computerized densitometer using the National Institutes of Health image 1.61 software (19). Each field was scanned once to avoid signal quenching. Each section was scanned at a z-level yielding the maximum signal intensity. Density was taken from the center of the cell body layer of the bend of CA3a and from CA1, CA3c, and the upper blade of stratum granulosum of DG about 2 mm from midline. To enable comparisons between experimental and control sections, scanning parameters including scan head pin hole aperture, gain, laser intensity, brightness/contrast settings, and image size were kept constant throughout the analysis. The fluorescence intensity range was 16 bits. Two sections were analyzed per animal (eight values/subfield per animal). Analyses were performed blind with respect to treatment, and pixel intensities were averaged for each of the hippocampal subfields for each animal.
Western Blot Analysis.
For quantitation of protein expression, Western blot analysis was performed on hippocampi of experimental and control rats as described (9). Rats were anesthetized with chloroform and decapitated 6, 16, 20, and 24 h after i.p. injection of KA (n = 3 per time point) or 24 h after i.p. injection of saline (n = 4). Slices of dorsal hippocampus (1 mm) were cut by using a Mcllwain tissue chopper. CA1, CA3a-b, and DG-CA3c subfields were rapidly micro-dissected (see Fig. 6A Inset) and stored at −70°C until use. Tissue was sonicated in the presence of PMSF (1 mM, Sigma) and incubated in Laemmli sample buffer overnight. Protein samples (10 g) were separated by gel electrophoresis (10% polyacrylamide mini-gels; Bio-Rad) and transferred to nitrocellulose for immunolabeling with the GluR1 and GluR2 antibodies described above. Membranes were blocked (25 mM Tris⋅HCl buffer, pH 8.0, 125 mM NaCl, 0.1% Tween 20, and 4% skim milk for 30 min) before incubation with primary (GluR2: 0.5 μg/ml; GluR1: 1.5 μg/ml) and secondary (1:1,000) antibodies. Enhanced chemiluminescence reagents were applied to the blots (ECL, Amersham Pharmacia), which then were apposed to XAR-5 x-ray film (Kodak).
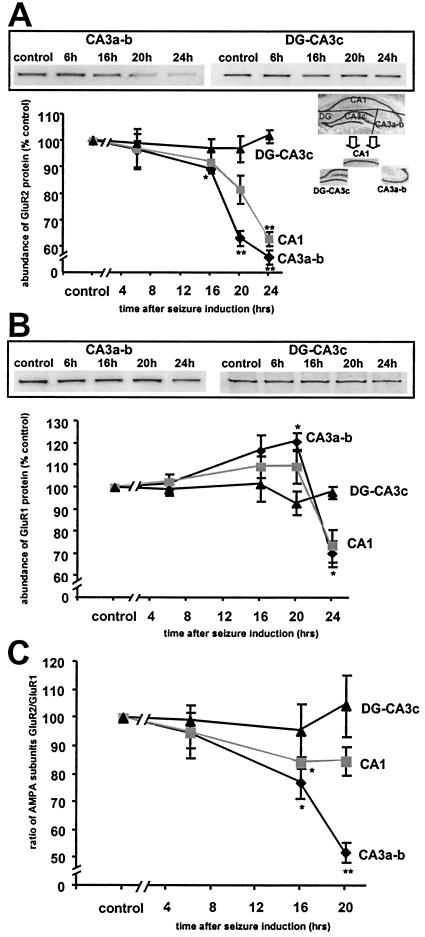
Figure 6.
Status epilepticus decreased GluR2 but not GluR1 protein levels in CA1 and CA3. Quantitative Western blot analysis of GluR1 and GluR2 protein expression in microdissected hippocampal subfields (see Inset in A) after status epilepticus. (A) GluR2 protein levels decreased in CA3a-b at 16 h after KA injection, and further declined at 20 and 24 h. Similar but smaller changes were seen in CA1, but were not significant until 24 h. No changes in the level of GluR2 protein were observed in DG-CA3c. (B) GluR1 protein levels increased slightly in CA1 and CA3a-b at 16 and 20 h after KA injection (significant only for CA3a-b at 20 h), but exhibited a reduction (presumably because of onset of neuronal damage) at 24 h. No changes in GluR1 protein occurred in DG-CA3c. (C) The ratio of levels of GluR2 and GluR1 proteins decreased significantly in hippocampal CA1 and CA3a-b at 16 h after KA injection and in CA3a-b at 20 h. The plotted ratios are the means of the ratios for individual animals. Error bars represent SEM. **, P < 0.001; *, P < 0.05; Student's unpaired t test.
Films from Western blots were analyzed with a Scan Jet 4-C computing densitometer using National Institutes of Health image 1.61 image-analysis software. ODs were background-subtracted and analyzed (two samples per animal). Final ODs were expressed as grand means (± SEMs) of individual means. OD values for KA rats were normalized to OD values for corresponding brain regions of controls run on the same gels.
Results
Behavioral Manifestations of Status Epilepticus.
Kainate (15 mg/kg, i.p.) induced status epilepticus in 67% of injected rats, with 7% mortality. Rats exhibited generalized seizures including repetitive rearing, jumping, and loss of postural control. Seizures were accompanied by copious salivation and foaming at the mouth.
Status Epilepticus Induces Neurodegeneration in Adult Rats.
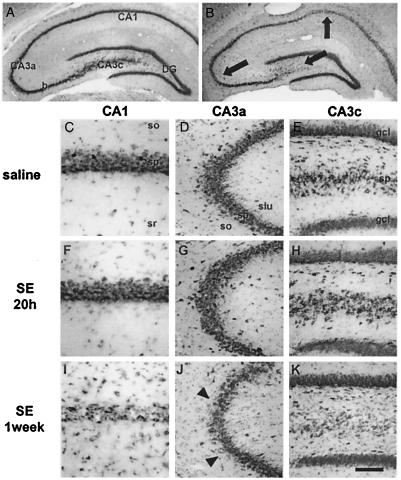
Toluidine blue-stained sections at the level of the hippocampus revealed no detectable loss of pyramidal neurons at 16 h (data not shown) or 20 h (Fig. 1 F–H) after status epilepticus in the CA1 or CA3 pyramidal cell layers. At 24 h after status epilepticus, three of four animals showed a small degree of cell loss in CA3, primarily in CA3a, and two of four showed some degeneration in CA1. At 1 week, loss of pyramidal neurons was moderate to severe in CA1 (Fig. 1I) and CA3a (Fig. 1J), and virtually complete in CA3c (Fig. 1K). Pyknotic neurons within the cell body layer of CA1 were prominent, and there was accumulated cellular debris. Neurodegeneration was accompanied by marked gliosis. There was no histologically detectable degeneration in the granule cell layer of the DG (Fig. 1K).
Figure 1.
Status epilepticus (SE) induces delayed neurodegeneration of CA1 and CA3 pyramidal cells. Histological analysis of toluidine blue-stained brain sections at the level of the dorsal hippocampus. Control brain from an animal sacrificed 7 days after saline injection (A and C–E). There is no detectable neuronal damage 20 h after KA administration (F–H), but at 1 week, loss of CA1 and CA3 pyramidal neurons is prominent (B and I–K). Arrows in B indicate location of higher magnification views in C–K. Arrowheads in J indicate a region of extensive necrosis. Many neurons in I show pyknotic nuclei. (Scale bar: 3,690 μm in A and B; 150 μm in D, E, G, H, J, and K; 75 μm in C, F, and I.)
Status Epilepticus Induces Down-Regulation of GluR2 (But Not GluR1) mRNA in CA1 and CA3.
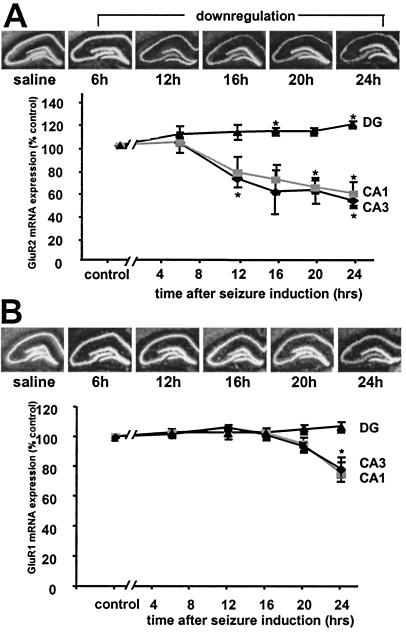
To examine expression of mRNA of AMPA receptor subunits in hippocampus after status epilepticus, in situ hybridization was carried out on brain sections of saline- and kainate-injected adult rats. Changes in expression were assessed quantitatively by computerized image analysis of autoradiographic film. In control rats, intense labeling of GluR1 and GluR2 mRNA was observed throughout the pyramidal cell layer of CA1 and CA3 and in the granule cell layer of the DG as described (9, 11, 15). Status epilepticus induced a decrease in GluR2 mRNA expression as early as 12–16 h in CA1 and CA3 (P < 0.05 only for CA3 at 12 h). Decreases were greater at 20 h (CA1: to 60.5 ± 5.7% of control, P = 0.01; CA3: to 63.7 ± 11.1% of control, P = 0.07; n = 3 for all time points) and 24 h (CA1: to 51.9 ± 10.7%, P = 0.05; CA3: to 57.7 ± 5.6%, P = 0.03) after KA injection. In contrast, GluR2 mRNA expression slightly increased in the granule cell layer of the DG after status epilepticus (at 16 h: 111.9 ± 1.6%, n = 3, P = 0.05; 20 h: 112.0 ± 0.35%, n = 3, P = 0.07; 24 h: 118.3 ± 0.15%, n = 3, P = 0.03). Expression of GluR1 mRNA was not significantly changed in CA1, CA3, or DG through 20 h after status epilepticus (Fig. 2B), and there was a small decrease in CA1 (to 75.9 ± 11.3%, n = 3, P = 0.16) and CA3 (to 78.8 ± 4.6%, n = 3, P = 0.03) at 24 h.
Figure 2.
Status epilepticus induced down-regulation of GluR2 (but not GluR1) mRNA. (A) (Upper) Film autoradiograms of GluR2 mRNA expression in a saline-injected control and at 6, 12, 16, 20, and 24 h after KA-induced status epilepticus. Down-regulation of GluR2 mRNA expression occurred 12 h after KA in CA1 and CA3, but remained stable in DG. (Lower) Mean densities of GluR2 mRNA decreased in hippocampal CA1 and CA3 and increased slightly in DG. (B) (Upper) Film autoradiograms of GluR1 mRNA expression at 6, 12, 16, 20, and 24 h after KA-induced status epilepticus and in a saline control. GluR1 mRNA expression remained stable up to 24 h, at which time there was a modest decrease in CA1 and CA3. (Lower) Mean densities of GluR1 mRNA decreased at 24 h in CA1 and CA3 and remained stable in DG. Values are expressed as a percentage of saline-injected controls. Error bars represent the SEM. *, P ≤ 0.05 using the Student's unpaired t test.
Status Epilepticus Reduces GluR2 (But Not GluR1) Immunolabeling in CA1 and CA3 Pyramidal Neurons.
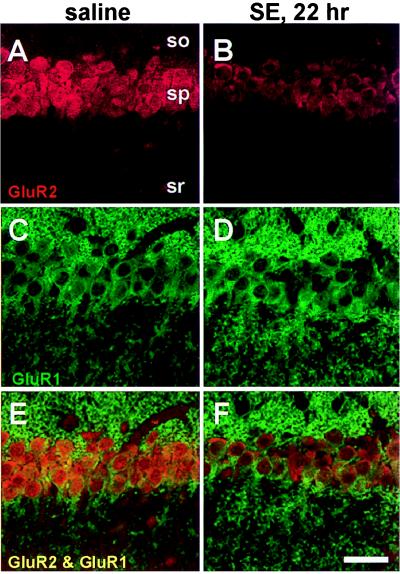
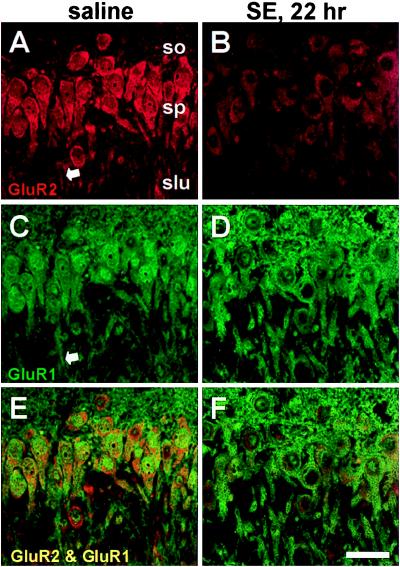
To examine patterns of AMPA receptor protein expression after status epilepticus, immunolabeling was performed with subunit-specific antibodies. In the stratum pyramidale of CA1 and CA3a, the somata (including the cytoplasm and often the nuclei) of pyramidal neurons exhibited intense GluR1 immunolabeling in control animals and at 22–23 h after status epilepticus (Figs. 3 C and D and 4 C and D, data typical of four animals). The stratum oriens was diffusely stained for GluR1 in control sections, presumably representing immunolabeling of basilar dendrites (Fig. 3C). GluR2 staining was also intense in pyramidal neurons of control animals, but after status epilepticus, GluR2 immunolabeling decreased (Figs. 3 A and B and 4 A and B). Within stratum lucidum of CA3a, apical dendrites displayed diffuse cytoplasmic labeling for both GluR1 and GluR2 (arrows, Fig. 4 A and C). In separate experiments, quantitative assessment of GluR2 single labeling ≈17 h after status epilepticus indicated decreased staining to 65.4 ± 8.7% of control values in CA3a (P = 0.04, n = 7 for all measurements), to 61.9 ± 11.9% in CA3c (P = 0.04), and to 66.3 ± 13.2% in CA1 (P = 0.06).
Figure 3.
Status epilepticus (SE) is followed by down-regulation of GluR2 but not GluR1 immunolabeling in CA1 pyramidal neurons. GluR1 and GluR2 immunoreactivity in sections from a saline-injected control (A and C) and an animal 22 h after KA injection (B and D). GluR2 immunoreactivity decreased in pyramidal cells after KA administration (B), although GluR1 immunoreactivity was unchanged (D). (E and F) Superposition of images of GluR1 and GluR2 labeling show colocalization. (Scale bar, 50 μm.) so, stratum oriens; sp, stratum pyramidale; sr, stratum radiatum.
Figure 4.
Status epilepticus (SE) is followed by down-regulation of GluR2 but not GluR1 immunolabeling in CA3a pyramidal neurons. Presentation as in Fig. 3. GluR1 and GluR2 immunoreactivity in sections from a saline-injected control (A and C) and an animal 22 h after KA injection (B and D). GluR2 immunoreactivity decreased in pyramidal cells after KA administration (B), although GluR1 immunoreactivity was unchanged (D). (E and F) Superposition of images of GluR1 and GluR2 labeling show colocalization. (Scale bar, 50 μm.) so, stratum oriens; sp, stratum pyramidale; slu, stratum lucidum.
In DG, granule cell somata and proximal dendrites were intensely labeled by the GluR1 and GluR2 antibodies (Fig. 5 A and C). No changes in immunolabeling were observed 22–23 h after status epilepticus (Fig. 5 B and D). The inner molecular layer showed less intense, essentially uniform immunoreactivity for the GluR1 antibodies. Similar patterns of cell-specific GluR1 (20, 21) and GluR2 (18) immunolabeling are described in normal rat and monkey brain.
Figure 5.
Status epilepticus (SE) does not alter GluR2 immunolabeling in DG granule cells. Same presentation as in Figs. 3 and 4. GluR2 immunoreactivity was unchanged 22 h after KA administration (B) when compared with control sections (A). GluR1 immunoreactivity also was unchanged after status epilepticus (C and D). (E and F) Superposition of images of GluR1 and GluR2 labeling show colocalization. iml, inner molecular layer; gcl, granule cell layer; h, hilus. (Scale bar: 50 μm.)
Status Epilepticus Induces Down-Regulation of GluR2 (But Not GluR1) Protein Assessed by Western Blotting in Hippocampal CA1 and CA3.
Seizure-induced changes in GluR1 and GluR2 protein levels were examined by quantitative Western blot analysis of microdissected hippocampal subfields (Fig. 6A Inset). Representative blots are shown in Fig. 6 A and B. In CA3a-b, GluR2 protein expression was unchanged at 6 h, but was reduced at 16 h after KA injection (to 89.5% ± 1.3% of control, P = 0.02, n = 3), and further reduced at 20 h (to 63.3 ± 2.8%, P = 0.0003, n = 3; Fig. 6A), and at 24 h (to 56.2% ± 2.6% of control, P = 0.001, n = 3). In CA1, GluR2 protein expression was changed little at 16 h, reduced but not significantly so at 20 h (to 81.7% ± 5.4%, P = 0.07, n = 3; Fig. 6A), and significantly reduced at 24 h (to 63.0% ± 2.8%, P = 0.001, n = 3).
In CA3a-b and CA1 the level of GluR1 protein was slightly enhanced at 16 and 20 h after KA injection, but the change was significant only for CA3a-b at 20 h (120.9% ± 3.9% of control, P = 0.02, n = 3 for all points, Fig. 6B). At 24 h after KA injection GluR1 protein was decreased in both CA3a-b and CA1 (to 70.3% ± 5.8%, P = 0.03, n = 3, and to 73.8% ± 7.3%, P = 0.06, n = 3, respectively).
Neither GluR2 nor GluR1 protein expression was significantly altered in DG-CA3c at any time examined (Fig. 6 A and B).
The specificity of the reduction in GluR2 at earlier times in CA3 indicates that the effect is not simply caused by cell death and is consistent with a role for this down-regulation in the delayed neurodegeneration that is to follow. The decrease in protein expression of GluR2 and GluR1 in CA3a-b and CA1 at the 24-h time point is ascribable to a decline in the viability of pyramidal cells at this late time point.
The GluR2/GluR1 Ratio Decreases in Hippocampal Pyramidal Cells After Status Epilepticus.
The ratio of levels of GluR2 protein to GluR1 protein may predict Ca2+ permeability of AMPA receptors (22). The mean value of this ratio was calculated from the values for individual animals (Fig. 6C). In CA3a-b, the GluR2/GluR1 ratio decreased significantly 16 h after KA injection (to 77.0 ± 5.3% of control, P = 0.03, n = 3) and at 20 h reached 52.4 ± 3.6% of control (P = 0.0006, n = 3). In CA1, the change in the GluR2/GluR1 ratio was more modest at 16 h (to 84.11 ± 2.0% of control, P = 0.006, n = 3) and was not significant at 20 h (85.05 ± 4.9% of control, P = 0.15, n = 3). At 24 h, the ratio returned to near control values for both CA3a-b and CA1, which is caused by the drop in GluR1 level, ascribable to deterioration of the cells. These findings suggest increased formation of Ca2+-permeable AMPA receptors in pyramidal neurons after status epilepticus but before cell death.
Discussion
This work examined expression of GluR2 mRNA and protein in the hippocampus preceding histologically detectable cell death caused by KA-induced status epilepticus. We found that GluR2 mRNA expression was reduced in CA1 and CA3 pyramidal cells, which show degeneration, and increased in DG neurons, which are resistant. Immunolabeling for GluR2 was reduced in CA1 and CA3 but not DG, whereas GluR1 labeling was unchanged in all hippocampal subfields. The change in GluR2 protein also was measured by Western analysis of microdissected tissue and occurred in CA3a-b with a time lag of about 8 h relative to the mRNA change. Similar although smaller changes in GluR2 protein occurred in CA1. These changes did not reach significance by Western analysis or quantitative immunolabeling at ≈17 h but were obvious in sections taken 22 h after seizures and labeled for both GluR1 and GluR2 (Fig. 3). Western analysis showed a small increase or no change in levels of GluR1 in CA1 and CA3a-b after status epilepticus but before cell death. Status epilepticus did not alter GluR1 or GluR2 in DG by immunolabeling or in DG-CA3c by Western analysis.
Although signs of cellular damage can be observed in the hippocampus as early as 3–5 h after KA injection by electron microscopy (2), neuronal death was minor in Nissl-stained sections at 24 h. These observations and the maintained expression of GluR1 protein levels demonstrate the specificity of the down-regulation of GluR2, and the timing is consistent with a role in triggering cell death. Because AMPA receptors lacking the GluR2 subunit are relatively Ca2+ permeable, the decrease in GluR2 expression could lead to increased formation of Ca2+-permeable AMPA receptors and increased Ca2+ influx in response to endogenous glutamate. This abnormal Ca2+ influx could contribute to the delayed cell death that occurs after KA-induced status epilepticus.
The importance of GluR2 down-regulation in delayed neurodegeneration after transient forebrain ischemia is indicated by data similar but more extensive than those for the delayed neurodegeneration after status epilepticus (the “GluR2 hypothesis” of delayed neurodegeneration) (7, 8). GluR2 mRNA and protein are decreased in the vulnerable CA1 but maintained in the resistant CA3, whereas GluR1 mRNA and protein are maintained in both regions as well as in DG. Furthermore, increased influx of Ca2+ in response to AMPA application is observed in postischemic CA1 neurons before neurodegeneration (22). Intracerebroventricular injection of specific GluR2 antisense oligonucleotides causes degeneration in both CA1 and CA3, suggesting that the GluR2 down-regulation occurring after forebrain ischemia or status epilepticus is sufficient to cause neurodegeneration (9).
An important issue with respect to the role of GluR2 down-regulation in delayed neurodegeneration is the time for removal of existing GluR2-containing receptors and insertion of new GluR2-lacking receptors. A half-life of GluR2/3 of ≈18 h has been measured in cultured cerebellar neurons (23). Removal of GluR2 from the surface occurs within minutes when interaction with NSF, an N-ethylmaleimide-sensitive fusion protein necessary for exocytosis, is blocked (24, 25). Further study of trafficking of AMPA receptor subunits is required, but present data are not inconsistent with rapid insertion of newly formed Ca2+-permeable receptors.
Influx of Zn2+ during transient forebrain ischemia is implicated in initiation of the cascade of changes leading to delayed neurodegeneration after this neurological insult (26). Zn2+ chelation by intracerebroventricular injection of CaEDTA just before induction of ischemia prevents the subsequent neurodegeneration and also the down-regulation of GluR2 (A. Calderone, K. Oguro, M. V. L. B. and R. S. Z., unpublished work). Although GluR2-lacking AMPA receptors are permeable to Zn2+ as well as Ca2+ (27), it has not been determined whether there is a late protective action of Zn2+ chelation after formation of GluR2-lacking receptors. Zn2+ chelation during status epilepticus protects against the subsequent neurodegeneration (28), which suggests that the mechanism of initiating down-regulation of GluR2 in CA1 and CA3 after status epilepticus is similar to that after global ischemia.
Some previous reports indicate KA-induced hippocampal damage and prior down-regulation of GluR2 mRNA are restricted to CA3 pyramidal cells (ref. 11, using a probe reacting with all splice variants; ref. 12, with a probe specific for the flip splice variant). Reduction in GluR2 protein restricted to CA3 also has been reported (29). However, Lason et al. (13) report decreased GluR2 flip expression in both CA1 and CA3 at 24 h after KA-induced status epilepticus with little change in GluR2 flop. In addition, Ong et al. (30) observed loss of CA1 and/or CA3 pyramidal neurons after KA treatment (i.v.). At 18 h, which was before cell loss, there was decrease in immunolabeling with a GluR1 antibody and an antibody reacting to both GluR2 and GluR3; there were no changes in immunolabeling of DG, and the granule cells were resistant to degeneration (30). Differences in experimental technique and timing of observations may explain these differences.
In conclusion, we have extended the evidence for GluR2 down-regulation in hippocampal neurons vulnerable to degeneration after status epilepticus induced by KA injection. GluR2 protein as well as mRNA is decreased before cell death as indicated by immunocytochemistry and Western blotting and in situ hybridization, respectively. Decrease in GluR2 protein is expected to lead to assembly of Ca2+-permeable AMPA receptors lacking GluR2. The newly formed receptors could lead to cell death by permitting excessive Ca2+ and possibly Zn2+ influx in response to endogenous glutamate (the GluR2 hypothesis, refs. 7 and 8). These and other data indicate that formation of Ca2+-permeable AMPA receptors is an important component of the cascade of events leading to delayed neurodegeneration after seizures.
Acknowledgments
We are indebted to Drs. Agata Calderone and Teresa Jover for helpful discussions. This work was supported by a Merck/UNCF postdoctoral fellowship (to S.Y.G.) and National Institute of Health Grants NS 20752 and NS 31282 (to R.S.Z.). M.V.L.B. is the Sylvia and Robert S. Olnick Professor of Neuroscience.
Abbreviations
- KA
kainic acid
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- GluR
glutamate receptor
- DG
dentate gyrus
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050586497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050586497
References
- 1.Nadler J V. Life Sci. 1981;29:2031–2042. doi: 10.1016/0024-3205(81)90659-7. [DOI] [PubMed] [Google Scholar]
- 2.Schwob J E, Fuller T, Price J L, Olney J W. Neuroscience. 1980;5:991–1014. doi: 10.1016/0306-4522(80)90181-5. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Ari Y. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 4.Frerking M, Malenka R C, Nicoll R A. Nat Neurosci. 1998;1:479–486. doi: 10.1038/2194. [DOI] [PubMed] [Google Scholar]
- 5.Cossart R, Esclapez M, Hirsch J C, Bernard C, Ben-Ari Y. Nat Neurosci. 1998;1:470–478. doi: 10.1038/2185. [DOI] [PubMed] [Google Scholar]
- 6.Lerma J. FEBS Lett. 1998;430:100–104. doi: 10.1016/s0014-5793(98)00462-1. [DOI] [PubMed] [Google Scholar]
- 7.Bennett M V L, Pellegrini-Giampietro D E, Gorter J A, Aronica E, Connor J A, Zukin R S. Cold Spring Harbor Symp Quan Biol. 1996;61:373–384. [PubMed] [Google Scholar]
- 8.Pellegrini-Giampietro D E, Gorter J A, Bennett M V L, Zukin R S. Trends Neurosci. 1997;20:464–470. doi: 10.1016/s0166-2236(97)01100-4. [DOI] [PubMed] [Google Scholar]
- 9.Oguro K, Oguro N, Kojima T, Grooms S Y, Calderone A, Zheng X, Bennett M V L, Zukin R S. J Neurosci. 1999;19:9218–9227. doi: 10.1523/JNEUROSCI.19-21-09218.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Washburn M S, Numberger M, Zhang S, Dingledine R. J Neurosci. 1997;17:9393–9406. doi: 10.1523/JNEUROSCI.17-24-09393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman L K, Pellegrini-Giampietro D E, Sperber E F, Bennett M V L, Moshe S L, Zukin R S. J Neurosci. 1994;14:2697–2707. doi: 10.1523/JNEUROSCI.14-05-02697.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollard H, Heron A, Moreau J, Ben-Ari Y, Khrestchatisky M. Neuroscience. 1993;57:545–554. doi: 10.1016/0306-4522(93)90004-y. [DOI] [PubMed] [Google Scholar]
- 13.Lason W, Turchan J, Przewlocka B, Labuz D, Mika J, Przewlocki R. J Neural Transm. 1997;104:125–133. doi: 10.1007/BF01273175. [DOI] [PubMed] [Google Scholar]
- 14.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic; 1991. [DOI] [PubMed] [Google Scholar]
- 15.Pellegrini-Giampietro D E, Bennett M V L, Zukin R S. Proc Natl Acad Sci USA. 1991;88:4157–4161. doi: 10.1073/pnas.88.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin L J, Blackstone C D, Huganir R L, Price D L. J Neurosci. 1993;13:782–792. doi: 10.1523/JNEUROSCI.13-02-00782.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin L J, Blackstone C D, Levey A I, Huganir R L, Price D L. Neuroscience. 1993;53:327–358. doi: 10.1016/0306-4522(93)90199-p. [DOI] [PubMed] [Google Scholar]
- 18.Vissavajjhala P, Janssen W G, Hu Y, Gazzaley A H, Moran T, Hof P R, Morrison J H. Exp Neurol. 1996;142:296–312. doi: 10.1006/exnr.1996.0199. [DOI] [PubMed] [Google Scholar]
- 19.Gazzaley A H, Weiland N G, McEwen B S, Morrison J H. J Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers S W, Hughes T E, Hollmann M, Gasic G P, Deneris E S, Heinemann S. J Neurosci. 1991;11:2713–2724. doi: 10.1523/JNEUROSCI.11-09-02713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackstone C D, Moss S J, Martin L J, Levey A I, Price D L, Huganir R L. J Neurochem. 1992;58:1118–1126. doi: 10.1111/j.1471-4159.1992.tb09370.x. [DOI] [PubMed] [Google Scholar]
- 22.Gorter J A, Petrozzino J J, Aronica E M, Rosenbaum D M, Opitz T, Bennett M V L, Connor J A, Zukin R S. J Neurosci. 1997;17:6179–6188. doi: 10.1523/JNEUROSCI.17-16-06179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huh K H, Wenthold R J. J Biol Chem. 1999;274:151–157. doi: 10.1074/jbc.274.1.151. [DOI] [PubMed] [Google Scholar]
- 24.Song I, Kamboj S, Xia J, Dong H, Liao D, Huganir R L. Neuron. 1998;21:393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- 25.Noel J, Ralph G S, Pickard L, Williams J, Molnar E, Uney J B, Collingridge G L, Henley J M. Neuron. 1999;23:365–376. doi: 10.1016/s0896-6273(00)80786-2. [DOI] [PubMed] [Google Scholar]
- 26.Koh J Y, Suh S W, Gwag B J, He Y Y, Hsu C Y, Choi D W. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 27.Yin H Z, Ha D H, Carriedo S G, Weiss J H. Brain Res. 1998;781:45–55. doi: 10.1016/s0006-8993(97)01208-0. [DOI] [PubMed] [Google Scholar]
- 28.Choi D W, Koh J Y. Annu Rev Neurosci. 1998;21:347–375. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- 29.Friedman L K. Hippocampus. 1998;8:511–525. doi: 10.1002/(SICI)1098-1063(1998)8:5<511::AID-HIPO9>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 30.Ong W Y, Leong S K, Garey L J, Reynolds R, Liang A W. Exp Brain Res. 1996;109:251–267. doi: 10.1007/BF00231785. [DOI] [PubMed] [Google Scholar]