Abstract
Stanniocalcin (STC) is a glycoprotein hormone originally found in bony fish, in which it regulates calcium/phosphate homeostasis and protects against hypercalcemia. The recently characterized human STC shows about 70% homology with fish STC. We previously reported a constitutive expression of STC in terminally differentiated neurons. Here, we show that exposure of human neural-crest-derived cell line Paju to hypercalcemic culture medium induced expression of STC. Treatment of Paju cells with recombinant human STC increased their uptake of inorganic phosphate. Paju cells expressing STC by cDNA transfection displayed increased resistance to ischemic challenge and to elevated intracellular free calcium induced by treatment with thapsigargin. An up-regulated and redistributed expression of STC was observed in neurons surrounding the core of acute infarcts in human and rat brains. Given that mobilization and influx of calcium is considered a main neurotoxic mechanism following ischemia, our results suggest that the altered expression of STC contributes to the protection of cerebral neurons against hypoxic/ischemic damage. Manipulation of the STC expression may therefore offer a therapeutic approach to limit the injury after ischemic brain insults.
Fish stanniocalcin (STC) is synthesized in a specialized organ adjacent to the kidney called the corpuscles of Stannius (1). An elevated level of calcium in plasma is a major stimulus for the secretion of STC (2, 3). It regulates calcium and phosphate homeostasis by acting on the gills to lower the calcium uptake (4, 5), on the kidney to increase phosphate reabsorption (6), and on the gut to inhibit intestinal calcium transport (7).
The cDNAs for human and mouse STC were recently cloned (8, 9). Human STC shares 60% identity and 80% similarity with fish STC. Infusion of recombinant human STC into rats reduced the renal excretion of phosphate (10). Addition of STC to the serosal surface of rat or pig duodenal mucosa reduced the net absorption of calcium and increased the uptake of phos-phate (11).
We previously investigated gene expression during neural differentiation by using a human neural-crest-derived cell line, Paju, as an in vitro model. Induced terminal differentiation of Paju cells was found to strongly up-regulate the expression of STC. We also found an expression of STC restricted to mature neurons in human and mouse brain (12).
Cerebral neurons are highly vulnerable to tissue ischemia. Mobilization and influx of calcium has long been considered a major mechanism of ischemic cell death (13–16). Our histochemical stainings revealed the most prominent STC expression in the pyramidal cells of the cerebral cortex and hippocampus and in the Purkinje cells of the cerebellum (12), i.e., brain neurons known to be highly sensitive to ischemia (13). Given this, we hypothesized that STC may be involved in the protection against hypoxic damage.
Here, we show that treatment of cultivated neural cells with recombinant STC stimulated their uptake of phosphate. Expression of STC by transfection of STC cDNA conferred increased resistance to hypoxic stress and to mobilization of intracellular calcium induced by treatment with thapsigargin. An up-regulated and intracellular redistribution of STC expression was seen in human and rat brain neurons in the “penumbra” of infarcted areas. Taken together, these findings suggest that STC plays an important role in maintaining and guarding the integrity of terminally differentiated neuronal cells challenged by ischemia and calcium-mediated cell death.
Materials and Methods
Cell Culture and Reagents.
The Paju cell line (Paju/WT) (17) was established in our laboratory from the pleural fluid of a 16-yr-old girl who had a widespread metastatic neural-crest-derived tumor. The cells grow surface adherent in RPMI 1640 medium, supplemented with 10% FCS, penicillin G (50 mg/ml), streptomycin sulfate (50 mg/ml), and 1 mM glutamine. For subculturing, the cells were detached by treatment with 0.5 M EDTA. Human recombinant STC (hSTC) and rabbit antiserum against hSTC were prepared as described (18). Thapsigargin was purchased from Calbiochem.
Western Blotting.
Cells were lysed for 10 min in an ice-cold buffer containing 20 mM Tris⋅HCl (pH 8.0), 0.2 mM EDTA, 3% Nonidet P-40, 2 mM orthovanadate, 50 mM NaF, 10 mM NaPPi, 100 mM NaCl, and 10 μg/ml each of aprotinin and leupeptin. The samples were centrifuged at 14,000 × g for 15 min, and the supernatants recovered. A total of 30 μg of protein from each sample was separated by SDS/PAGE under reducing conditions and transferred electrophoretically to nitrocellulose filters. The filters were treated with 3% BSA in 20 mM Tris⋅HCl (pH 7.5)/150 mM NaCl/Triton X-100 for 2 h. Immunoblotting was done with 1:1,000 diluted rabbit antibodies to human STC antibody followed by peroxidase-conjugated secondary goat antibodies to anti-rabbit Ig. The blots were developed by enhanced chemiluminescence (Amersham Pharmacia).
Phosphate Uptake.
Phosphate (32Pi) uptake was measured as described (19) at 37°C in Locke's buffer (20) (pH 7.2–7.4) consisting of 5.5 mM KCl, 1.0 mM MgCl2, 2.5 mM CaCl2, 5.5 mM glucose, 8.5 mM Hepes, and 160 mM NaCl. After preincubation in the assay medium for 10 min, the uptake was initiated by addition of 200 ng/ml of recombinant STC together with 125 μM KH232PO4 (200 μCi/μmol). At indicated time points, 32Pi uptake was terminated by washing with cold stop solution [10 mM Tris⋅HCl (pH 7.2)]. The cells were lysed in 0.1% SDS in water, and the 32Pi activity was measured by liquid scintillation.
Expression Vector Constructs and Transfection.
Human stc cDNA containing the full-length ORF was cloned into the BamHI site of a pcDNA3 expression vector (Invitrogen). Paju cells were transfected with 5 μg of the vector construct by using Lipofectamine Reagent according to the instructions of the manufacturer (GIBCO/BRL). Transfected cells were selected for resistance to G418 (700 μg/ml) for 3 wk and single-cell cloned (Paju/STC). Three Paju/STC clones were analyzed in this study with identical results. Control cells were transfected with the empty vector (Paju/C).
Cell Viability Assay and Luciferase Assay for ATP Monitoring.
Cell viability was assessed by trypan blue exclusion (BDH). ATP was quantitated by an ATP monitoring kit (BioOrbit, Tampere, Finland) according to the manufacturer's instructions. In brief, cells were lysed in luciferase buffer with 1% Triton X-100, and the ATP-dependent activity was monitored by a luminometer (BioOrbit).
In Situ Hybridization.
Single-stranded RNA probes were generated in vitro by T7 RNA polymerase from the pcDNA3 vector (Invitrogen) in which a cDNA for human stc was cloned in either sense or anti-sense orientation. The product was labeled with digoxigenin (Roche Molecular Biochemicals). A sense probe was used as control. The hybridization was carried out as described by the manufacturer (Roche Molecular Biochemicals). The bound label was detected by a peroxidase-conjugated anti-digoxygenin antibody (Roche Molecular Biochemicals).
Immunohistochemistry.
The tissues were fixed in 4% buffered formaldehyde, routinely processed, and embedded in paraffin. Sections of 4 μm thickness were mounted on slides coated with 3-aminopropyltriethoxysilane (Sigma) and dried for 12 h at 37°C. The deparaffinized and rehydrated sections were processed in a microwave oven (21) and treated with a methanol-perhydrol solution (0.5% hydrogen peroxide in absolute methanol) for 30 min at room temperature to block endogenous peroxidase activity. Immunohistochemical stainings were performed as described (12). Staining with STC antibodies preabsorbed with recombinant STC protein and with normal rabbit serum served as controls.
Samples of Infarcted Human Brains.
Specimens were collected at autopsy from the infarcted hemisphere and the corresponding contralateral brain area of patients who had died at different times after the onset of ischemic stroke symptoms. The samples were dissected, processed, and histologically analyzed as described in the Helsinki Stroke Study (22).
Experimental Animals and Induction of Focal Brain Ischemia.
All animal experiments and surgical procedures were approved by The Animal Research Committee of the Helsinki University Central Hospital and the governmental animal health authorities. A total of 37 male Wistar rats weighing 310–380 g were used. Focal cerebral ischemia was induced by the suture occlusion of the medial cerebral artery of anesthetized animals (23). Reperfusion was established by withdrawing the suture occluder after 90 min. The control animals underwent the same procedure, but the suture occluder was inserted only 10 mm above the carotid bifurcation and withdrawn 1 min later. After postischemia periods of 2, 6, 24, 72 h, and 7 days, the experimental animals were anesthetized and subjected to transcardial perfusion with 200 ml of 0.9% saline for 5 min or until the perfusion fluid was clear. The controls underwent transcardial perfusion 24 h after the sham occlusion. The brains were immediately removed, 2-mm-thick coronal slices were dissected at the level of the optic chiasm, fixed in phosphate-buffered (pH 7) 4% formaldehyde, and embedded in paraffin.
Results
Elevated Extracellular Ca2+ Induces STC Expression in Cultivated Neural Cells.
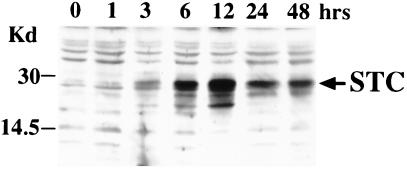
Elevated environmental calcium is a major trigger of STC synthesis in fish (2). Given this, we cultivated Paju cells in RPMI 1640 medium containing 5.4 mM CaCl2 or MgCl2. The expression of STC protein increased strongly after 6 h of culture in hypercalcemic medium and reached plateau levels at 12 h (Fig. 1). Addition of equimolar concentrations of Mg2+ to cultures of Paju cells had no effect on the STC expression (data not shown).
Figure 1.
Elevated extracellular calcium induces STC expression in the neural cell line Paju. The cells were cultivated in 5.4 mM CaCl2 and lysed at indicated time points (1–48 h). Western blotting with rabbit antibodies revealed accumulation of STC. Time point 0 shows Western blotting of lysate from Paju cells cultivated in normocalcemic (0.7 mM) medium (control).
STC Stimulates Pi Uptake in Cultured Neural Cells.
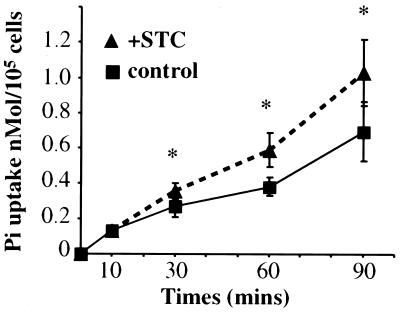
STC has been reported to stimulate tubular phosphate reabsorption in rat kidney by acting on the renal Na-phosphate cotransporter (10). Addition of 200 ng/ml hSTC to Paju/WT cells significantly increased their rate of Pi uptake (Fig. 2).
Figure 2.
Effects of extracellular STC on Pi uptake in Paju cells. The cells were incubated in Lockes' buffer containing 160 mM NaCl. The phosphate uptake was initiated by addition of 200 ng/ml recombinant STC together with 125 μM KH232PO4, and the radioactivity was measured at indicated time points. No STC was added to the control samples. The data are presented as mean ± SD. *, P < 0.05 compared with control samples (Student's t test, n = 6).
STC Confers Increased Resistance to Hypoxia and Hypercalcemia.
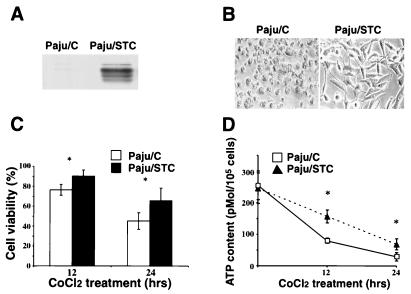
Treatment with CoCl2 is commonly used to mimic hypoxic insults both in vitro and in vivo (24, 25). Paju cells overexpressing STC after transfection with stc cDNA (Paju/STC, clone-27) and control cells transfected with the empty vector (Paju/C) (Fig. 3A) were cultivated for 12 and 24 h in the presence of 300 μM CoCl2. Only the Paju/STC cells retained high viability, whereas the control Paju/C cells had rounded up and mostly detached (Fig. 3 B and C). The protective role of STC was further substantiated by the fact that, in the presence of CoCl2, Paju/STC cells maintained an efficient ATP synthesis in comparison to Paju/C cells (Fig. 3D).
Figure 3.
Overexpression of STC increases cell resistance to hypoxic insult. (A) Paju cells transfected with stc cDNA (Paju/STC) expressed STC as shown by Western blotting. (B) Morphology of control Paju/C (Paju cells transfected with empty vector) and Paju/STC cells after 24 h in the presence of 300 μM CoCl2. (C) Cell viability assay of Paju/C and Paju/STC cells treated with CoCl2 for indicated time periods. (D) ATP contents of Paju/C and Paju/STC cells treated with 300 μM CoCl2 for indicated times. The data are presented as mean ± SD. *, P < 0.05 compared with control samples (Student's t test, n = 5–6).
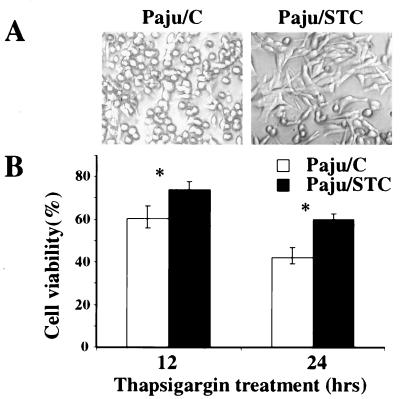
Thapsigargin, an inhibitor of Ca2+ ATPases in the endoplasmic reticulum, mobilizes intracellular calcium stores and leads to increased levels of intracellular free calcium (26). Paju/STC cells displayed clearly elevated resistance to treatment with thapsigargin at concentrations toxic to Paju/C cells (Fig. 4).
Figure 4.
Overexpression of STC increases cell resistance to mobilization of intracellular calcium induced by treatment with thapsigargin. (A) Morphology of Paju/STC and Paju/C after treatment for 12 h with 10 μM thapsigargin in serum-free culture medium. (B) Cell viability assay of Paju/C and Paju/STC cells treated with thapsigargin for indicated time periods. The data are presented as mean ± SD. *, P < 0.05 compared with control samples (Student's t test, n = 5).
Transiently Up-Regulated and Redistributed Neuronal Expression of STC in Human Parietal Cortex Surrounding Brain Infarcts.
We reported that the constitutive expression of STC in mammalian brain was restricted to neurons. Immunohistochemistry revealed STC expression in the cytoplasm and the nuclei, except for the nucleoli. The cytoplasm of larger neurons, like the pyramidal cells of the parietal cortex, hippocampus, and the Purkinje cells of cerebellum and the large neurons in basal nuclei also stained for STC (12).
Immunohistochemical stainings of sections from the brain of a patient who died within 15 h after onset of ischemic stroke revealed a clearly altered distribution of STC in the neurons close to the infarcted area. When compared with neurons in corresponding areas of the contralateral hemisphere, there was an overall increased intensity of staining with a prominent reactivity in the cytoplasm of larger cortical neurons and in the neuronal processes (Fig. 5 A and B). This increased and redistributed neuronal staining of STC was less notable in brain sections obtained from a similar location of the ipsilateral hemisphere harboring a 3-day old ischemic infarct (Fig. 5C). Control stainings with normal rabbit serum or with STC antibodies preabsorbed with recombinant STC protein gave no neuronal staining (data not shown).
Figure 5.
Immunohistochemical demonstration of STC in infarcted human parietal brain cortex. (A) Staining of a corresponding area from the contralateral hemisphere of a brain with a 15-h-old infarct (control). (B) Staining of the “penumbra” of the damaged area in the infarcted hemisphere of the same brain. (C) Staining of an ischemic area from another brain with a 3-day-old infarct. Arrows indicate staining of neuronal processes.
Altered STC Expression in Experimental Ischemic Brain Insults in Rat.
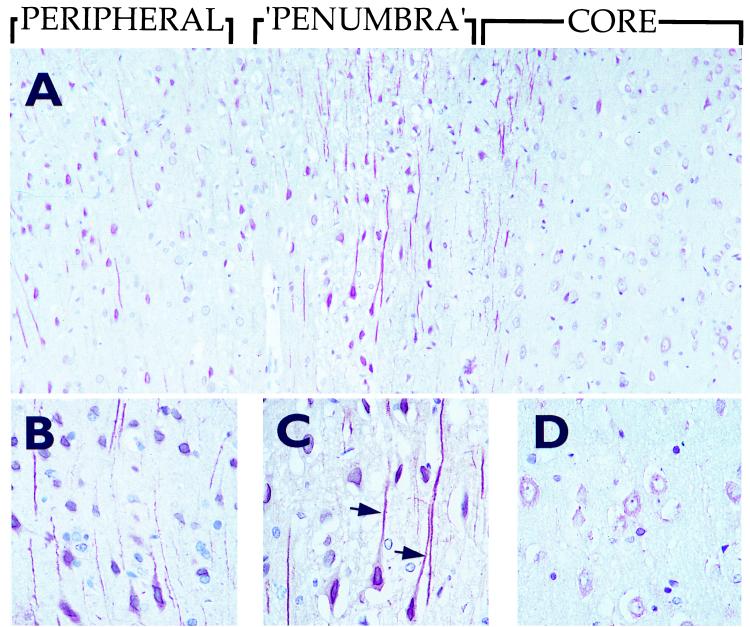
To further investigate the changes in STC expression in cerebral neurons in response to ischemia, we studied brains from rats subjected to experimental transient focal ischemia. In sections from the ischemic core, a slight decrease in STC staining in neurons was seen already after 2 h. After 6 h, only a faint cytoplasmic STC staining remained in the swollen neurons (Fig. 6), and it decreased further on the third day in parallel with the maturation of the infarct. A redistributed and up-regulated expression of STC, corresponding to that observed 15 h after an infarct in the human brain, was seen in the “penumbra” zone surrounding the infarct core. The neurons displayed a strong cytoplasmic immunoreactivity for STC, which was also translocated to the neuronal processes (Fig. 6). This accentuated and redistributed pattern of STC in the neurons of the “penumbra” area was most prominent at 2 and 6 h. In situ hybridization using an stc anti-sense RNA probe revealed an up-regulated stc gene expression in the neurons of the “penumbra” zone (Fig. 7). Hybridization with a control, sense-stc RNA probe did not give any specific reaction (data not shown). The expression of STC declined gradually, and, by day 7, returned to the pattern observed in sections of brains from sham-operated animals or from the noninfarcted, contralateral hemisphere (Fig. 8). The neurons did not stain with normal rabbit serum or antibodies preabsorbed with recombinant STC (data not shown).
Figure 6.
Immunohistochemical staining of STC in brains of rats subjected to experimental ischemia. (A) Six hours after induced focal brain ischemia covering the infarct core, “penumbra” and peripheral area. (B–D) Greater magnifications of the corresponding areas shown in A. Arrows indicate staining of neuronal processes.
Figure 7.
In situ hybridization with an stc anti-sense RNA probe showing an up-regulated neuronal expression of stc mRNA in the “penumbra” zone surrounding the core of a 6-h-old experimental infarct in the rat brain.
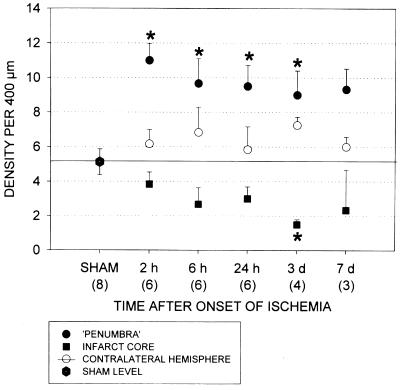
Figure 8.
The density of STC-stained neuronal processes was histologically quantitated in the cerebral cortex in three locations of the infarcted rat brains: the ischemic core, the narrow rim surrounding the infarct (“penumbra”), and the homologous area in the contralateral, nonischemic hemisphere. Corresponding cortical areas were examined in sham-operated animals. An investigator blinded to the source of the samples counted the numbers of vertically oriented STC-stained neuronal processes cut by a 400-μM bar placed horizontally on the cerebral cortex. The data are expressed as mean ± SE. Statistical tests were performed according to one-way ANOVA followed by Dunn's post hoc test. *, P < 0.05 for the given number of animals in each group.
Discussion
We originally reported that expression of STC is induced in mammalian neurons during terminal differentiation both in vitro and in vivo (12). It raised the question why this evolutionarily highly conserved glycoprotein hormone, which regulates calcium, and phosphate homeostasis in fish (3) is constitutively expressed in the human nervous system? Here, we show a redistributed and up-regulated expression of STC in brain neurons in response to cerebral ischemia in rats and humans. We also present data demonstrating that neural cells expressing STC display increased resistance against hypoxic challenge in vitro.
High concentrations of environmental calcium are known to trigger increased STC synthesis in fish (2). Analogously, we found that cultivation of the human neural-crest-derived Paju cells in hypercalcemic medium induced STC expression independently of neural differentiation. It is therefore conceivable that calcium may also trigger the up-regulated and redistributed expression of STC that we observed in the neurons surrounding the ischemic core.
An elevated intracellular concentration of free calcium is considered a primary neurotoxic mechanism in brain damage induced by different means, including ischemia (13, 14). The increased concentrations of cytoplasmic free calcium after hypoxia are derived both from an influx and a release from intracellular stores, such as mitochondria and endoplasmic reticulum (27). Recent evidence indicates that mobilization of the intracellular calcium stores is in itself sufficient to activate neuronal apoptosis (28).
The pyramidal neurons of the cerebral cortex and hippocampus are known to carry the highest density of calcium channels, especially agonist-gated channels such as NMDA-channels. This seems to correlate with their selective vulnerability to ischemic, epileptic, and hypoglycemic cell death through calcium-mediated necrosis (13).
Our present results indicate that the elevated STC expression protects neurons against potentially harmful calcium levels after hypoxia. This notion is supported by our finding that neural cells constitutively overexpressing transfected STC display elevated resistance to treatment with CoCl2, which mimics hypoxic stress and leads to influx of calcium (29). STC-expressing cells also displayed increased resistance to mobilization of intracellular calcium accomplished by treatment with thapsigargin. It is tempting to speculate that, during phylogenesis, terminally differentiated cerebral neurons have retained STC as a means to guard the cellular integrity.
Although the molecular details of the mode of action of STC in neurons still need to be elucidated, in vivo studies have demonstrated that transfusion of rats with hSTC increases the reabsorption of Pi in renal tubular epithelium (10). STC itself does not bind or sequester calcium, but recent findings indicate that the sodium-dependent phosphate cotransporter (NaPi) is a primary target of STC activity (10). Here, we show that addition of STC in vitro to Paju cells stimulated uptake of Pi. Moreover, we have observed that Paju cells overexpressing STC display a higher steady-state rate of Pi uptake (our unpublished observations). Pi has been shown to buffer intracellular free Ca2+ by increasing its sequestration to organelles (30). These findings are interesting in view of a recent study demonstrating that addition of inorganic phosphate increases neuronal survival in vitro during the acute phase after oxidative and excitotoxic insults (31). Glinn et al. (32, 33) reported that Pi influx stimulates ATP synthesis and enhances the energy charge of neurons cultivated from fetal rat brain. They also found that neurons preexposed to Pi had higher steady state levels of ATP than Pi-starved cells. Elevated stores of high-energy phosphate have been found to improve neuronal survival under excitotoxic conditions (31, 33).
There were, however, no measurably increased levels of ATP in stc-transfected or STC-treated cells (data not shown), whereas Paju/STC cells showed a significantly increased ability to maintain the ATP synthesis in a hypoxic environment.
Taken together, our findings demonstrate a previously uncharacterized neurochemical control mechanism by which STC, known to regulate calcium and phosphate homeostasis in fish, contributes to the protection of neurons challenged by hypoxia or ischemia. Similar patterns of STC expression were found in rat and human stroke. Manipulation of the STC system may therefore offer an approach to therapeutic interventions aimed at limiting the damage after brain insults.
Acknowledgments
This study was supported by the Academy of Finland, the Sigrid Juelius Foundation, the Novo Nordisk Foundation, and the Wilhelm and Elise Stockmann Foundation. K.Z. is enrolled in the Haartman Institute Graduate School at the University of Helsinki. P.J.L. and T.T. are supported by grants from the University of Helsinki, the Helsinki University Central Hospital, and the Maire Taponen Foundation.
Abbreviations
- STC
stanniocalcin
- hSTC
human recombinant STC
- Pi
inorganic phosphate
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.070045897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.070045897
References
- 1.Stannius H. Arch Anat Physiol. 1839;6:97–101. [Google Scholar]
- 2.Wagner G F, Gellersen B, Friesen H G. Mol Cell Endocrinol. 1989;62:31–39. doi: 10.1016/0303-7207(89)90110-x. [DOI] [PubMed] [Google Scholar]
- 3.Wagner G F, Milliken C, Friesen H G, Copp D H. Mol Cell Endocrinol. 1991;79:129–138. doi: 10.1016/0303-7207(91)90103-y. [DOI] [PubMed] [Google Scholar]
- 4.Fenwick J C, So Y P. J Exp Zool. 1974;188:125–131. doi: 10.1002/jez.1401880112. [DOI] [PubMed] [Google Scholar]
- 5.Lafeber F P, Flik G, Wendelaar Bonga S E, Perry S F. Am J Physiol. 1988;254:R891–R896. doi: 10.1152/ajpregu.1988.254.6.R891. [DOI] [PubMed] [Google Scholar]
- 6.Lu M, Wagner G F, Renfro J L. Am J Physiol. 1994;267:R1356–R1362. doi: 10.1152/ajpregu.1994.267.5.R1356. [DOI] [PubMed] [Google Scholar]
- 7.Sundell K, Björnsson B T, Itoh H, Kawauchi H. J Comp Physiol B. 1992;162:489–495. doi: 10.1007/BF00264807. [DOI] [PubMed] [Google Scholar]
- 8.Chang A C, Janosi J, Hulsbeek M, de Jong D, Jeffrey K J, Noble J R, Reddel R R. Mol Cell Endocrinol. 1995;112:241–247. doi: 10.1016/0303-7207(95)03601-3. [DOI] [PubMed] [Google Scholar]
- 9.Chang A C, Dunham M A, Jeffrey K J, Reddel R R. Mol Cell Endocrinol. 1996;124:185–187. doi: 10.1016/s0303-7207(96)03929-9. [DOI] [PubMed] [Google Scholar]
- 10.Wagner G F, Vozzolo B L, Jaworski E, Haddad M, Kline R L, Olsen H S, Rosen C A, Davidson M B, Renfro J L. J Bone Miner Res. 1997;12:165–171. doi: 10.1359/jbmr.1997.12.2.165. [DOI] [PubMed] [Google Scholar]
- 11.Madsen K L, Tavernini M M, Yachimec C, Mendrick D L, Alfonso P J, Buergin M, Olsen H S, Antonaccio M J, Thomson A B, Fedorak R N. Am J Physiol. 1998;274:G96–G102. doi: 10.1152/ajpgi.1998.274.1.G96. [DOI] [PubMed] [Google Scholar]
- 12.Zhang K Z, Westberg J A, Paetau A, von Boguslawsky K, Lindsberg P, Erlander M, Guo H, Su J, Olsen H S, Andersson L C. Am J Pathol. 1998;153:439–445. doi: 10.1016/S0002-9440(10)65587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siesjö B K. J Cereb Blood Flow Metab. 1981;1:155–185. doi: 10.1038/jcbfm.1981.18. [DOI] [PubMed] [Google Scholar]
- 14.Siesjö B K, Bengtsson F. J Cereb Blood Flow Metab. 1989;9:127–140. doi: 10.1038/jcbfm.1989.20. [DOI] [PubMed] [Google Scholar]
- 15.Choi D W. Trends Neurosci. 1995;18:58–60. [PubMed] [Google Scholar]
- 16.Kristian T, Siesjö B K. Stroke. 1998;29:705–718. doi: 10.1161/01.str.29.3.705. [DOI] [PubMed] [Google Scholar]
- 17.Zhang K Z, Westberg J A, Hölttä E, Andersson L C. Proc Natl Acad Sci USA. 1996;93:4504–4508. doi: 10.1073/pnas.93.9.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen H S, Cepeda M A, Zhang Q Q, Rosen C A, Vozzolo B L. Proc Natl Acad Sci USA. 1996;93:1792–1796. doi: 10.1073/pnas.93.5.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glinn M, Ni B, Paul S M. J Neurochem. 1995;65:2358–2365. doi: 10.1046/j.1471-4159.1995.65052358.x. [DOI] [PubMed] [Google Scholar]
- 20.Anner B, Ferrero J, Jirounek P, Straub R W. J Physiol (London) 1975;247:759–771. doi: 10.1113/jphysiol.1975.sp010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Boguslawsky K. APMIS. 1994;102:641–646. doi: 10.1111/j.1699-0463.1994.tb05215.x. [DOI] [PubMed] [Google Scholar]
- 22.Lindsberg P J, Carpén O, Paetau A, Karjalainen-Lindsberg M-L, Kaste M. Circulation. 1996;94:939–945. doi: 10.1161/01.cir.94.5.939. [DOI] [PubMed] [Google Scholar]
- 23.Takano K, Tatlisumak T, Bergmann A G, Gibson D G, III, Fisher M. J Neurol Sci. 1997;153:8–11. doi: 10.1016/s0022-510x(97)00184-6. [DOI] [PubMed] [Google Scholar]
- 24.Chandel N S, Maltepe E, Goldwasser E, Mathieu C E, Simon M C, Schumacker P T. Proc Natl Acad Sci USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badr G A, Zhang J Z, Tang J, Kern T S, Ismail-Beigi F. Brain Res Mol Brain Res. 1999;64:24–33. doi: 10.1016/s0169-328x(98)00301-5. [DOI] [PubMed] [Google Scholar]
- 26.Thastrup O, Cullen P J, Drobak B K, Hanley M R, Dawson A P. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paschen W. Med Hypotheses. 1996;47:283–288. doi: 10.1016/s0306-9877(96)90068-7. [DOI] [PubMed] [Google Scholar]
- 28.Nath R, Raser K J, Hajimohammadreza I, Wang K K. Biochem Mol Biol Int. 1997;43:197–205. doi: 10.1080/15216549700203971. [DOI] [PubMed] [Google Scholar]
- 29.Yamagami K, Nishimura S, Sorimachi M. Brain Res. 1994;646:295–298. doi: 10.1016/0006-8993(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 30.Fulceri R, Bellomo G, Gamberucci A, Romani A, Benedetti A. Biochem J. 1993;289:299–306. doi: 10.1042/bj2890299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glinn M, Ni B, Irwin R P, Kelley S W, Lin S Z, Paul S M. J Neurochem. 1998;70:1850–1858. doi: 10.1046/j.1471-4159.1998.70051850.x. [DOI] [PubMed] [Google Scholar]
- 32.Glinn M, Paul S M. J Neurochem. 1995;65:2358–2365. doi: 10.1046/j.1471-4159.1995.65052358.x. [DOI] [PubMed] [Google Scholar]
- 33.Glinn M, Ni B, Paul S M. Brain Res. 1997;757:85–92. doi: 10.1016/s0006-8993(97)00162-5. [DOI] [PubMed] [Google Scholar]