Abstract
Background and purpose:
Activation of poly(ADP-ribose) polymerase (PARP) is deleterious during cerebral ischemia. We assessed the influence of PARP activation induced by cerebral ischemia on the synthesis of proinflammatory mediators including the cytokines, tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) and the adhesion molecules, E-selectin and intercellular adhesion molecule-1 (ICAM-1).
Experimental approach:
Ischemia was induced by intravascular occlusion of the left middle cerebral artery for 1 h in male Swiss mice anaesthetized with ketamine and xylazine. The PARP inhibitor PJ34 (1.25–25 mg kg−1) was administered intraperitoneally 15 min before and 4 hours after, the onset of ischemia. Animals were killed 6 h or 24 h after ischemia and cerebral tissue removed for analysis.
Key results:
Ischemia increased TNF-α protein in cerebral tissue at 6 and 24 h after ischemia. All doses of PJ34 blocked the increase in TNF-α at 6 h and 25 mg kg−1 PJ34 had a sustained effect for up to 24 h. Quantitative real time polymerase chain reaction showed that PJ34 (25 mg kg−1) reduced the increase in TNF-α mRNA by 70% at 6 h. PJ34 also prevented the increase in mRNAs encoding IL-6 (−41%), E-selectin (−81%) and ICAM-1 (−54%). PJ34 (25 mg kg−1) reduced the infarct volume (−26%) and improved neurological deficit, 24 h after ischemia.
Conclusions and Implications:
PJ34 inhibited the increase in the mRNAs of four inflammatory mediators, caused by cerebral ischemia. The contribution of this effect of PJ34 to neuroprotection remains to be clarified.
Keywords: poly (ADP-ribose) polymerase, cerebral ischemia/reperfusion, PJ34
Introduction
The poly(ADP-ribose)polymerases (PARP) are a large family of enzymes that catalyze the transfer of ADP-ribose units from β nicotinamide adenine dinucleotide (NAD+) to protein acceptors (Ame et al., 2004). PARP-1, the most abundant isoform in the brain, is a constitutive enzyme involved in a variety of physiological events such as DNA repair, DNA replication and gene expression (Nguewa et al., 2005).
Cerebral ischemia induces strand breaks in DNA, mainly due to oxidative stress, leading to the activation of repair enzymes like PARP-1. Paradoxically, a large body of evidence obtained over the past decade demonstrates that activation of PARP-1 is a harmful event during cerebral ischemia: various PARP inhibitors have neuroprotective effects and PARP-1-deficient mice (PARP-1−/−) subjected to ischemia have reduced infarct volumes (for a review see: Chiarugi (2005b); Jagtap and Szabo, 2005). A recent report showed that deletion of the gene encoding PARP-2 also leads to neuroprotection in focal cerebral ischemia (Kofler et al., 2006). NAD+ consumption and subsequent energy failure are thought to be the primary mechanisms underlying PARP-mediated post-ischemic cell death (Berger, 1985). However, more recent observations suggest a role for PARP-1 in the release of apoptosis-inducing factor (AIF) from mitochondria (Yu et al., 2003; Zhang et al., 2005) and in inflammation, both of which are known to aggravate ischemic brain damage (Chiarugi, 2005a).
In peripheral inflammation, PARP has been involved in experimental colitis, arthritis, lung inflammation and asthma, as well as in multiple organ failure and septic shock. PARP also plays a role in the inflammation of the central nervous system in multiple sclerosis. The anti-inflammatory effects of PARP inhibitors, or of deleting the PARP gene, included decreased oedema and leukocyte infiltration into the injured tissue (see for review: Cuzzocrea (2005); Szabo (2006)). In cerebral ischemia, we have demonstrated that 3-aminobenzamide (3-AB), a PARP inhibitor, reduces the infiltration of neutrophils into the ischemic brains of neonatal rats (Ducrocq et al., 2000) and adult mice (Couturier et al., 2003). Similar results were recently reported in adult rats (Koh et al., 2005).
The pro-inflammatory action of PARP-1 has been attributed to its capacity to alter the transcription of various cytokines and adhesion molecules implicated in edema production and cell infiltration. However, this transcriptional activity of PARP appears to be tissue-dependent. For instance, PARP contributes to the increase of the mRNA of the adhesion molecule E-selectin in hepatic (Khandoga et al., 2002), but not in myocardial ischemia/reperfusion (Zingarelli et al., 2004). E-selectin and cytokines such as tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) are known to be increased after cerebral ischemia (Berti et al., 2002). However, there is no report, so far, on the role of PARP in the expression of these inflammatory mediators in models of this pathology. Furthermore contradictory results have been reported on the involvement of PARP in the expression of the intercellular adhesion molecule-1 (ICAM-1) in cerebral ischemia (Koh et al., 2004; Park et al., 2004).
This study therefore investigated, for the first time, in a model of transient focal cerebral ischemia in mice, whether PJ34 (N-(6-oxo-5,6-dihydrophenanthridin-2-yl)-2-(N,N-dimethylamino)acetamide), a potent PARP inhibitor, modulated the transcription of TNF-α and IL-6, and E-selectin and aimed to clarify the role of PARP in ICAM-1 transcription. The effect of PJ34 on brain damage was also assessed.
Methods
Animals
All animal experiments complied with the French regulations on the protection of animals used for experimental and other scientific purposes (D2001-486), and with the EC regulations (OJ of ECL358 12/18/1986). Male Swiss albino mice (27–32 g, Janvier, Le genest-St-Isle, France) were housed under standard conditions with a 12 h light/dark cycle and allowed access to food and water ad libitum. A total of 137 mice were used in these experiments.
Model of cerebral ischemia
Mice were anesthetized with intraperitoneal ketamine (50 mg kg−1) and xylazine hydrochloride (6 mg kg−1). Body temperature was monitored throughout surgery by a rectal probe and maintained at 37±0.5°C with a homeothermic blanket control unit (Harvard Apparatus, Edenbridge, Kent, UK). Transient focal cerebral ischemia was induced by occlusion and reperfusion of the left middle cerebral artery (MCA) using an intraluminal filament technique (Connolly et al., 1996). The left common carotid artery, external carotid artery and internal carotid artery were isolated through a midline cervical skin incision under a microscope. A nylon monofilament (Drennan; diameter, 83 μm) coated with ‘thermomelting' glue (4 mm long, diameter 180 μm) was introduced through an arteriotomy performed on the external carotid artery and advanced into the internal carotid artery. Occlusion of the MCA was controlled by monitoring the cerebral blood flow in the MCA territory by laser Doppler flowmetry (Moor Instruments Ltd, Millwey, England) for 5 min after the insertion of the filament. Mice with less than a 50% drop in blood flow were excluded from the studies. The filament was withdrawn 1 h after occlusion to allow reperfusion. After surgery, the wound was sutured and mice were returned to their cage maintained at 29°C with free access to food and water. Sham-operated mice were subjected to the same surgical procedure, except that the filament was not advanced to occlude the MCA.
Neurological deficit
Sensorimotor neurological deficits were assessed by a grip test 24 h after ischemia (Hall, 1985). Mice were picked up by the tail and placed on a taut string 60 cm long suspended 40 cm above a table. Grip score was measured as the length of time (in seconds) that mice remained on the string in some manner (using one or more paws, tail, tail plus paws), for a maximum of 30 s. Each experiment was conducted randomly and blindly. Thereafter mice were killed and the infarct volume determined (see below).
Infarct volume
Mice were killed with sodium pentobarbitone (200 mg kg−1, i.p.) 24 h after ischemia. Brains were removed and cut into six 1-mm-thick coronal sections using a MacIlwain Tissue Chopper (Mickle Laboratory Engineering, Gomshall, Surrey, UK). Brain slices were quickly immersed in 2% 2,3,5-triphenyltetrazolium chloride in 0.1 M phosphate-buffered saline (PBS, pH 7.4) for 20 min at room temperature and then stored in phosphate-buffered 4% paraformaldehyde overnight before analysis (Bederson et al., 1986). The area of damaged parenchyma (unstained tissue) was measured on the posterior surface of each slice using a computer image analysis system (Imstar, Paris, France). Each infarct area was then multiplied by the ratio of the surface of the infarcted (ipsilateral) to the intact (contralateral) hemispheres at the same level to correct for brain swelling (Golanov and Reis, 1995). The total volume of damaged tissue (in cubic millimeters) was then calculated by linear integration of the corrected lesion areas.
Assays for TNF-α protein
Assays were performed at two different times. Mice were killed, as described above, 6 or 24 h after the onset of ischemia. Brains were removed and the ipsilateral sides of 1 mm thick coronal slices cut in the ischemic core were used to assay the concentrations of TNF-α with a commercially available ELISA performed according to the manufacturer's instructions. Briefly, ipsilateral brain slices were homogenized in PBS with an Ultra-turrax homogenizer and centrifuged (20 min, 3000 g, 4°C). The protein content of the supernatant was determined by the Bradford assay (Bradford, 1976) and the remaining supernatant was stored at −20°C. 96-well microplates were coated with anti-TNF-α capture antibody (0.8 μg ml−1 in 50 μl PBS) and incubated overnight. The wells were then saturated for 2 h at room temperature with 150 μl PBS containing 1% bovine serum albumin (BSA) and 5% sucrose. The recombinant human TNF-α standard (twofold serial dilutions from 2000 pg ml−1 to 31.25 pg ml−1) and the samples were diluted in 1% BSA in PBS. All reagents were incubated for 2 h at room temperature before adding a biotinylated detection antibody (300 ng ml−1 in PBS containing 1% BSA) for 2 more hours. Plates were then incubated with streptavidin conjugated to horseradish-peroxidase for 30 min at room temperature and immunostained wells revealed with tetramethylbenzidine. Staining was stopped by adding of 25 μl 2 N sulphuric acid, and the absorbance was read at 450 nm. Results are expressed as pg TNF-α mg−1 protein.
Assays for mRNA for cytokines and adhesion molecules
In a separate set of experiments, mice were killed 6 h after the onset of ischemia. Brains were removed and the ipsilateral side of a 1 mm thick coronal slice cut in the ischemic core was immediately placed in RNAlater RNA stabilization reagent and kept at −80°C for further extraction of total RNA using the Rneasy kit. Total (1 μg) RNA was reverse transcribed in a final volume of 20 μl containing 4 μl 5 × RT buffer, 20 U RNasin RNase inhibitor, 10 mM DTT, 100U Superscript II RNase H-reverse transcriptase, and 3 mM random hexamers. Mixes were incubated at 20°C for 10 min and 42°C for 30 min, and reverse transcriptase was inactivated by heating at 99°C for 5 min and cooling at 5°C for 5 min.
PCR primers were designed using Oligo6 software (Molecular Biology Insights, Cascade, CO, USA). Specific primer sets were as follows (5′ → 3′): for TNF-α, CGATGGGTTGTACCTTGTCT (forward) and CTTGACGGCAGAGAGGAG (reverse); for IL-6, GACTGATGCTGGTGACAAC (forward) and TCTCATTTCCACGATTTCC (reverse); for E-selectin, ACTGCGAGAAGAACGGATAG (forward) and GCCACCAGATGTGTGTAGTC (reverse); for ICAM-1, GCTTCCGCTACCATCACC (forward) and GCTCAGTATCTCCTCCCCAC (reverse); and for ribosomal RNA 18S, TTGACGGAAGGGCACCACCAG (forward) and GCACCACCACCCACGGAATCG (reverse). For each experiment a reaction mix was prepared; the volumes given hereafter were multiplied by the number of capillaries that were used for the run: 2 μl RNAse-free, DNAse-free H2O, 0.5 μl forward primer (0.5 μM), 0.5 μl reverse primer (0.5 μM) and 2.0 μl Fast Start DNA Master SYBR® Green I Master Mix. Aliquots (5 μl) of this mix were placed in the LightCycler glass capillaries and 5 μl cDNA solution was added to each. All solutions were processed in duplicate. Capillaries were closed, centrifuged and placed in the LightCycler (Roche Diagnostics, Meylan, France) rotor. The LightCycler experimental run protocol was: denaturation (95°C for 10 min), amplification and quantification repeated 40 times (95°C for 15 s, 68°C for 6 s, 72°C for 6 s (ICAM-1) or 8 s (TNF-α, IL-6, E-selectin, ribosomal RNA 18S) with a single fluorescence measurement), melting curve program (60–95°C with heating at 0.1°C per second and continuous fluorescence measurement), plus a final cooling to 40°C.
The mRNA concentrations were normalized to that of the ribosomal 18S RNA in each sample. Results are expressed as fold increases in mRNA compared to those in the brains of naive (unoperated) animals.
Experimental protocols
The PARP inhibitor, PJ34 (1.25, 12.5 or 25 mg kg−1) was dissolved in isotonic saline (NaCl, 0.9%) and injected intraperitoneally, in a volume of 10 ml kg−1, 15 min before ischemia and again 4 h after the onset of ischemia. Control ischemic mice and sham animals were given vehicle (saline). Naive animals were also included in the studies.
Statistical analysis
Data are expressed as means±s.e.m. Differences between groups were evaluated using one way analysis of variance (ANOVA) followed by a Bonferroni test. For the grip test, non-parametric Kruskall–Wallis and Mann–Whitney tests were used. The dose response effect of PJ34 was analysed by a linear regression. A P-value of <0.05 was considered significant. All tests were performed with Statview 5.0 (Abacus Concepts, Berkeley, CA, USA).
Drugs and reagents
All chemicals and reagents were purchased from Sigma Chemical Co. (Saint-Quentin Fallavier, France), with the following exceptions. Sodium pentobarbitone was from Centravet, Plancoet, France; paraformaldehyde from Acros, Noisy-le-Grand, France and the PARP inhibitor PJ34 from Inotek Corporation, Beverly, USA. The ELISA for TNF-α (Duoset ELISA) was from R&D Systems, Abingdon, UK. Kits for RNA extraction (RNAlater RNA stabilization reagent and Rneasy kit) were obtained from QIAGEN, Valencia, CA, USA; RNAse-free, DNAse-free H2O from GIBCO, Cergy-Pontoise, France and RNase inhibitor from Promega, Madison, WI, USA. Superscript II RNase H-reverse transcriptase was from Invitrogen, Cergy-Pontoise, France and the Fast Start DNA Master SYBR Green I Master Mix from Roche Diagnostics, Meylan, France.
Results
Effect of PJ34 on inflammatory mediators
Effect of PJ34 on TNF-α protein
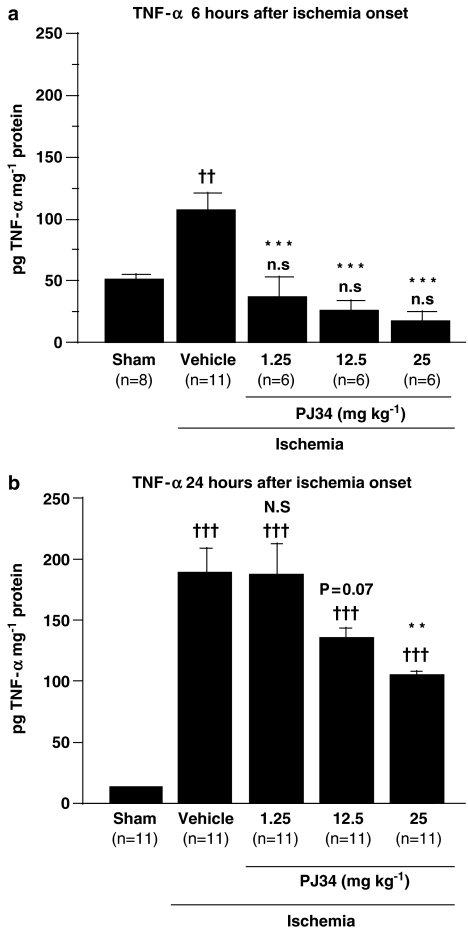
The concentrations of TNF-α in the supernatants from the brains of naive mice were below the detection threshold. The TNF-α concentration 6 h after the onset of ischemia was double that of sham-operated animals (Figure 1a). All three doses of PJ34 significantly blocked the increase in the concentration of TNF-α 6 h after the onset of ischemia. These concentrations in PJ34-treated ischemic mice did not differ from that of sham animals (Figure 1a).
Figure 1.
Effect of PJ34 on brain concentrations of TNF-α protein 6 h (a) and 24 h (b) after the onset of ischemia. PJ34 (1.25–25 mg kg−1) was administered i.p., 15 min before ischemia and again 4 h after the onset of ischemia. Bars show means±s.e.m. and group sizes are shown at the foot of each bar. n.s: non significant, ††P<0.01, †††P<0.001 vs sham mice; N.S: non significant, P=0.07, **P<0.01, ***P<0.001 vs vehicle-treated ischemic mice (ANOVA with Bonferroni post hoc test).
The TNF-α concentration in cerebral tissue was further increased 24 h after ischemia, to almost double the value at 6 h (Figure 1b). Treatment with PJ34 produced a dose-dependent reduction of this increase (y=−3.491x+187; P<0.001) over the dose range used. However, at this time of assay (24 h), the TNF-α concentration in mice treated with the highest dose of PJ34 (25 mg kg−1) was still above that of sham animals (Figure 1b).
Effect of PJ34 on mRNA for TNF-α, IL-6, E-selectin and ICAM-1
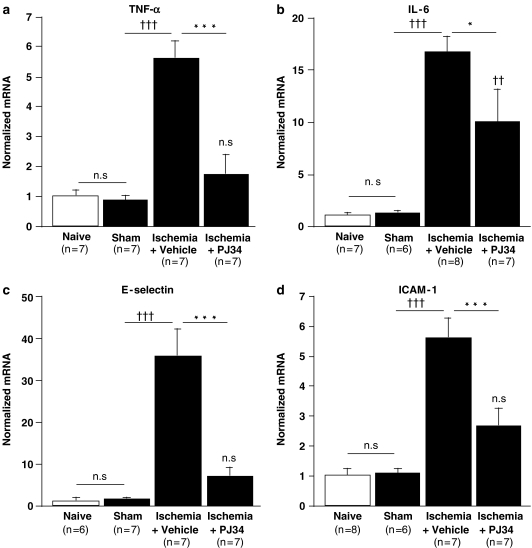
The amounts of the four mRNAs in cerebral tissue did not differ between naive and sham operated animals (Figure 2) 6 h after the onset of ischemia, TNF-α mRNA was sixfold higher in vehicle-treated ischemic mice than in sham-operated mice (Figure 2a). PJ34 (25 mg kg−1) reduced the levels of TNF-α mRNA in ischemic animals by 70% and these values in treated mice did not differ from that of sham or naive animals. There was also a 14-fold increase in mRNA for IL-6 in cerebral tissue following ischemia (Figure 2b) and this effect of ischemia was 41% lower in mice treated with PJ34. However, the mRNA levels for IL-6 in PJ34 treated ischemic mice were still increased relative to those in naive and sham animals.
Figure 2.
Effect of PJ34 on the changes in the cerebral levels of the mRNAs encoding TNF-α (a), IL-6 (b), E-selectin (c) and ICAM-1 (d) 6 h after the onset of ischemia. PJ34 (25 mg kg−1, i.p.) was administered 15 min before ischemia and again 4 h after the onset of ischemia and the mRNAs were determined by quantitative RT–PCR 6 h after the onset of ischemia. The mRNA concentrations were normalized to that of ribosomal RNA 18S and expressed as means±s.e.m. The fold increase in specific mRNAs were compared to naive mice. ns: non significant, ††P<0.01, †††P<0.001 vs sham mice; *P<0.05, ***P<0.001 vs vehicle-treated ischemic mice (ANOVA with the Bonferroni post hoc test).
The levels of the mRNAs encoding the adhesion molecules E-selectin and ICAM-1 were also increased 6 h after the onset of ischemia (36-fold for E-selectin mRNA and sixfold for ICAM-1 mRNA), compared to sham-operated mice (Figures 2c and d). Treatment of ischemic mice with PJ34 reduced the level of E-selectin mRNA by 81% and that of ICAM-1 mRNA by 54%, compared to vehicle-treated ischemic mice. The levels of both adhesion molecules after PJ34 treatment did not differ from that of sham or naive animals.
Dose–response effect of PJ34 on the infarct volume and grip score 24 h after ischemia
The fall in cerebral blood flow produced by MCA occlusion was not statistically different among the four groups (79±2% of pre-ischemic value in the vehicle-treated mice; 83±2% in mice treated with 1.25 mg kg−1 PJ34, 75±3% in mice given 12.5 mg kg−1 PJ34 and 78±2% given 25 mg kg−1 PJ34).
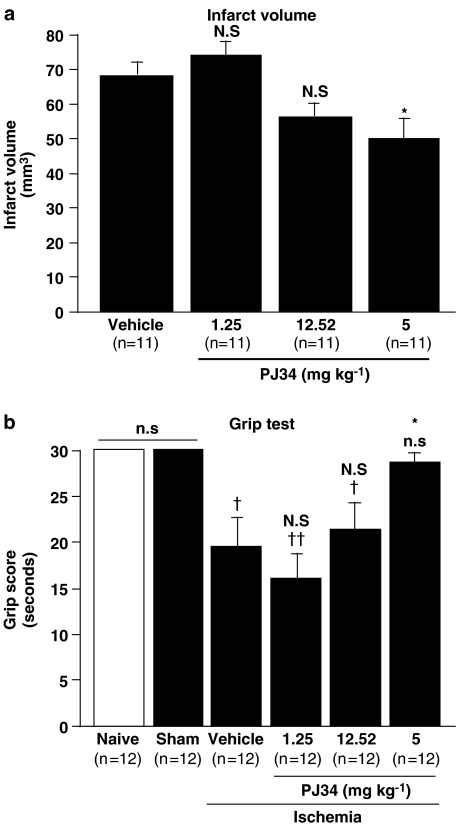
Infarct volume was affected by treatments with PJ34 (Figure 3a) and showed a dose-dependent reduction in this variable (y=−0.9x+72.6; P<0.05). The lower doses of PJ34 did not decrease the size of the lesion below the vehicle-treated value, but a 26% decrease was observed with the highest dose of PJ34 (25 mg kg−1).
Figure 3.
Effect of PJ34 on the infarct volume (a) and grip score (b) 24 h after the onset of ischemia. PJ34 (25 mg kg−1, i.p.) was administered 15 min before ischemia and again 4 h after the onset of ischemia. Bars show means±s.e.m. ns: non significant, †P<0.05, ††P<0.01 vs sham mice; n.s.: non significant, *P<0.05 vs vehicle-treated ischemic mice (a: ANOVA with the Bonferroni post hoc test; b: Kruskall–Wallis and Mann–Whitney tests).
The grip scores for naive and sham-operated mice 24 h after surgery were the same for both these groups (Figure 3b). Ischemia significantly decreased the grip score, indicating some neurological deficit, in the vehicle-treated group of mice, to about 60% of the score in naive mice. PJ34 increased in a dose-dependent manner the grip scores of ischemic animals (y=15x+0.5; P<0.01). The lower doses of PJ34 did not improve the grip score relative to those receiving vehicle alone, but ischemic mice treated with 25 mg kg−1 PJ34 had a grip score that did not differ from that of naive and sham animals.
Discussion and conclusion
The major finding of this study is that treatment with PJ34 decreased the raised levels of TNF-α protein and of mRNAs for TNF-α, IL-6, ICAM-1 and E-selectin in brain tissue that followed focal cerebral ischemia.
PJ34 is a potent PARP inhibitor that presently, has no other known effects. PJ34 has none of the antioxidant properties reported for other PARP inhibitors, like 3-AB (Garcia Soriano et al., 2001). Which PARP is inhibited by PJ34 to cause the effects described herein is not clear. PARP-1 (see for review: Chiarugi, 2005b; Cuzzocrea, 2005; Jagtap and Szabo 2005; Szabo, 2006) and PARP-2 (Popoff et al., 2002; Kofler et al., 2006) are reported to have deleterious effects in focal cerebral ischemia and to exert pro-inflammatory actions at the periphery.
In the late 1990s, PARP was implicated in the activation of transcription through the transcription factor NF-κB (Hassa and Hottiger, 1999; Oliver et al., 1999) but several other transcription factors are regulated by PARP, including activator protein-1 (AP-1) (Ha et al., 2002). Many of the genes whose NF-κB and AP-1 dependent transcription is upregulated by PARP encode inflammatory mediators including cytokines, chemokines, adhesion molecules and enzymes (e.g.: inducible nitric oxide synthase and cyclooxygenase 2) (Hassa and Hottiger, 2002; Carrillo et al., 2004; Zingarelli et al., 2004). We first focused on TNF-α, whose regulation by PARP had not been yet evaluated in cerebral ischemia. TNF-α appeared to be particularly interesting because the concentration of this cytokine is increased in focal cerebral ischemia (Liu et al., 1994; Wang et al., 1994; Gong et al., 1998; Lambertsen et al., 2005). TNF-α is also thought to be harmful in cerebral ischemia (Dawson et al., 1996; Meistrell et al., 1997; Lavine et al., 1998; Yang et al., 1999). Lastly, PARP inhibitors block the increase in TNF-α mRNA in the spinal cord tissues of mice and rats in models of multiple sclerosis (Chiarugi, 2002; Scott et al., 2004). The present study showed detectable levels of TNF-α protein at 6 h and 24 h post-ischemia in sham animals in contrast with naive animals. Thus, surgery per se seems to induce brain inflammation. How exposure of the three carotid arteries and the arteriotomy may induce this reaction remains to be clarified. Ischemia induced a further twofold increase in TNF-α that even the lowest dose of PJ34 abolished in the early phase (6 h) after MCA occlusion. A reduction in TNF-α at the later time of 24 h was, however, obtained only with the highest dose of PJ34 (25 mg kg−1). This may be due to a stronger effect and/or a slower elimination of PJ34 at this dose. This dose of PJ34 also suppresses the increase in TNF-α mRNA induced by ischemia.
Increased amounts of IL-6 mRNA and protein are reported in the brain of rats subjected to cerebral ischemia (Wang et al., 1995; Ali et al., 2000; Legos et al., 2000). The role of PARP in IL-6 overproduction in inflammation appears to depend on the stimulus and the type of tissue (Oliver et al., 1999; Soriano et al., 2002; Mota et al., 2005). We have now demonstrated that the PARP inhibitor PJ34 reduces the increase in IL-6 mRNA caused by ischemia/reperfusion affecting the brain. Few studies published to date have investigated the role of IL-6 in the outcomes of cerebral ischemia. The infarct volumes in IL-6 deficient mice are not different from those of wild-type mice (Clark et al., 2000; Herrmann et al., 2003). However, a recent study found that blocking IL-6 signalling aggravated brain damage, suggesting that endogenous IL-6 acts as a neuroprotective cytokine in cerebral ischemia (Yamashita et al., 2005). Thus, the consequences of the decrease in IL-6 induced by PJ34 are difficult to evaluate.
E-selectin and ICAM-1 are required for neutrophil adhesion and infiltration and may thus be pro-inflammatory in cerebral ischemia by contributing to the no-reflow phenomenon and by releasing cytotoxic mediators (see for review: Emerich et al., 2002; Sughrue et al., 2004). E-selectin upregulation by PARP in ischemia/reperfusion appears to depend upon the injured tissue as mentioned in the Introduction. Our study clearly shows that PARP is implicated in the increase in E-selectin mRNA in brain tissue that follows in cerebral ischemia. The contribution of PARP-1 to the overproduction of ICAM-1 has been demonstrated in models of ischemia/reperfusion affecting the liver, kidney and heart and in experimental splanchnic artery occlusion/reperfusion (Zingarelli et al., 1998; Khandoga et al., 2002; Mazzon et al., 2002; Mota-Filipe et al., 2002; Di Paola et al., 2004; Zingarelli et al., 2004; Zheng et al., 2005). However, in cerebral ischemia, two recent studies reported contradictory results. Immunohistochemical and Western blotting studies by Koh et al. (2004) found that the increase in ICAM-1 after 8 h, 24 h and 72 h of cerebral ischemia was reduced by the PARP inhibitor 3-AB. But Park et al. (2004) reported that PJ34 potentiated the increase in ICAM-1 mRNA 6 h after ischemia and that PJ34 only blocked ICAM-1 production much later (24 h and 72 h). We found that PJ34 markedly decreases the ICAM-1 mRNA level after 6 h of ischemia, suggesting, as did Koh et al., that PARP increases ICAM-1 mRNA production in the early phase of ischemia. The discrepancies between the three studies still have to be resolved.
Our demonstration of the drastic decreases in the mRNAs of both adhesion molecules by PJ34 suggests that PARP influences the synthesis of adhesion molecules following cerebral ischemia. This may explain the decrease in post-ischemic neutrophil infiltration caused by 3-AB, another PARP inhibitor (Ducrocq et al., 2000; Couturier et al., 2003; Koh et al., 2005).
The transcriptions of the four mediators studied here are regulated by the nuclear factor NF-κB (Kumar et al., 2004) that, as mentioned above, is activated by PARP. Thus PJ34 may decrease the amounts of both cytokine and adhesion molecule mRNAs by inhibiting their transcription. PJ34 may also interact with other transcription factors, notably AP-1, and so alter its influence on transcription. Finally, TNF-α is also known to be responsible for stimulating numerous inflammatory genes, including those studied here (see for review: Munoz-Fernandez and Fresno, 1998). Thus, PJ34 may have reduced IL-6, ICAM-1 and E-selectin mRNAs by a direct effect on transcription and/or by indirectly reducing TNF-α. However, Carrillo et al. (2004) demonstrated that the increases in IL-6 and ICAM-1 mRNAs in murine heart cells triggered by TNF-α were the same in both PARP-1−/− mice and wild-type mice, suggesting that PARP does not interfere with the action of TNF-α on the transcription of these genes. By contrast, the same study found that the increase in E-selectin mRNA by TNF-α was partly PARP-dependent. Further experiments should investigate the mechanisms involved in the effect of PJ34.
Some studies have already reported the neuroprotective effect of PJ34 in cerebral ischemia (Abdelkarim et al., 2001; Iwashita et al., 2004; Park et al., 2004; McCullough et al., 2005). Interestingly in our experiment, the reduction in the infarct volume and neurological deficit was observed only with the highest dose of PJ34, the dose that decreased TNF-α protein concentration at 24 h. The effect of PJ34 both on brain damage and inflammatory mediators at longer times after ischemia will be the subject of future studies.
The extent to which the reductions in TNF-α, ICAM-1 and E-selectin mRNAs contribute to the neuroprotection elicited by PJ34 in our study remains to be established. Whether or not the reduction by PJ34 of the potentially neuroprotective IL-6 minimized the beneficial effect of the PARP inhibitor is another question that needs answering.
In conclusion, we found that PJ34 reduced the transcription of the genes encoding TNF-α, IL-6, ICAM-1 and E-selectin following cerebral ischemia, suggesting that PARP plays a major role in the production of inflammatory mediators. Further studies are needed to determine whether limiting post-ischemic inflammation with PJ34 or other anti-PARP strategies contributes to their neuroprotective effect.
Acknowledgments
We thank Inotek Corporation (Beverly, USA) for the gift of PJ34. CS was supported by the Hungarian Scientific Research Fund (OTKA). We thank Owen Parkes for checking the English text.
Abbreviations
- AP-1
activator protein-1
- AIF
apoptosis inducing factor
- BSA
bovine serum albumin
- ICAM-1
intercellular adhesion molecule-1
- IL-6
interleukin-6
- MCA
middle cerebral artery
- NAD+
β nicotinamide adenine dinucleotide
- NF-κB
nuclear factor-κB
- PARP
poly(ADP-ribose)polymerase
- PBS
phosphate buffer saline
- PJ34
N-(6-oxo-5,6-dihydrophenanthridin-2-yl)-2-(N,N-dimethylamino)acetamide
- RT-PCR
real time polymerase chain reaction
- TNF-α
tumor necrosis factor-α
Conflict of interest
CS is a stockholder in Inotek, a pharmaceutical firm involved in the development of PARP inhibitors. The authors state no conflict of interest.
References
- Abdelkarim GE, Gertz K, Harms C, Katchanov J, Dirnagl U, Szabo C, et al. Protective effects of PJ34, a novel, potent inhibitor of poly(ADP-ribose) polymerase (PARP) in in vitro and in vivo models of stroke. Int J Mol Med. 2001;7:255–260. [PubMed] [Google Scholar]
- Ali C, Nicole O, Docagne F, Lesne S, MacKenzie ET, Nouvelot A, et al. Ischemia-induced interleukin-6 as a potential endogenous neuroprotective cytokine against NMDA receptor-mediated excitotoxicity in the brain. J Cereb Blood Flow Metab. 2000;20:956–966. doi: 10.1097/00004647-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–893. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- Berger NA. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat Res. 1985;101:4–15. [PubMed] [Google Scholar]
- Berti R, Williams AJ, Moffett JR, Hale SL, Velarde LC, Elliott PJ, et al. Quantitative real-time RT-PCR analysis of inflammatory gene expression associated with ischemia-reperfusion brain injury. J Cereb Blood Flow Metab. 2002;22:1068–1079. doi: 10.1097/00004647-200209000-00004. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carrillo A, Monreal Y, Ramirez P, Marin L, Parrilla P, Oliver FJ, et al. Transcription regulation of TNF-alpha-early response genes by poly(ADP-ribose) polymerase-1 in murine heart endothelial cells. Nucleic Acids Res. 2004;32:757–766. doi: 10.1093/nar/gkh239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarugi A. Inhibitors of poly(ADP-ribose) polymerase-1 suppress transcriptional activation in lymphocytes and ameliorate autoimmune encephalomyelitis in rats. Br J Pharmacol. 2002;137:761–770. doi: 10.1038/sj.bjp.0704934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarugi A. Intrinsic mechanisms of poly(ADP-ribose) neurotoxicity: three hypotheses. Neurotoxicology. 2005a;26:847–855. doi: 10.1016/j.neuro.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Chiarugi A. Poly(ADP-ribosyl)ation and stroke. Pharmacol Res. 2005b;52:15–24. doi: 10.1016/j.phrs.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Clark WM, Rinker LG, Lessov NS, Hazel K, Hill JK, Stenzel-Poore M, et al. Lack of interleukin-6 expression is not protective against focal central nervous system ischemia. Stroke. 2000;31:1715–1720. doi: 10.1161/01.str.31.7.1715. [DOI] [PubMed] [Google Scholar]
- Connolly ES, Jr, Winfree CJ, Stern DM, Solomon RA, Pinsky DJ.Procedural and strain-related variables significantly affect outcome in a murine model of focal cerebral ischemia Neurosurgery 199638523–531.discussion 532 [DOI] [PubMed] [Google Scholar]
- Couturier JY, Ding-Zhou L, Croci N, Plotkine M, Margaill I. 3-Aminobenzamide reduces brain infarction and neutrophil infiltration after transient focal cerebral ischemia in mice. Exp Neurol. 2003;184:973–980. doi: 10.1016/S0014-4886(03)00367-4. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S. Shock, inflammation and PARP. Pharmacol Res. 2005;52:72–82. doi: 10.1016/j.phrs.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Martin D, Hallenbeck JM. Inhibition of tumor necrosis factor-alpha reduces focal cerebral ischemic injury in the spontaneously hypertensive rat. Neurosci Lett. 1996;218:41–44. doi: 10.1016/0304-3940(96)13116-5. [DOI] [PubMed] [Google Scholar]
- Di Paola R, Genovese T, Caputi AP, Threadgill M, Thiemermann C, Cuzzocrea S. Beneficial effects of 5-aminoisoquinolinone, a novel, potent, water-soluble, inhibitor of poly (ADP-ribose) polymerase, in a rat model of splanchnic artery occlusion and reperfusion. Eur J Pharmacol. 2004;492:203–210. doi: 10.1016/j.ejphar.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Ducrocq S, Benjelloun N, Plotkine M, Ben-Ari Y, Charriaut-Marlangue C. Poly(ADP-ribose) synthase inhibition reduces ischemic injury and inflammation in neonatal rat brain. J Neurochem. 2000;74:2504–2511. doi: 10.1046/j.1471-4159.2000.0742504.x. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Dean RL, III, Bartus RT. The role of leukocytes following cerebral ischemia: pathogenic variable or bystander reaction to emerging infarct. Exp Neurol. 2002;173:168–181. doi: 10.1006/exnr.2001.7835. [DOI] [PubMed] [Google Scholar]
- Garcia Soriano F, Virag L, Jagtap P, Szabo E, Mabley JG, Liaudet L, et al. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med. 2001;7:108–113. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- Golanov EV, Reis DJ. Contribution of cerebral edema to the neuronal salvage elicited by stimulation of cerebellar fastigial nucleus after occlusion of the middle cerebral artery in rat. J Cereb Blood Flow Metab. 1995;15:172–174. doi: 10.1038/jcbfm.1995.19. [DOI] [PubMed] [Google Scholar]
- Gong C, Qin Z, Betz AL, Liu XH, Yang GY. Cellular localization of tumor necrosis factor alpha following focal cerebral ischemia in mice. Brain Res. 1998;801:1–8. doi: 10.1016/s0006-8993(98)00489-2. [DOI] [PubMed] [Google Scholar]
- Ha HC, Hester LD, Snyder SH. Poly(ADP-ribose) polymerase-1 dependence of stress-induced transcription factors and associated gene expression in glia. Proc Natl Acad Sci USA. 2002;99:3270–3275. doi: 10.1073/pnas.052712399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED. High-dose glucocorticoid treatment improves neurological recovery in head-injured mice. J Neurosurg. 1985;62:882–887. doi: 10.3171/jns.1985.62.6.0882. [DOI] [PubMed] [Google Scholar]
- Hassa PO, Hottiger MO. A role of poly (ADP-ribose) polymerase in NF-κB transcriptional activation. Biol Chem. 1999;380:953–959. doi: 10.1515/BC.1999.118. [DOI] [PubMed] [Google Scholar]
- Hassa PO, Hottiger MO. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-κB in inflammatory disorders. Cell Mol Life Sci. 2002;59:1534–1553. doi: 10.1007/s00018-002-8527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann O, Tarabin V, Suzuki S, Attigah N, Coserea I, Schneider A, et al. Regulation of body temperature and neuroprotection by endogenous interleukin-6 in cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:406–415. doi: 10.1097/01.WCB.0000055177.50448.FA. [DOI] [PubMed] [Google Scholar]
- Iwashita A, Tojo N, Matsuura S, Yamazaki S, Kamijo K, Ishida J, et al. A novel and potent poly(ADP-ribose) polymerase-1 inhibitor, FR247304 (5-chloro-2-[3-(4-phenyl-3,6-dihydro-1(2 H)-pyridinyl)propyl]-4(3 H)-quinazo linone), attenuates neuronal damage in in vitro and in vivo models of cerebral ischemia. J Pharmacol Exp Ther. 2004;310:425–436. doi: 10.1124/jpet.104.066944. [DOI] [PubMed] [Google Scholar]
- Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- Khandoga A, Enders G, Biberthaler P, Krombach F. Poly(ADP-ribose) polymerase triggers the microvascular mechanisms of hepatic ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2002;283:G553–G560. doi: 10.1152/ajpgi.00085.2002. [DOI] [PubMed] [Google Scholar]
- Kofler J, Otsuka T, Zhang Z, Noppens R, Grafe MR, Koh DW, et al. Differential effect of PARP-2 deletion on brain injury after focal and global cerebral ischemia. J. Cereb Blood Flow Metab. 2006;26:135–141. doi: 10.1038/sj.jcbfm.9600173. [DOI] [PubMed] [Google Scholar]
- Koh SH, Chang DI, Kim HT, Kim J, Kim MH, Kim KS, et al. Effect of 3-aminobenzamide, PARP inhibitor, on matrix metalloproteinase-9 level in plasma and brain of ischemic stroke model. Toxicology. 2005;214:131–139. doi: 10.1016/j.tox.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Koh SH, Park Y, Song CW, Kim JG, Kim K, Kim J, et al. The effect of PARP inhibitor on ischaemic cell death, its related inflammation and survival signals. Eur J Neurosci. 2004;20:1461–1472. doi: 10.1111/j.1460-9568.2004.03632.x. [DOI] [PubMed] [Google Scholar]
- Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-κB: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- Lambertsen KL, Meldgaard M, Ladeby R, Finsen B. A quantitative study of microglial-macrophage synthesis of tumor necrosis factor during acute and late focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:119–135. doi: 10.1038/sj.jcbfm.9600014. [DOI] [PubMed] [Google Scholar]
- Lavine SD, Hofman FM, Zlokovic BV. Circulating antibody against tumor necrosis factor-alpha protects rat brain from reperfusion injury. J Cereb Blood Flow Metab. 1998;18:52–58. doi: 10.1097/00004647-199801000-00005. [DOI] [PubMed] [Google Scholar]
- Legos JJ, Whitmore RG, Erhardt JA, Parsons AA, Tuma RF, Barone FC. Quantitative changes in interleukin proteins following focal stroke in the rat. Neurosci Lett. 2000;282:189–192. doi: 10.1016/s0304-3940(00)00907-1. [DOI] [PubMed] [Google Scholar]
- Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, et al. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25:1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- Mazzon E, Dugo L, De SA, Li JH, Caputi AP, Zhang J, et al. Beneficial effects of GPI 6150, an inhibitor of poly(ADP-ribose) polymerase in a rat model of splanchnic artery occlusion and reperfusion. Shock. 2002;17:222–227. doi: 10.1097/00024382-200203000-00011. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- Meistrell ME, III, Botchkina GI, Wang H, Di Santo E, Cockroft KM, Bloom O, et al. Tumor necrosis factor is a brain damaging cytokine in cerebral ischemia. Shock. 1997;8:341–348. [PubMed] [Google Scholar]
- Mota RA, Sanchez-Bueno F, Saenz L, Hernandez-Espinosa D, Jimeno J, Tornel PL, et al. Inhibition of poly(ADP-ribose) polymerase attenuates the severity of acute pancreatitis and associated lung injury. Lab Invest. 2005;85:1250–1262. doi: 10.1038/labinvest.3700326. [DOI] [PubMed] [Google Scholar]
- Mota-Filipe H, Sepodes B, McDonald MC, Cuzzocrea S, Pinto R, Thiemermann C. The novel PARP inhibitor 5-aminoisoquinolinone reduces the liver injury caused by ischemia and reperfusion in the rat. Med Sci Monit. 2002;8:BR444–BR453. [PubMed] [Google Scholar]
- Munoz-Fernandez MA, Fresno M. The role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog Neurobiol. 1998;56:307–340. doi: 10.1016/s0301-0082(98)00045-8. [DOI] [PubMed] [Google Scholar]
- Nguewa PA, Fuertes MA, Valladares B, Alonso C, Perez JM. Poly(ADP-ribose) polymerases: homology, structural domains and functions. Novel therapeutical applications. Prog Biophys Mol Biol. 2005;88:143–172. doi: 10.1016/j.pbiomolbio.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Oliver FJ, Menissier-de Murcia J, Nacci C, Decker P, Andriantsitohaina R, Muller S, et al. Resistance to endotoxic shock as a consequence of defective NF-κB activation in poly (ADP-ribose) polymerase-1 deficient mice. EMBO J. 1999;18:4446–4454. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EM, Cho S, Frys K, Racchumi G, Zhou P, Anrather J, et al. Interaction between inducible nitric oxide synthase and poly(ADP-ribose) polymerase in focal ischemic brain injury. Stroke. 2004;35:2896–2901. doi: 10.1161/01.STR.0000147042.53659.6c. [DOI] [PubMed] [Google Scholar]
- Popoff I, Jijon H, Monia B, Tavernini M, Ma M, McKay R, et al. Antisense oligonucleotides to poly(ADP-ribose) polymerase-2 ameliorate colitis in interleukin-10-deficient mice. J Pharmacol Exp Ther. 2002;303:1145–1154. doi: 10.1124/jpet.102.039768. [DOI] [PubMed] [Google Scholar]
- Scott GS, Kean RB, Mikheeva T, Fabis MJ, Mabley JG, Szabo C, et al. The therapeutic effects of PJ34 [N-(6-oxo-5,6-dihydrophenanthridin-2-yl)-N,N-dimethylacetamide.HCl], a selective inhibitor of poly(ADP-ribose) polymerase, in experimental allergic encephalomyelitis are associated with immunomodulation. J Pharmacol Exp Ther. 2004;310:1053–1061. doi: 10.1124/jpet.103.063214. [DOI] [PubMed] [Google Scholar]
- Soriano FG, Liaudet L, Szabo E, Virag L, Mabley JG, Pacher P, et al. Resistance to acute septic peritonitis in poly(ADP-ribose) polymerase-1-deficient mice. Shock. 2002;17:286–292. doi: 10.1097/00024382-200204000-00008. [DOI] [PubMed] [Google Scholar]
- Sughrue ME, Mehra A, Connolly ES, Jr, D'Ambrosio AL. Anti-adhesion molecule strategies as potential neuroprotective agents in cerebral ischemia: a critical review of the literature. Inflamm Res. 2004;53:497–508. doi: 10.1007/s00011-004-1282-0. [DOI] [PubMed] [Google Scholar]
- Szabo C. Poly(ADP-ribose) polymerase activation by reactive nitrogen species-Relevance for the pathogenesis of inflammation. Nitric Oxide. 2006;14:169–179. doi: 10.1016/j.niox.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Wang X, Yue TL, Barone FC, White RF, Gagnon RC, Feuerstein GZ. Concomitant cortical expression of TNF-alpha and IL-1 beta mRNAs follows early response gene expression in transient focal ischemia. Mol Chem Neuropathol. 1994;23:103–114. doi: 10.1007/BF02815404. [DOI] [PubMed] [Google Scholar]
- Wang X, Yue TL, Young PR, Barone FC, Feuerstein GZ. Expression of interleukin-6, c-fos, and zif268 mRNAs in rat ischemic cortex. J Cereb Blood Flow Metab. 1995;15:166–171. doi: 10.1038/jcbfm.1995.18. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Sawamoto K, Suzuki S, Suzuki N, Adachi K, Kawase T, et al. Blockade of interleukin-6 signaling aggravates ischemic cerebral damage in mice: possible involvement of Stat3 activation in the protection of neurons. J Neurochem. 2005;94:459–468. doi: 10.1111/j.1471-4159.2005.03227.x. [DOI] [PubMed] [Google Scholar]
- Yang GY, Gong C, Qin Z, Liu XH, Lorris Betz A. Tumor necrosis factor alpha expression produces increased blood-brain barrier permeability following temporary focal cerebral ischemia in mice. Brain Res Mol Brain Res. 1999;69:135–143. doi: 10.1016/s0169-328x(99)00007-8. [DOI] [PubMed] [Google Scholar]
- Yu SW, Wang H, Dawson TM, Dawson VL. Poly(ADP-ribose) polymerase-1 and apoptosis inducing factor in neurotoxicity. Neurobiol Dis. 2003;14:303–317. doi: 10.1016/j.nbd.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang X, Park TS, Gidday JM. Cerebral endothelial cell apoptosis after ischemia-reperfusion: role of PARP activation and AIF translocation. J Cereb Blood Flow Metab. 2005;25:868–877. doi: 10.1038/sj.jcbfm.9600081. [DOI] [PubMed] [Google Scholar]
- Zheng J, Devalaraja-Narashimha K, Singaravelu K, Padanilam BJ. Poly(ADP-ribose) polymerase-1 gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol. 2005;288:F387–F398. doi: 10.1152/ajprenal.00436.2003. [DOI] [PubMed] [Google Scholar]
- Zingarelli B, Salzman AL, Szabo C. Genetic disruption of poly (ADP-ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ Res. 1998;83:85–94. doi: 10.1161/01.res.83.1.85. [DOI] [PubMed] [Google Scholar]
- Zingarelli B, Hake PW, O'Connor M, Denenberg A, Wong HR, Kong S, et al. Differential regulation of activator protein-1 and heat shock factor-1 in myocardial ischemia and reperfusion injury: role of poly(ADP-ribose) polymerase-1. Am J Physiol Heart Circ Physiol. 2004;286:H1408–H1415. doi: 10.1152/ajpheart.00953.2003. [DOI] [PubMed] [Google Scholar]