Abstract
Background and purpose:
The acute vascular inflammatory dysfunction associated with endotoxaemia may reflect an imbalance between matrix metalloproteinases (MMPs) and their natural inhibitors (TIMPs), induced by the endotoxin. This possibility was tested in rat aortic tissue.
Experimental approaches:
Tone induced by phenylephrine in aortic rings was measured after exposure in vitro to ambient lipopolysaccharide (LPS) or the proinflammatory cytokine interleukin-1β (IL-1β) for 6h, with or without MMP inhibitors (doxycycline or GM6001). Gelatinase and MMP activities, TIMP proteins and contractility were measured in aortae taken from rats 6h after receiving LPS in vivo.
Key results:
Inhibition of MMP prevented the loss of phenylephrine–induced tone in aortic rings after LPS or IL-1β. IL-1β also increased release of MMP-2 activity from aortic tissue. In aortae exposed in vivo to LPS, net gelatinase, MMP-9 activities and TIMP-1 protein levels were increased, whereas TIMP-4 was reduced. These aortae were hypocontractile to both phenylephrine and KCl. Hypocontractility was partially reversed by doxycycline ex vivo.
Conclusions and Implications:
MMP inhibitors ameliorate vascular hyporeactivity induced by either LPS or IL-1β in vitro. LPS in vivo alters the balance between MMPs and TIMPs, contributing to vascular dysfunction which is partially reversed by MMP inhibitors. Vascular MMPs are activated as a result of LPS or IL-1β-induced stress and contribute to the hyporeactivity of blood vessels to vasoconstrictors.
Keywords: matrix metalloproteinases; tissue inhibitors of matrix metalloproteinases; septic shock; sepsis; endotoxaemia; vascular dysfunction; blood vessel, aorta
Introduction
Matrix metalloproteinases (MMPs) are a family of zinc dependent endopeptidases that are key regulators of the extracellular matrix. The gelatinases, MMP-2 and MMP-9, contribute to a wide variety of chronic cardiovascular pathologies including heart failure, atherosclerosis and abdominal aneurysms (reviewed in Galis and Khatri (2002); Newby (2005)). Alterations in the levels of their main endogenous inhibitors, the tissue inhibitors of MMPs (TIMPs), may also play a role in these chronic pathologies.
Recently, MMP-2 has been implicated in a number of acute cardiovascular processes such as myocardial ischemia-reperfusion injury (Cheung et al., 2000; Wang et al., 2002; Schulze et al., 2003; Sawicki et al., 2005), regulation of normal vascular tone (Fernandez-Patron et al., 1999; Jeyabalan et al., 2003; Chew et al., 2004), platelet aggregation (Sawicki et al., 1997), and modulation of the inflammatory response (McQuibban et al., 2000). As MMPs are involved in inflammation and control of vascular tone, we hypothesized that they may be involved in the vascular alterations that occur in septic shock.
Septic shock is a potentially fatal condition in which a systemic bacterial infection generates an unencumbered inflammatory response. This inflammatory response produces excessive vasodilatation, hyporeactivity to contractile agents, and cardiac dysfunction. It is the chief cause of death and disability in intensive care units (Angus et al., 2001). A similar response and cardiovascular dysfunction can be provoked by administering endotoxin (lipopolysaccharide, LPS) to animals or human volunteers (Suffredini et al., 1989; Rees et al., 1998).
In both sepsis and endotoxaemia the initial inflammatory response due to LPS is largely mediated by proinflammatory cytokines (including interleukin-1β (IL-1β) (Hesse et al., 1988) and tumor necrosis factor-α (Tracey et al., 1986, 1987)) which increase the production of a number of downstream effectors. One effector is peroxynitrite (Beckman and Koppenol, 1996), the toxic reaction product of nitric oxide (Rees et al., 1990) and superoxide anion (Javesghani et al., 2003) which are produced in excess during severe acute inflammation. Peroxynitrite biosynthesis is enhanced in aortae from LPS-treated rats (Szabo et al., 1995). Interestingly, both proinflammatory cytokines and peroxynitrite increase MMP activity and decrease TIMP activity in vitro (Frears et al., 1996; Rajagopalan et al., 1996; Okamoto et al., 2001). Thus, it was speculated that an imbalance between MMPs and TIMPs occurs during acute inflammatory stress.
To date, few investigations have examined the role of MMPs in septic shock or endotoxaemia. LPS was found to increase MMP-2 and MMP-9 activities in experiments using endothelial cells, neutrophils or macrophages (Xie et al., 1994; Pugin et al., 1999; Kim and Koh, 2000). In both animal and human models of endotoxaemia, circulating MMP-9 activity is increased (Paemen et al., 1997; Pugin et al., 1999; Albert et al., 2003; Lalu et al., 2004). In sepsis, circulating MMP-2 and MMP-9 are elevated in both animal models and patients (Paemen et al., 1997; Nakamura et al., 1998; Pagenstecher et al., 2000). Despite these insights no study has investigated the functional role of MMPs in the vascular dysfunction that arises from severe, acute inflammatory stress.
In order to address this issue, we employed three previously established models of vascular inflammatory stress: (a) aortae isolated from normal rats stimulated with ambient LPS, (b) aortae isolated from normal rats stimulated with IL-1β and (c) aortae isolated from rats administered LPS in vivo. Both functional, pharmacological and biochemical approaches were taken to determine changes in vascular contractility and the MMPs and TIMPs in the vessel wall.
Methods
This investigation conforms to the Guide to the Care and Use of Laboratory Animals published by the Canadian Council on Animal Care (revised 1993). Male Sprague Dawley rats (250–300 g) were used in all experiments.
MMP inhibitors
Doxycycline (Sigma, Oakville, Canada) and GM6001 (Chemicon, Temecula, USA), two chemically distinct MMP inhibitors, were used in these studies (Figure 1, structures drawn with ChemSketch 8.17, ACD Labs, Toronto, Canada). Doxycycline is the most potent of the tetracycline class antibiotics that are recognized to have MMP inhibitory activity distinct from their antimicrobial property (Golub et al., 1998). Specifically, doxycycline has been shown to interact with structural ions of MMPs, including Ca2+ and Zn2+ (Garcia et al., 2005). GM6001 is a hydroxamic acid-based MMP inhibitor designed to act as a bidentate ligand for the catalytic Zn2+ in the active site (Galardy et al., 1994).
Figure 1.
Structure of MMP inhibitors (a) doxycycline and (b) GM6001.
Spontaneous loss of contractile tone in aortic rings
Untreated rats were killed by sodium pentobarbital overdose (100 mg kg−1, i.p.). Aortae were rapidly excised and connective tissue was trimmed away in gassed (95% O2-5% CO2) Krebs buffer (118 mM NaCl, 4.75 mM KCl, 1.19 mM KH2PO4, 1.19 mM MgSO4·7 H2O, 2.5 mM CaCl2·2 H2O, 11.1 mM D-glucose, 25 mM NaHCO3) bubbled with carbogen at room temperature. One to three aortic rings (5 mm in length) were dissected from each animal. If more than one ring was prepared from one rat, the individual rings were used in different experimental groups. The rings were then mounted in organ baths filled with Krebs buffer at 37°C which was continuously bubbled with 95% O2-5% CO2. Isometric tension was measured using force transducers (Grass FT03) and recorded using AcqKnowledge 3.1 Software. A tension of 1 g was applied and the rings were equilibrated for 60 min with fresh Krebs buffer added at intervals of 20 min. Following equilibration, rings were contracted with phenylephrine (750 nM, Sigma). At the plateau of contraction one of the following was added: polymyxin B (10 μg ml−1, Sigma), a drug which binds and inactivates LPS (Danner et al., 1989), or the MMP inhibitors doxycycline (30 μM), GM6001 (10 or 30 μM), or their vehicles (doxycycline: ddH2O, GM6001: 0.1% ethanol in ddH2O). Vascular tone was then monitored for another 4.5 h. This model has been previously used as a model of vascular hyporeactivity caused by the presence of ambient levels of LPS in experimental environments in which LPS is not controlled (Rees et al., 1990).
IL-1β-induced vascular dysfunction
Two models of IL-1β-induced vascular dysfunction were used. In the first, aortae were isolated and contracted with phenylephrine (750 μM) as above in the organ bath. At the plateau of contraction IL-1β (10 ng ml−1, R&D Systems, Minneapolis, USA) was added in the presence of either doxycycline (30 or 100 μM) or its vehicle (ddH2O). Vascular tone was then monitored for 6 h and the final tension was reported.
In the second model, aortae isolated as above were washed three times in sterile phosphate-buffered saline (supplemented with an antibiotic cocktail: 100 μg ml−1 streptomycin, 100 U ml−1 penicillin, 5 μg ml−1 gentamicin, Sigma) and then dissected under a tissue culture hood. Rings were cut (5 mm in length) and then washed three times in phosphate-buffered saline before a final wash in Dulbecco's modified Eagle's medium (with 1000 mg l−1 glucose, pyroxidine HCl, NaHCO3; supplemented with antibiotic cocktail, Sigma). Rings were then placed in fresh cell culture medium with one or more of the following added: IL-1β (10 ng ml−1, R&D Systems), GM6001 (10 or 30 μM), or GM6001 vehicle (100% ethanol). Rings were then incubated at 37°C in a humidified atmosphere containing 5% CO2. After 6 h the rings were removed from the medium and mounted in organ baths as described above. The medium was sampled for MMP activity by zymography (see below). Following equilibration (60 min) under 1 g of tension, a concentration–response curve to phenylephrine was obtained. Rings were then washed and the maximum contractile response to KCl (75 mM) was determined.
Rat endotoxaemia
Rats were given either a non-lethal dose of LPS (Salmonella typhosa, Sigma, 4 mg kg−1 i.p.) or pyrogen-free water (control). Rats were then killed with a sodium pentobarbital overdose (100 mg kg−1, i.p.) at 6 h. Previous investigations have revealed that in this model of endotoxaemia, nitric oxide production is increased by this time point and that cardiovascular function is significantly depressed (Schulz et al., 1992; Lalu et al., 2003, 2004).
In both sets of rats a blood sample was drawn from the chest cavity immediately upon sacrifice. The plasma fraction was obtained following centrifugation (6500 g for 5 min at 4°C) and stored at −20°C for later determination of plasma nitrite and nitrate levels. Aortae were rapidly excised and connective tissue was trimmed away in carbogen-gassed Krebs buffer. The dissected aortae were blotted to remove excess water and then rapidly frozen in liquid nitrogen and stored at −80°C for later processing.
Assessment of vascular function of aortae taken from endotoxemic rats
As described above rats were given either a non-lethal dose of LPS or pyrogen-free water and then killed by sodium pentobarbital overdose (100 mg kg−1, i.p.) at 6 h. Aortae were isolated from either LPS- or vehicle-treated rats as above, and two 5 mm rings from each animal were mounted in organ baths. Following equilibration and washes with Krebs buffer (60 min) while under 1 g of tension, a concentration–response curve to phenylephrine was obtained in order to confirm that vessels from LPS-treated animals were hypocontractile compared to vessels from control animals. Vessels were then washed and incubated with either doxycycline (100 μM) or ddH2O vehicle for 10 min. Following incubation, all vessels were subject to a second concentration response curve to phenylephrine. Vessels were then washed and maximal contractile response to KCl (75 mM) was determined.
Determination of plasma nitrate/nitrite levels
Plasma was diluted 1:1 with deionized water and then deproteinized by centrifugal ultrafiltration (Ultrafree-MC microcentrifuge tubes UFC3, Millipore, Mississauga, Canada). Ultrafiltrates were analyzed for total nitrate and nitrite content according to the method of Green et al. (1982).
Preparation of aorta homogenates
Frozen aortae were crushed using a mortar and pestle that was cooled to dry ice temperature. The resulting powder was diluted 1:4 w/v in 50 mM Tris-HCl (pH 7.4) buffer containing 3.1 mM sucrose, 1 mM dithiothreitol, 10 μg ml−1 leupeptin, 10 μg ml−1 soybean trypsin inhibitor, 2 μg ml−1 aprotinin and 0.1% Triton X-100. This solution was then homogenized by hand on ice using a microcentrifuge tube pestle. The homogenate was centrifuged at 10 000 g for 5 min at 4°C and the supernatant was kept on ice for immediate assay of MMP activities.
Determination of protein content
Aortic homogenate protein content was determined by the bicinchoninic acid method (Sigma) using bovine serum albumin as a standard.
Gelatinase and collagenase assays
In order to measure the net activity of gelatinases (MMP-2 and MMP-9), aortic homogenate (100 μg of protein) was analyzed using a gelatinase assay kit (E-12055, Molecular Probes, Burlington, Canada). Nonactivated samples were incubated at room temperature in the presence of DQ gelatin fluorescein conjugate. Digestion of this product yields fluorescent peptides that are detectable using a fluorometer (λex 495 nm, λem 515 nm). The MMP inhibitor o-phenanthroline (100 μM) was added to duplicate samples in order to determine MMP-related gelatinase activity. Pretreament of aortic homogenate from a control rat with p-aminophenylmercuric acetate, a synthetic chemical activator of metalloproteinases, increased gelatinolytic activity approximately 10-fold.
In order to measure the activities of collagenases (MMP-1, -8, and -13), aortic homogenates (50 μg of protein) were analyzed using an MMP collagenase assay kit (ECM710, Chemicon) according to the manufacturer's instructions. The samples, however, were not chemically treated to activate latent collagenase activity. Biotinylated collagen was digested by collagenase activity in the samples at 37°C. The biotinylated fragments were then transferred to a biotin binding 96 well plate and detected with a streptavidin-enzyme complex. In order to control for baseline collagen degradation a series of wells were loaded with only biotinylated collagen and no sample. Addition of a colorimetric substrate produced a colored reaction product which was detectable at 450 nm. Addition of 10 μM GM6001 abolished all collagenase activity.
Measurement of MMP activity by zymography
Gelatinolytic activities of MMPs were examined by gelatin zymography as previously described (Heussen and Dowdle, 1980; Cheung et al., 2000). 8% polyacrylamide gels copolymerized with gelatin (2 mg ml−1, type A from porcine skin, Sigma) were prepared. Nonheated samples were diluted with ddH2O in order to load a constant amount of protein per lane (10 μg of protein from aorta incubation media, 20 μg of protein from aorta homogenate). A standard was loaded into one lane of each gel (supernatant of phorbol ester activated HT-1080 cells, American Type Culture Collection) as an internal standard used to normalize activities between gels. Following 1.5 h of electrophoresis, the gels were washed with 2.5% Triton X-100 for 1 h at room temperature (with three changes of solution) to remove sodium dodecyl sulphate. Gels were then incubated for 20–30 h at 37°C in incubation buffer (50 mM Tris-HCl, 150 mM NaCl, 5 mM CaCl2, and 0.05% NaN3). After incubation the gels were stained with 0.05% Coomassie Brilliant Blue (G-250, Sigma) in a mixture of methanol:acetic acid:water (2.5:1:6.5, v/v) and destained in aqueous 4% methanol:8% acetic acid (v/v). Gelatinolytic activities were detected as transparent bands against the dark blue background. Zymograms were digitally scanned and band intensities were quantified using SigmaGel software (Jandel Corporation, Chicago, USA) and expressed as a ratio to the internal standard. In order to confirm that quantified gelatinolytic activities were of MMP origin, addition of either o-phenanthroline (100 μM) or GM6001 (10 μM) to incubation buffer was found to abolish all gelatinolytic activities.
Immuoblot analysis
Aorta homogenate (10–20 μg protein) was loaded onto 12% polyacrylamide gels, electrophoresed under reducing conditions, and then electroblotted onto polyvinyllidene difluoride membranes (BioRad, Hercules, USA). Positive standards and/or molecular weight standards were also loaded into gels in order to confirm the identity of proteins to be probed. Samples were probed with either: a mouse anti-human MMP-2 antibody (1:1000 dilution, MAB3308, Chemicon), a rabbit anti-rat MMP-9 antibody which detects the 92 kDa form of this protein (1:4000 dilution, courtesy of Dr Mieczyslaw Wozniak, Medical University, Wroclaw, Poland), a mouse anti-human TIMP-1 antibody (2 μg ml−1, MS-608, NeoMarkers, Fremont, USA), a rabbit anti-human TIMP-2 antibody (10 μg ml−1 dilution, RB-1489, NeoMarkers), a mouse anti-human TIMP-3 (1 μg ml−1, 136-13H4, Calbiochem) or a rabbit anti-human TIMP-4 antibody (0.2 μg ml−1, AB19087, Chemicon). All blots were subsequently probed with appropriate horseradish peroxidase conjugated antibodies (either anti-mouse or anti-rabbit, Transduction Laboratories, San Jose, USA) and visualized using the horseradish peroxidase-luminol chemiluminesence reaction kit (Amersham Pharmacia Biotech, Piscataway, USA). As a negative control blots were probed with appropriate non-immune IgGs before incubation with secondary antibodies.
Statistical analysis
Results are expressed as the mean±standard error of the mean (s.e.m.) for n animals. The results were analyzed by using Statistical Package for the Social Sciences. Independent samples t-test, repeated measures two-way ANOVA, or one-way ANOVA followed by Fisher's least significant difference test were used as indicated to evaluate differences between groups. Differences were considered significant at P<0.05.
Results
MMP inhibition ameliorates spontaneous loss of phenylephrine-induced vascular tone
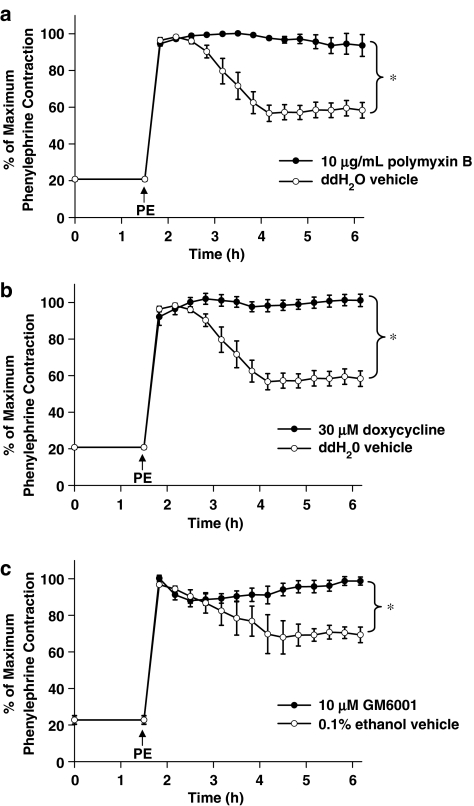
In order to assess whether MMP inhibition affects LPS-mediated vascular dysfunction, aortae from normal rats were mounted in organ baths, contracted with phenylephrine, and their tone was then monitored to a total time of 6 h. At the end of the observation period a spontaneous and significant loss of vascular tone was noted in these contracted vessels (to 58±4% of original phenylephrine-induced tone (Figure 2a)). This spontaneous loss of tone has previously been demonstrated to be due to ambient levels of LPS (Rees et al., 1990). This was confirmed in our experimental conditions by abolishing the spontaneous loss of tone with polymyxin B (10 μg ml−1), a drug that binds and inactivates LPS (Figure 2a). In order to evaluate the role of MMPs in this spontaneous loss of contractile tone, two pharmacologically distinct MMP inhibitors were tested. Doxycycline (30 μM) or GM6001 (10 μM) abolished the spontaneous loss in vascular tone (Figure 2b and c).
Figure 2.
Response of aortic rings taken from normal rats to a time-dependent loss of phenylephrine (PE)-induced tone. PE was added as indicated by the arrow after 1.5 h equilibration and then rings were treated with either (a) polymyxin B (10 μg ml−1) (b) doxycycline (30 μM) or ddH2O vehicle or (c) 10 μM GM6001 or 0.1% ethanol vehicle (*P<0.05, two-way repeated measures ANOVA, n=4–5 aortic rings taken from distinct animals/group).
MMP inhibition protects IL-1β-mediated vascular hyporeactivity
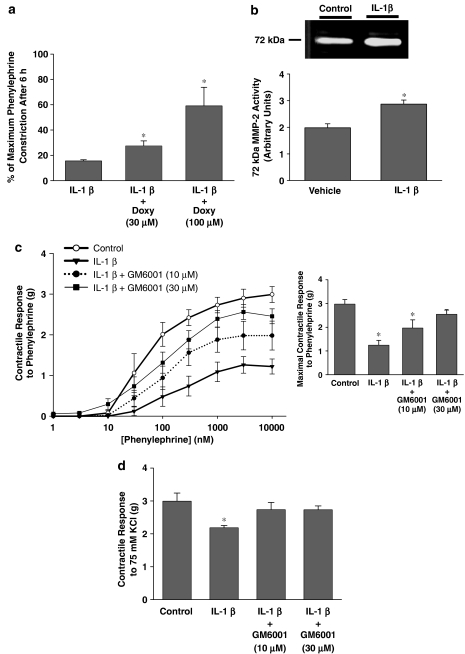
The above experiments demonstrate that MMP inhibition could improve LPS-mediated relaxation, however, it is unclear whether the beneficial effects of MMP inhibition were upstream or downstream of cytokine processing. This is a significant issue since MMPs are known to process cytokine precursors. In order to determine whether MMP inhibition could protect against vascular dysfunction downstream of its possible effects on cytokine processing, we incubated aortae with IL-1β. In the first set of experiments, aortae from normal rats were mounted in organ baths and contracted with phenylephrine. At the plateau of contraction IL-1β (10 ng ml−1) was added and vessel tone was then monitored for 6 h. IL-1β at this concentration produced a greater spontaneous loss of tone compared to ambient levels of LPS (vessels relaxed to 16±0.2% of original phenylephrine-induced tone, Figure 3a). Doxycycline significantly inhibited this IL-1β-induced loss of tone in a concentration-dependent manner (Figure 3a).
Figure 3.
(a) Response of aortic rings taken from normal rats to an IL-1β (10 ng ml−1) spontaneous loss of PE-induced tone. Rings were treated with either doxycycline (30 or 100 μM) or ddH2O vehicle (*P<0.05 vs IL-1β, one-way ANOVA, n=3 rings taken from distinct animals/group). (b) A representative zymogram of MMP-2 activity in incubation media of aortic rings taken from normal rats and incubated for 6 h at 37°C in the presence or absence of IL-1β (10 ng ml−1) (*P<0.05, independent samples t-test, n=6 rings taken from distinct animals/group). (c) Left: contractile response of aortic rings taken from normal rats and incubated for 6 h at 37°C in the presence or absence of IL-1β (10 ng ml−1)±GM6001 (10 or 30 μM) and then placed in organ baths and exposed to increasing concentrations of PE. Right: summary data of maximal contractile response to PE (*P<0.05 vs Control, one-way ANOVA followed by Fisher's LSD test, n=8–13 aortic rings taken from distinct animals/group). (d) Contractile response of aortic rings as prepared in (b) to 75 mM KCl (*P<0.05 vs Control, one-way ANOVA followed by Fisher's LSD test, n=8–13 aortic rings taken from distinct animals/group).
The effect of GM6001 on IL-1β-induced hyporeactivity was tested in a separate group of experiments that also facilitated measurement of MMP activity. Fresh aortae were incubated at 37°C with IL-1β (10 ng ml−1) under sterile conditions in culture medium for 6 h. IL-1β treatment significantly increased MMP-2 activity in the aorta incubation media compared to vehicle-treated rings (P<0.05, Figure 3b). IL-1β decreased the contractile response to increasing concentrations of phenylephrine, relative to control vessels that were incubated without the cytokine (P<0.05, Figure 3c). The addition of 10 or 30 μM GM6001 to the cell culture medium significantly protected the vessels from cytokine-mediated dysfunction in a concentration-dependent manner (P<0.05, Figure 3c). GM6001 had no effect on the contractile response of control vessels, nor did the drug vehicle have any effect on IL-1β-treated vessels (data not shown).
The maximum response to KCl (75 mM), a vasoconstrictor that acts by electromechanical coupling and not via plasmalemmal membrane receptors, was also significantly decreased with IL-1β treatment (P<0.05, Figure 3d). The addition of 10 μM or 30 μM GM6001 restored vascular reactivity to levels not significantly different from control vessels.
LPS administration in vivo causes overt signs of endotoxaemia
Overt symptoms of endotoxaemia were apparent in rats 6 h following LPS administration. These included lethargic behavior, piloerection and porphyrin secretion from the eyes. Plasma nitrate/nitrite, measured as a marker of NO biosynthesis, was significantly elevated at this time point relative to vehicle-treated (control) rats (330±37 μM vs 32±4 μM, respectively, n=10 per group, P<0.05).
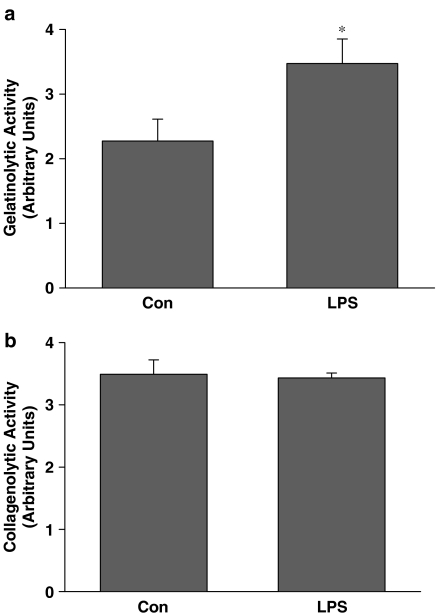
Aortic gelatinolytic activity is increased following LPS administration in vivo
At 6 h after LPS administration, net gelatinolytic activity in excised aortae was significantly increased relative to aortae from control rats (P<0.05, Figure 4a). Collagenolytic activity was also measured since gelatin is susceptible to cleavage by collagenases (MMP-1, -8, -13). Net collagenolytic activity in the aortae, however, was not increased relative to control rats (Figure 4b).
Figure 4.
(a) Gelatinolytic and (b) collagenolytic activies of homogenates prepared from aortae removed 6 h after i.p. injection of either LPS-(LPS, 4 mg kg−1) or pyrogen-free water vehicle (Con) (*P<0.05, independent samples t-test, n=5 rats/group).
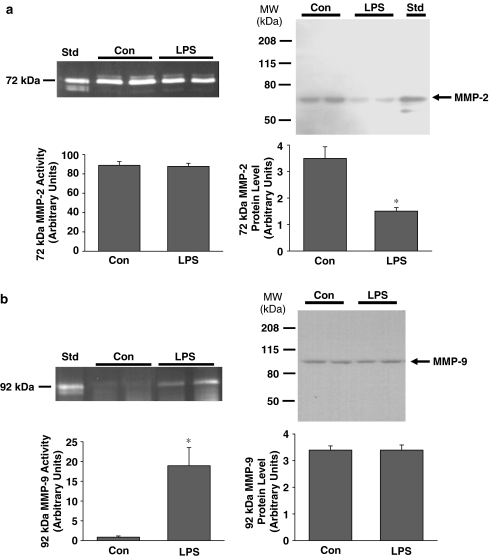
Zymographic analysis of control aortic tissue (Figure 5a) revealed robust 72 kDa MMP-2 activity, as well as minor 75 and 64 kDa MMP-2 activities. The rank order of MMP-2 activities was 72>75>64 kDa. The 72 and 64 kDa bands corresponded to MMP-2 by comparison to the standard, and the 75 kDa band corresponded to a rodent-specific glycosylated form of proMMP-2 (Sang et al., 1990). The 72 kDa MMP-2 activity was not significantly changed following LPS treatment (Figure 5a). In contrast, however, 72 kDa MMP-2 protein content was significantly decreased following LPS (P<0.05, Figure 4b).
Figure 5.
(a) Left: A representative zymogram of vascular homogenate MMP-2 activities. Aortae from two control rats (Con) and two LPS-treated rats (LPS) show primarily 72 kDa activity. ‘Std' represents culture media from HT-1080 cells. Gel incubation time: 20 h. Right: a representative immunoblot showing 72 kDa MMP-2 protein content. Position of molecular weight markers is shown on the left of the immunoblot. (b) Left: a representative zymogram of vascular homogenate MMP-9 activity. 92 kDa activity appears in aortae from two LPS-treated rats but not from Con rats. Gel incubation time: 30 h. Right: a representative immunoblot showing 92 kDa MMP-9 protein content (*P<0.05, independent samples t-test, n=9–13 rats per group).
In aortae from control rats, gelatinolytic activity of molecular weight higher than 75 kDa (the region for MMP-9) was not detectable. However, when zymographic gels were incubated for a longer period of time, 92 kDa MMP-9 activity could be detected in aortae from LPS-treated rats only (P<0.05, Figure 5b). Interestingly, immunoblot analysis revealed that 92 kDa MMP-9 protein content was not significantly different between control and LPS-treated rats (Figure 5b). As a negative control for all immunoblots, no bands were detected when blots were probed with appropriate non-immune IgG.
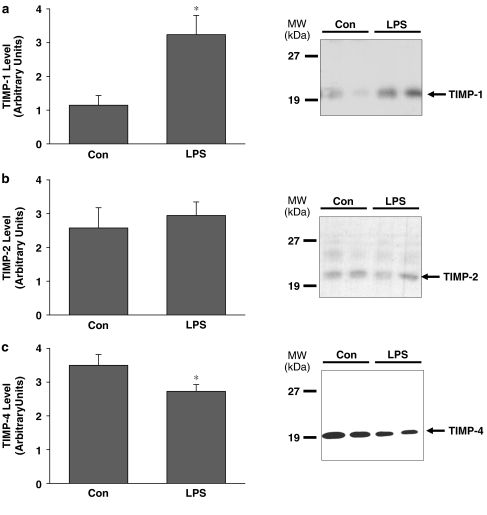
LPS administration in vivo affects TIMP-1 and TIMP-4 protein content
Immunoblot analysis was performed to assess aortic TIMP-1, -2, -3, and -4 content. TIMP-1 protein was found to be increased almost threefold in aorta from LPS-treated rats relative to control rats (P<0.05, Figure 6a). In contrast, TIMP-4 was significantly decreased (P<0.05, Figure 6c). Both glycosylated and non-glycosylated forms of TIMP-2 could be detected, however, they were unchanged in aortae from LPS-treated rats (Figure 6b). TIMP-3 could not be detected (data not shown).
Figure 6.
(a) TIMP-1, (b) TIMP-2 and (c) TIMP-4 protein content in aortae excised from lipopolyssaccharide (LPS) or vehicle (Con) – treated rats. Right panels show representative immunoblots taken from aortae from two control rats (Con) and two LPS-treated rats (LPS). TIMP-3 was not detectable (data not shown). Position of molecular weight markers is depicted on the left (*P<0.05, independent samples t-test, n=12–13 rats/group for TIMP-1 and TIMP-4, n=6 rats/group for TIMP-2).
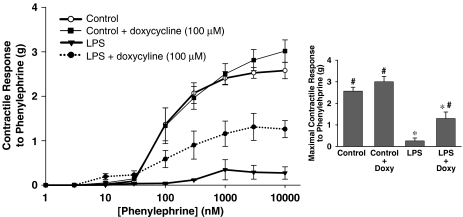
Inhibition of MMPs ex vivo protects against in vivo LPS-induced hyporeactivity
We investigated the ability of MMP inhibition to acutely reverse vascular hyporeactivity following in vivo LPS administration (6 h). Two aortic rings were mounted in organ baths from each LPS-treated or control rat. Half of the rings were incubated ex vivo with doxycycline (100 μM, 10 min), and the other half were treated with ddH2O vehicle. Incubation with doxycycline did not affect baseline resting tension in any vessels. Vessels from LPS-treated rats were significantly hypocontractile compared to vessels from control animals (Figure 7). Doxycycline significantly improved contractile responses to phenylephrine (P<0.05 Figure 7a). Ex vivo doxycycline was able to improve the diminished contractile response to KCl in aortae isolated from LPS-treated animals (data not shown). Doxycycline did not significantly change the contractile response of vessels from control animals to either phenylephrine or KCl.
Figure 7.
Left: contractile response of aortic rings taken from rats 6 h after i.p. injection of either lipopolysaccharide (LPS, 4 mg kg−1) or pyrogen-free water vehicle (Con). Rings were treated ex vivo with either doxycyline (100 μM) or ddH2O vehicle. Right: summary data of maximal contractile response to PE (*P<0.05 vs Control, #P<0.05 vs LPS, one-way ANOVA followed by Fisher's LSD test, n=3–4 aortic rings/group).
Discussion
We studied the effect of MMP inhibition on LPS- and IL-1β-mediated vascular dysfunction in vitro, as well as the regulation of vascular MMP and TIMPs during endotoxemia, an in vivo model of acute inflammation. Both the LPS-mediated spontaneous loss of contractile tone and IL-1β-mediated vascular hyporeactivity were ameliorated by inhibition of MMP activity. During endotoxaemia in rats, both MMP-2 and MMP-9 were detected while net gelatinolytic activity in the aorta was increased. Aortic TIMP-1 was increased, TIMP-4 was decreased, and TIMP-2 remained unchanged in aortae taken from LPS-treated rats. MMP inhibition improved vascular dysfunction in aortae taken from LPS-treated rats. This is the first study to demonstrate that MMP inhibition ameliorates inflammatory vascular dysfunction, and that vascular MMPs and TIMPs are acutely regulated in vivo by severe inflammatory stress.
Ambient levels of LPS, under standard laboratory conditions, were sufficient to cause a spontaneous and slowly developing relaxation in phenylephrine-contracted rat aortae incubated for up to 6 h in organ baths. The ability of polymyxin B, a known chelator of LPS (Danner et al., 1989), to prevent this hyporeactivity suggests that the loss of tone was mediated by ambient LPS under our experimental conditions (Rees et al., 1990). We showed that doxycycline also prevents this loss of tone. Doxycycline is a tetracycline class antibiotic that exhibits MMP inhibitory activity independent of its antibacterial effects (Golub et al., 1998). The chemically distinct MMP inhibitor GM6001, which is devoid of antibacterial action, also prevented the LPS-mediated spontaneous loss of contractile tone. Interestingly, previous work demonstrated that this hypocontractility is related to enhanced inducible NO synthase activity (Rees et al., 1990; Gui et al., 2000). Enhanced NO production under inflammatory conditions leads to increased peroxynitrite biosynthesis (Szabo et al., 1995) and cytokine production in the aortic wall, both of which are known to increase MMP activity and decrease TIMPs (Galis et al., 1994; Frears et al., 1996; Rajagopalan et al., 1996; Okamoto et al., 2001). Thus, it is possible that MMPs are downstream mediators of the well characterized increase in endogenous oxidative stress in this model.
Incubating blood vessels ex vivo with IL-1β is another well-established model of vascular hyporeactivity to vasoconstrictor agonists (French et al., 1991; Gui et al., 2000). This model was used to test whether MMP inhibitors were protective independent of their ability to block proteolytic cleavage of cytokine precursors. This is particularly important since MMP-2 is known to cleave and activate the IL-1β precursor (Schonbeck et al., 1998). IL-1β is thought to be a principal player in the cardiovascular dysfunction associated with endotoxaemia since its administration causes marked hypotension in rabbits (Okusawa et al., 1988). Moreover, IL-1β receptor antagonists reduce cardiovascular dysfunction and mortality in models of sepsis (Ohlsson et al., 1990; Fisher et al., 1994). In the present investigation, treatment with IL-1β produced a time-dependent spontaneous loss of phenylephrine-induced tone. This loss of tone was more pronounced than the loss of tone produced by ambient LPS, suggesting that IL-1β at the concentration used was a more efficacious stimulus for vascular dysfunction than ambient LPS alone. Doxycycline could significantly diminish IL-1β mediated loss of tone in a concentration dependent manner. Incubating aortae with IL-1β in cell culture conditions stimulated an increase in MMP-2 activity released from the isolated vessels, and MMP inhibition with GM6001 significantly improved the contractile response to both phenylephrine and KCl in treated vessels. These results suggest that part of the IL-1β-mediated response is dependent on MMP activity and that MMP inhibitors work downstream of cytokine processing in these models of vascular dysfunction. As well, GM6001's ability to improve vascular reactivity to both phenylephrine and KCl suggests that it acts at a point downstream of where both receptor and nonreceptor dependent contractile pathways converge.
A well-established model of endotoxaemia was used to assess whether MMP and TIMPs are regulated acutely in vivo during severe inflammatory stress. Previously, we demonstrated that significant cardiovascular dysfunction occurs six hours post LPS administration in the rat. This dysfunction is characterized by hypotension and severe depression of cardiac mechanical function (Khadour et al., 2002; Lalu et al., 2003; Lalu et al., 2004). Here, we demonstrated that severe vascular hyporeactivity also occurs 6 h post-LPS administration. At this time point nitric oxide, superoxide anion, markers of circulating and cardiac peroxynitrite production, and proinflammatory cytokines are all significantly increased 6 h following LPS administration (Szabo et al., 1995; Khadour et al., 2002). Alterations of in vivo MMP activity were anticipated since MMPs and TIMPs are regulated by proinflammatory cytokines and peroxynitrite. Net gelatinolytic activity increased in aortae taken from LPS-treated animals. MMP-2 and -9 are largely responsible for gelatinolytic activity, however, the collagenases (MMP-1, -8, and -13) are also recognized to cleave gelatin in vitro. In order to determine if collagenases were contributing to the increase in net gelatinolytic activity, we also measured collagenolytic activity. The lack of increase in collagenolytic activity suggested that the measurable gelatinolytic activity was due to MMP-2 and -9 and not collagenases.
Increased aortic MMP-9 activity following LPS administration adds to mounting evidence implicating this MMP in in vivo models of endotoxaemia and septic shock. We previously demonstrated that circulating 92 kDa MMP-9 activity correlated inversely with mean arterial blood pressure in endotoxemic rats (Lalu et al., 2004). As well, in vivo administration of MMP inhibitors (doxycycline or Ro 31-9790) significantly decreased 92 kDa MMP-9 activity and improved cardiac mechanical dysfunction in the hearts from endotoxemic rats (Lalu et al., 2003). Opdenakker and co-workers have demonstrated that both MMP inhibition and MMP-9 gene deletion significantly protect mice against lethal doses of LPS (Dubois et al., 2002; Hu et al., 2005). A significant increase in circulating MMP-9 activity was also noted in human volunteers administered LPS (Albert et al., 2003). Finally, in septic shock patients, plasma MMP-9 protein correlated with circulating LPS concentration and was significantly higher in non-survivors than survivors (Nakamura et al., 1998).
A dysregulation of aortic TIMPs was also seen following LPS administration in vivo. Interestingly, TIMP-4 content was significantly decreased. These observations are similar to the acute loss of TIMP-4 seen in isolated perfused rat hearts following proinflammatory cytokine-mediated cardiac dysfunction (Gao et al., 2003) or in acute ischemia-reperfusion injury (Schulze et al., 2003). The increase in aortic TIMP-1 was not unexpected since it is regarded as an inducible TIMP (Brew et al., 2000). However, it should be noted that the ability of TIMP-1 to inhibit MMP activity may be diminished under conditions of enhanced oxidative stress characterized by increased peroxynitrite biosynthesis (Frears et al., 1996). Thus, even though TIMP-1 protein content was elevated, its net inhibitory effect may not have likewise increased.
The apparent discordance between protein levels (as measured by immunoblot) and activity (as measured by zymography) of both 72 kDa MMP-2 and 92 kDa MMP-9 was an interesting finding. This discordance might be attributable to peroxynitrite-induced activation of these enzymes (Okamoto et al., 2001). Peroxynitrite disrupts the MMP propeptide domain ‘cysteine switch' by S-glutathiolation of a critical cysteine residue in this domain, causing a conformational change resulting in an activated ‘proenzyme' (Rajagopalan et al., 1996; Okamoto et al., 2001). Thus, increased aortic peroxynitrite biosynthesis during endotoxaemia (Szabo et al., 1995) may have activated both the 72 kDa MMP-2 and the 92 kDa MMP-9 without loss of the propeptide. With such activation, an increase in MMP-9 activity could be detected in zymography despite unchanged protein content. Likewise, such activation could allow MMP-2 activity to appear equal between LPS and control aortae, despite a loss in MMP-2 protein content in the LPS aortae. Other explanations also exist for the observed discordance between zymography and immunoblot, such as possible epitope modification by peroxynitrite or protease activity.
Our study also addressed the functional significance of vascular hyporeactivity following in vivo LPS-mediated MMP-TIMP dysregulation. In vivo LPS administration produces a much more complex inflammatory stimuli than the ex vivo models used in this study. At 6 h after in vivo LPS administration, we found that ex vivo vascular reactivity was significantly impaired. Doxycycline significantly improved the vascular reactivity to phenylephrine. This improvement in contractility was elicited by an acute in vitro incubation with doxycycline (10 min) after the vascular dysfunction was already established. These results raise the possibility that MMP inhibition may be beneficial under in vivo conditions of inflammatory stress (e.g. sepsis) when blood pressure has fallen due to vascular hyporeactivty.
Overall, our results raise several interesting new questions. For instance, the specific target(s) of MMPs in these models of vascular hyporeactivity is unknown. A recent investigation by Chew et al. (2004) suggests that MMPs may interfere with Ca2+ entry. The addition of MMP-2 or MMP-9 to aortic strips isolated from normal rats blunted responses to phenylephrine and KCl, and at the same time interfered with entry of radiolabelled extracellular Ca2+. Whether this mechanism contributes to inflammation-associated hyporeactivity to vasoconstrictors remains to be determined.
Some possible limitations to these experiments should be considered. First is the use of micromolar amounts of GM6001, a concentration higher than the reported Ki of this drug when tested against isolated MMP-2 and MMP-9 proteins under cell-free conditions. Such micromolar concentrations are necessary to produce biological effects in intact cells and tissues (Hao et al., 2004; Haug et al., 2004). Thus, it is likely that higher concentrations of MMP inhibitors are required to produce their effects in a complex biological milieu. It should also be noted that, in cell culture models IL-1β is recognized to stimulate a variety of MMPs in vascular smooth muscle cells (Gurjar et al., 2001; Galis and Khatri, 2002) and endothelial cells (Rajavashisth et al., 1999). Thus, part of the protective effect of GM6001 could be explained by an inhibition of a variety of MMPs.
In summary, MMPs and TIMPs are acutely regulated in the vasculature during LPS- or IL-1β-mediated vascular hyporeactivity. MMP inhibitors are effective in preventing this vascular dysfunction. In vivo, acute endotoxaemia produces vascular dysfunction that is associated with an imbalance between MMPs and TIMPs. This vascular dysfunction was significantly ameliorated after incubation with an inhibitor of MMPs. These data suggest that MMPs play a role in acute inflammatory vascular dysfunction associated with conditions such as endotoxaemia.
Acknowledgments
This study was funded by a grant from the Heart and Stroke Foundation of Alberta, NWT, and Nunavut. Manoj Lalu was a graduate trainee supported by the Alberta Heritage Foundation for Medical Research (AHFMR) and the Canadian Institutes of Health Research. Jonathan Cena is a graduate trainee supported by the University of Alberta Faculty of Medicine Dentistry 75th Anniversary Award. Rezwan Chowdhury and Anna Lam were AHFMR summer students. Richard Schulz is an AHFMR scientist.
Abbreviations
- IL-1β
interleukin-1beta
- LPS
lipopolysaccharide
- MMP
matrix metalloproteinase
- TIMP
tissue inhibitor of matrix metalloproteinase
Conflict of interest
All authors state no conflict of interest.
References
- Albert J, Radomski A, Soop A, Sollevi A, Frostell C, Radomski MW. Differential release of matrix metalloproteinase-9 and nitric oxide following infusion of endotoxin to human volunteers. Acta Anaesthesiol Scand. 2003;47:407–410. doi: 10.1034/j.1399-6576.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good the bad, and the ugly. Am J Physiol Cell Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- Cheung P-Y, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation. 2000;101:1833–1839. doi: 10.1161/01.cir.101.15.1833. [DOI] [PubMed] [Google Scholar]
- Chew DK, Conte MS, Khalil RA. Matrix metalloproteinase-specific inhibition of Ca2+ entry mechanisms of vascular contraction. J Vasc Surg. 2004;40:1001–1010. doi: 10.1016/j.jvs.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Danner RL, Joiner KA, Rubin M, Patterson WH, Johnson N, Ayers KM, et al. Purification, toxicity, and antiendotoxin activity of polymyxin B nonapeptide. Antimicrob Agents Chemother. 1989;33:1428–1434. doi: 10.1128/aac.33.9.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Starckx S, Pagenstecher A, Oord J, Arnold B, Opdenakker G. Gelatinase B deficiency protects against endotoxin shock. Eur J Immunol. 2002;32:2163–2171. doi: 10.1002/1521-4141(200208)32:8<2163::AID-IMMU2163>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Fernandez-Patron C, Radomski MW, Davidge SM. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85:906–911. doi: 10.1161/01.res.85.10.906. [DOI] [PubMed] [Google Scholar]
- Fisher CJ, Jr, Slotman GJ, Opal SM, Pribble JP, Bone RC, Emmanuel G, et al. Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: a randomized, open-label, placebo-controlled multicenter trial. Crit Care Med. 1994;22:12–21. doi: 10.1097/00003246-199401000-00008. [DOI] [PubMed] [Google Scholar]
- Frears ER, Zhang Z, Blake DR, O'Connell JP, Winyard PG. Inactivation of tissue inhibitor of metalloproteinase-1 by peroxynitrite. FEBS Letters. 1996;381:21–24. doi: 10.1016/0014-5793(96)00065-8. [DOI] [PubMed] [Google Scholar]
- French JF, Lambert LE, Dage RC. Nitric oxide synthase inhibitors inhibit interleukin-1 beta-induced depression of vascular smooth muscle. J Pharmacol Exp Ther. 1991;259:260–264. [PubMed] [Google Scholar]
- Galardy RE, Grobelny D, Foellmer HG, Fernandez LA. Inhibition of angiogenesis by the matrix metalloprotease inhibitor N-[2R-2-(hydroxamidocarbonymethyl)-4-methylpentanoyl)]-L-tryptophan methylamide. Cancer Res. 1994;54:4715–4718. [PubMed] [Google Scholar]
- Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- Galis ZS, Muszynski M, Sukhova GK, Simon-Morrissey E, Unemori EN, Lark MW, et al. Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ Res. 1994;75:181–189. doi: 10.1161/01.res.75.1.181. [DOI] [PubMed] [Google Scholar]
- Gao CQ, Sawicki G, Suarez-Pinzon WL, Csont T, Wozniak M, Ferdinandy P, et al. Matrix metalloproteinase-2 mediates cytokine-induced myocardial contractile dysfunction. Cardiovasc Res. 2003;57:426–433. doi: 10.1016/s0008-6363(02)00719-8. [DOI] [PubMed] [Google Scholar]
- Garcia RA, Pantazatos DP, Gessner CR, Go KV, Woods VL, Jr, Villarreal FJ. Molecular interactions between matrilysin and the matrix metalloproteinase inhibitor doxycycline investigated by deuterium exchange mass spectrometry. Mol Pharmacol. 2005;67:1128–1136. doi: 10.1124/mol.104.006346. [DOI] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non- antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Gui Y, Zheng XL, Hollenberg MD. Interleukin-1beta, Src- and non-Src tyrosine kinases, and nitric oxide synthase induction in rat aorta in vitro. Am J Physiol Heart Circ Physiol. 2000;279:H566–H576. doi: 10.1152/ajpheart.2000.279.2.H566. [DOI] [PubMed] [Google Scholar]
- Gurjar MV, Deleon J, Sharma RV, Bhalla RC. Role of reactive oxygen species in IL-1 beta-stimulated sustained ERK activation and MMP-9 induction. Am J Physiol Heart Circ Physiol. 2001;281:H2568–H2574. doi: 10.1152/ajpheart.2001.281.6.H2568. [DOI] [PubMed] [Google Scholar]
- Hao L, Du M, Lopez-Campistrous A, Fernandez-Patron C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ Res. 2004;94:68–76. doi: 10.1161/01.RES.0000109413.57726.91. [DOI] [PubMed] [Google Scholar]
- Haug C, Lenz C, Diaz F, Bachem MG. Oxidized low-density lipoproteins stimulate extracellular matrix metalloproteinase Inducer (EMMPRIN) release by coronary smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:1823–1829. doi: 10.1161/01.ATV.0000142806.59283.11. [DOI] [PubMed] [Google Scholar]
- Hesse DG, Tracey KJ, Fong Y, Manogue KR, Palladino MA, Jr, Cerami A, et al. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet. 1988;166:147–153. [PubMed] [Google Scholar]
- Heussen C, Dowdle EB. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hu J, Van den Steen PE, Dillen C, Opdenakker G. Targeting neutrophil collagenase/matrix metalloproteinase-8 and gelatinase B/matrix metalloproteinase-9 with a peptidomimetic inhibitor protects against endotoxin shock. Biochem Pharmacol. 2005;70:535–544. doi: 10.1016/j.bcp.2005.04.047. [DOI] [PubMed] [Google Scholar]
- Javesghani D, Hussain SN, Scheidel J, Quinn MT, Magder SA. Superoxide production in the vasculature of lipopolysaccharide-treated rats and pigs. Shock. 2003;19:486–493. doi: 10.1097/01.shk.0000054374.88889.37. [DOI] [PubMed] [Google Scholar]
- Jeyabalan A, Novak J, Danielson LA, Kerchner LJ, Opett SL, Conrad KP. Essential role for vascular gelatinase activity in relaxin-induced renal vasodilation, hyperfiltration, and reduced myogenic reactivity of small arteries. Circ Res. 2003;93:1249–1257. doi: 10.1161/01.RES.0000104086.43830.6C. [DOI] [PubMed] [Google Scholar]
- Khadour FH, Panas D, Ferdinandy P, Schulze C, Csont T, Lalu MM, et al. Enhanced NO and superoxide generation in dysfunctional hearts from endotoxemic rats. Am J Physiol Heart Circ Physiol. 2002;283:H1108–H1115. doi: 10.1152/ajpheart.00549.2001. [DOI] [PubMed] [Google Scholar]
- Kim H, Koh G. Lipopolysaccharide activates matrix metalloproteinase-2 in endothelial cells through an NF-κB-dependent pathway. Biochem Biophys Res Commun. 2000;269:401–405. doi: 10.1006/bbrc.2000.2308. [DOI] [PubMed] [Google Scholar]
- Lalu MM, Csont T, Schulz R. Matrix metalloproteinase activities are altered in the heart and plasma during endotoxemia. Crit Care Med. 2004;32:1332–1337. doi: 10.1097/01.ccm.0000127778.16609.ec. [DOI] [PubMed] [Google Scholar]
- Lalu MM, Gao CQ, Schulz R. Matrix metalloproteinase inhibitors attenuate endotoxemia induced cardiac dysfunction: a potential role for MMP-9. Mol Cell Biochem. 2003;251:61–66. [PubMed] [Google Scholar]
- McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ebihara I, Shimada N, Shoji H, Koide H. Modulation of plasma metalloproteinase-9 concentrations and peripheral blood monocyte mRNA levels in patients with septic shock: effect of fiber-immobilized polymyxin B treatment. Am J Med Sci. 1998;316:355–360. doi: 10.1097/00000441-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- Ohlsson K, Bjork P, Bergenfeldt M, Hageman R, Thompson RC. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature. 1990;348:550–552. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Akaike T, Sawa T, Miyamoto Y, Van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem. 2001;276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- Okusawa S, Gelfand JA, Ikejima T, Connolly RJ, Dinarello CA. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988;81:1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paemen L, Jansen PM, Proost P, Van Damme J, Opdenakker G, Hack E, et al. Induction of gelatinase B and MCP-2 in baboons during sublethal and lethal bacteraemia. Cytokine. 1997;9:412–415. doi: 10.1006/cyto.1996.0183. [DOI] [PubMed] [Google Scholar]
- Pagenstecher A, Stalder AK, Kincaid CL, Volk B, Campbell IL. Regulation of matrix metalloproteinases and their inhibitor genes in lipopolysaccharide-induced endotoxemia in mice. Am J Pathol. 2000;157:197–210. doi: 10.1016/S0002-9440(10)64531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugin J, Widmer MC, Kossodo S, Liang CM, Preas HLn, Suffredini AF. Human neutrophils secrete gelatinase B in vitro and in vivo in response to endotoxin and proinflammatory mediators. Am J Respir Cell Mol Biol. 1999;20:458–464. doi: 10.1165/ajrcmb.20.3.3311. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavashisth TB, Liao JK, Galis ZS, Tripathi S, Laufs U, Tripathi J, et al. Inflammatory cytokines and oxidized low density lipoproteins increase endothelial cell expression of membrane type 1-matrix metalloproteinase. J Biol Chem. 1999;274:11924–11929. doi: 10.1074/jbc.274.17.11924. [DOI] [PubMed] [Google Scholar]
- Rees DD, Cellek S, Palmer RM, Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990;173:541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- Rees DD, Monkhouse JE, Cambridge D, Moncada S. Nitric oxide and the haemodynamic profile of endotoxin shock in the conscious mouse. Br J Pharmacol. 1998;124:540–546. doi: 10.1038/sj.bjp.0701815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang QX, Stetler-Stevenson WG, Liotta LA, Byers SW. Identification of type IV collagenase in rat testicular cell culture: influence of peritubular-Sertoli cell interactions. Biol Reprod. 1990;43:956–964. doi: 10.1095/biolreprod43.6.956. [DOI] [PubMed] [Google Scholar]
- Sawicki G, Leon H, Sawicka J, Sariahmetoglu M, Schulze CJ, Scott PG, et al. Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury: a new intracellular target for matrix metalloproteinase-2. Circulation. 2005;112:544–552. doi: 10.1161/CIRCULATIONAHA.104.531616. [DOI] [PubMed] [Google Scholar]
- Sawicki G, Salas E, Murat J, Miszta-Lane H, Radomski MW. Release of gelatinase A during platelet activation mediates aggregation. Nature. 1997;386:616–619. doi: 10.1038/386616a0. [DOI] [PubMed] [Google Scholar]
- Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998;161:3340–3346. [PubMed] [Google Scholar]
- Schulz R, Nava E, Moncada S. Induction and potential biological relevance of Ca2+-independent nitric oxide synthase in the myocardium. Br J Pharmacol. 1992;105:575–580. doi: 10.1111/j.1476-5381.1992.tb09021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze CJ, Wang W, Suarez-Pinzon WL, Sawicka J, Sawicki G, Schulz R. Imbalance between tissue inhibitor of metalloproteinase-4 and matrix metalloproteinases during acute myocardial ischemia-reperfusion injury. Circulation. 2003;107:2487–2492. doi: 10.1161/01.CIR.0000065603.09430.58. [DOI] [PubMed] [Google Scholar]
- Suffredini AF, Fromm RE, Parker MM, Brenner M, Kovacs JA, Wesley RA, et al. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989;321:280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- Szabo C, Salzman AL, Ischiropoulos H. Endotoxin triggers the expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in the rat aorta in vivo. FEBS Lett. 1995;363:235–238. doi: 10.1016/0014-5793(95)00322-z. [DOI] [PubMed] [Google Scholar]
- Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JR, Sawicki G, Schulz R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation. 2002;106:1543–1549. doi: 10.1161/01.cir.0000028818.33488.7b. [DOI] [PubMed] [Google Scholar]
- Xie B, Dong Z, Fidler IJ. Regulatory mechanisms for the expression of type IV collagenases/gelatinases in murine macrophages. J Immunol. 1994;152:3637–3644. [PubMed] [Google Scholar]