Abstract
Background and purpose:
In our previous study (see accompanying paper) we observed that all-trans retinoic acid (ATRA) p.o. induces changes in spinal cord neuronal responses similar to those observed in inflammation-induced sensitization. In the present study we assessed the it. effects of ATRA, and its mechanisms of action.
Experimental approach:
The effects of all drugs were studied after it. administration in nociceptive withdrawal reflexes using behavioural tests in awake male Wistar rats.
Key results:
The administration of ATRA in normal rats induced a dose-dependent enhancement of nociceptive responses to noxious mechanical and thermal stimulation, as well as responses to innocuous stimulation. The intensity of the responses was similar to that observed in non-treated animals after carrageenan-induced inflammation. The effect induced by ATRA was fully prevented by the previous administration of the retinoic acid receptor (RAR) pan-antagonist LE540 but not by the retinoid X receptor (RXR) pan-antagonist HX531, suggesting a selective action on spinal cord RARs. The COX inhibitor dexketoprofen and the interleukin-1 receptor antagonist IL-1ra inhibited ATRA effect. The results indicate that COX and interleukin-1 are involved in the effects of ATRA in the spinal cord, similar to that seen in inflammation.
Conclusions and implications:
In conclusion, ATRA induces changes in the spinal cord similar to those observed in inflammation. The sensitization-like effect induced by ATRA was mediated by RARs and associated with a modulation of COX-2 and interleukin-1 activities. ATRA might be involved in the mechanisms underlying the initiation and/or maintenance of sensitization in the spinal cord.
Keywords: vitamin A, retinoic acid, pain, hyperalgesia, COX, interleukin-1β, inflammation, spinal cord, intrathecal cannulation
Introduction
Spinal cord sensitization, usually as a consequence of an inflammatory reaction, results in an altered activity of nociceptive neurons that leads to the phenomena of allodynia and hyperalgesia. Changes in the activity of nociceptive neurons involve numerous mechanisms and neuromediators whose release triggers and maintains a state of high neuronal activity. As stated in the first part of this study (see accompanying paper), inflammation-induced sensitization is related with a rapid enhancement of the expression of cyclooxygenase-2 (COX-2; Vane et al., 1998) in the spinal cord (Samad et al., 2001), as well as an upregulation of interleukin-1β (IL-1; Maier et al., 1990; Watkins et al., 1994; Safieh-Garabedian et al., 1995; Samad et al., 2001). The intrathecal (it.) administration of recombinant IL-1 receptor antagonist (IL-1ra) only 30 min before the induction of inflammation reduces drastically the level of COX-2 mRNA, suggesting that IL-1 is responsible, at least in part, for central transcriptional activation of COX-2 after peripheral inflammation (Samad et al., 2001).
However, sensitization is a complex phenomenon far from being fully understood. For example, the mechanisms underlying the upregulation of IL-1 or COX-2 are not known and it seems that some endogenous systems involved in the initiation of sensitization are still unidentified. Retinoids might be one of these unidentified systems.
Our previous study (part I, see accompanying paper) shows that the oral treatment with all-trans retinoic acid (ATRA) in rats induces a sensitization-like effect on spinal cord neuronal responses similar to that observed in animals with inflammation, that is, a decrease of thresholds to natural and electrical stimulation and an enlargement of cutaneous receptive fields (Woolf, 1983; McMahon and Wall, 1984; Schaible and Schmidt, 1985; Laird and Cervero, 1989; Woolf and King, 1990; Dubner and Ruda, 1992; Treede et al., 1992). The effects of ATRA might explain the enhancement of allodynia and hyperalgesia observed in previously published experiments (Romero-Sandoval et al., 2004).
The aim of the present experiments was to answer four questions raised from the results observed in our previous study on the sensitization-like activity of ATRA after oral administration: (i) Is the sensitization-like effect of ATRA the result of an action on spinal cord and/or peripheral tissues, that is nociceptors, or is it an action mainly located in the spinal cord? (ii) Is the pronociceptive activity of ATRA unspecific or is it mediated by the retinoic acid receptor (RAR) and/or retinoid X receptor (RXR)? (iii) If the pronociceptive effect of ATRA increases the expression of COX enzymes, would a COX inhibitor block this effect within the spinal cord? (iv) Is the pronociceptive activity of ATRA only associated with an overexpression of COX enzymes or is there another different mechanism associated with the effect?
In order to answer these questions, we have carried out some behavioural experiments assessing the effects of it. ATRA, RAR and RXR antagonists, the non-selective COX inhibitor dexketoprofen and an IL-1 receptor antagonist. Preliminary data have been published in an abstract form (Molina et al., 2005). We conclude that ATRA induces changes in the spinal cord similar to those observed in inflammation. The sensitization-like effect induced by ATRA was mediated by RARs and was associated with a modulation of COX-2 and IL-1 activities. ATRA might be involved in the mechanisms underlying the initiation and/or maintenance of sensitization in the spinal cord.
Methods
Animals
The experiments were carried out in accordance with the European Union legislation and were approved and supervised by the University Animal Care facility. All efforts were made to minimize animal suffering and to reduce the number of animals used. The experiments were performed in male Wistar rats weighing 215–330 g housed individually in cages and maintained on a 12 h light–dark cycle. The animals had free access to food and water at all times and were allowed to habituate for 5 days to the testing environment and for at least 15 min to the experimenter before the test started. The animals were used for one procedure only and were humanely killed on completion of the experiments by an overdose of sodium pentobarbital (Euta-Lender, Normon Lab., Madrid, Spain).
It. catheter implantation
All drugs were administered it. Chronic lumbar catheters were implanted in rats under ketamine/xylazine anaesthesia (2:1; i.p. 0.1 ml, 100 g−1: 0.05 ml, 100 g−1) following the procedure described by Yaksh and Rudy (1976). A 7.6 cm long polyethylene catheter (PE-5) was inserted through an incision in the atlanto-occipital membrane and advanced caudally into the it. space terminating at the L1–3 spinal segments. The end of the catheter was tunnelled subcutaneously over the front dorsal skull bones and held in this place with dental acrylic. Cefazolin (0.08 ml, 100 g−1) was administered after surgery to prevent post-operative infection. Rats were housed individually after implantation under the same conditions described above. It. catheters were carried for at least 5 days after implantation. Rats showing motor weakness or signs of paresis upon recovery from anaesthesia were euthanized immediately. Location of catheters was assessed on completion of experiments by injecting 7 μl of pontamine sky blue (4% in 0.5 M sodium acetate; Sigma, St Louis, MO, USA).
It. drugs and experimental groups
The drugs studied were prepared fresh everyday, immediately before administration, and were diluted in 0.9% saline and injected it. in a total volume of 7 μl followed by another 5 μl of saline to flush the catheter. A summary of experimental protocols and groups of animals is shown in Table 1. ATRA (Tretinoin, Sigma) was dissolved in 100% ethanol and its effect studied at doses of 2.5, 5 and 10 ng, at 0, 15, 30, 60 and 90 min after administration. The possible effect of the solvent was studied in an independent group of experiments (see Table 1 and below). In order to compare the level of the sensitization-like effect observed after the administration of ATRA with the sensitization observed using a standard inflammatory condition, we carried out some experiments studying the increment of responses in animals with carrageenan-induced soft-tissue inflammation without any treatment, following the standard technique used previously in our laboratory (Ramos-Zepeda et al., 2004; Romero-Sandoval et al., 2004). Soft-tissue inflammation was induced under brief halothane anaesthesia (5% in oxygen for induction, 2% for maintenance) by the intraplantar administration of 100 ml of carrageenan λ (10 mg ml−1 in distilled water; Sigma) in the right hind paw (Table 1), 15 h before the experiment. Another 100 μl of saline was injected in the left hind paw as a control for inflammation. The effectiveness of carrageenan in the induction of inflammation was assessed by measuring the volume of the paw by plethysmometry (Letica plethysmometer) before the administration of carrageenan or saline, and after each of the tests performed.
Table 1.
Groups of animals and experimental protocols
| Group | Drug 1 | Dose | Time gap | Drug 2 | Dose | N |
|---|---|---|---|---|---|---|
| ATRA |
ATRA |
2.5 ng |
— |
— |
— |
12 |
| |
ATRA |
5 ng |
— |
— |
— |
12 |
| |
ATRA |
10 ng |
— |
— |
— |
14 |
| |
Solvent |
7 μl |
— |
— |
— |
10 |
| |
|
|
|
|
|
|
| Inflammation |
— |
— |
— |
— |
— |
12 |
| |
|
|
|
|
|
|
| RAR |
LE540 |
2 ng |
— |
— |
— |
6 |
| |
LE540 |
2 ng |
10 |
ATRA |
10 ng |
12 |
| |
Solvent |
7 μl |
10 |
ATRA |
10 ng |
6 |
| |
|
|
|
|
|
|
| RXR |
HX531 |
2 ng |
10 |
ATRA |
10 ng |
6 |
| |
HX531 |
4 ng |
10 |
ATRA |
10 ng |
6 |
| |
|
|
|
|
|
|
| DKT |
DKT |
4 μg |
10 |
ATRA |
10 ng |
4 |
| |
DKT |
6 μg |
10 |
ATRA |
10 ng |
12 |
| |
DKT |
8 μg |
10 |
ATRA |
10 ng |
6 |
| |
DKT |
6 μg |
— |
— |
— |
6 |
| |
DKT |
8 μg |
— |
— |
— |
6 |
| |
Solvent |
7 μl |
10 |
ATRA |
10 ng |
6 |
| |
|
|
|
|
|
|
| IL-1 |
IL-1ra |
20 μg |
— |
— |
— |
4 |
| |
IL-1ra |
10 μg |
— |
— |
— |
8 |
| |
ATRA |
10 ng |
15 |
IL-1ra |
10 μg |
12 |
| ATRA | 10 ng | 15 | Solvent | 7 μl | 12 |
The effect of it. administered drugs was studied on withdrawal reflexes evoked by low- and high-intensity mechanical stimulation and by high-intensity thermal stimulation in male Wistar rats. ATRA was studied at doses of 2.5, 5 and 10 ng and its effect was compared to that induced by carrageenan-induced soft-tissue inflammation (inflammation). The effect of ATRA was challenged by the RAR pan-antagonist LE540, the RXR pan-antagonist HX531, the COX inhibitor DKT and the IL-1ra. Control experiments were made with the solvents used and by the administration of the antagonists in the absence of ATRA.
Abbreviations: ATRA, all-trans retinoic acid; COX, cyclooxygenase; DKT, dexketoprofen; IL-1ra, interleukin-1 receptor antagonist; it., intrathecal; RAR, retinoic acid receptor; RXR, retinoid X receptor.
The effect of ATRA on retinoic acid and retinoids receptors was studied by challenging its activity with the RAR pan-antagonist LE540 (2 ng dissolved in DMSO, Kagechika, 2002), and the RXR pan-antagonist, HX531 (2 and 4 ng dissolved in DMSO, Ebisawa et al., 1999). Both antagonists were kindly donated by H Kagechika (University of Tokyo). As in the previous group, the effect of the solvent was also studied in a different group of animals (Table 1). The antagonists and vehicle were administered 10 min before administration of 10 ng of ATRA.
The possible activity of ATRA on COX enzymes was evaluated with the non-selective COX inhibitor dexketoprofen (DKT; NicOx S.A.), at doses of 4, 6 and 8 μg (dissolved in DMSO). As in previous experiments, DKT was injected 10 min before the administration of 10 ng of ATRA. In addition, separate experiments were conducted to test the possible effect of 6 and 8 μg of DKT in the absence of any other drug, as well as the possible effect of the solvent (Table 1). Finally, the possible modulation of ATRA on IL-1 activity was studied by challenging the effect of 10 ng of ATRA with the human recombinant IL-1ra (Amgen, Thousand Oaks, CA, USA). IL-1ra, at doses of 10 and 20 μg, was dissolved in SCE buffer (10 mM citrate, 140 nM NaCl, 0.5 mM EDTA, pH 7.0) and was studied alone or when administered 15 min after 10 ng of ATRA (Table 1). Control experiments were made with the solvent in an independent experimental group.
Analysis of nociception
Thermal hyperalgesia was assessed by measuring paw withdrawal latencies to 55°C radiant heat generated by an algesimeter (Ugo Basile plantar test; Hargreaves et al., 1988). Animals were placed in a clear plastic chamber and allowed to accommodate to the testing apparatus for 5 min. Two consecutive thermal stimuli were applied to each of the paws with an interval of 2–3 min between tests. The maximum cutoff time was set to 15 s to avoid tissue damage. Frequency of withdrawal reflex responses to mechanical stimulation was studied by applying Von Frey filaments. A response was considered as positive when a withdrawal of the paw due to the application of the stimulus was observed. The methods were adapted from those reported by Gilchrist et al. (1996), and have been described in detail elsewhere (Mazario et al., 2001; Lahdesmaki et al., 2003). The rats were placed on a raised wire mesh grid under plastic chambers. Low-intensity mechanical stimulation, which hardly evokes a withdraw in a normal animal (Romero-Sandoval et al., 2004), was applied with 60 and 80 mN hairs. High-intensity stimulation was carried out by means of 100, 200 and 300 filaments. Each filament was applied 10 times for approximately 2 s to the plantar surface of each hind paw and the number of positive responses counted. The experimenter was always blind to the treatment of animals.
Data analysis and statistics
Withdrawal responses were converted, for comparison between groups, to percentage of maximum possible effect (%MPE; Harris and Pierson, 1964; Romero-Sandoval et al., 2005) according to the following formula: %MPE=100 × ((post-drug value−pre-drug value)/(Max. effect−pre-drug value)), where the post-drug value represents the latency (in the case of thermal stimulation) or the number of positive responses (in the case of von Frey filaments stimuli) for a specific dose at a specific time after drug administration; pre-drug value is the latency or number of positive responses before drug was given. In the present experiments, maximal effect was 0 s, as the minimum latency that can possibly be observed with thermal stimulation if the drug induces a pronociceptive effect, whereas 20 and 30 are the maximum number of positive responses that can possibly be observed with low-intensity mechanical stimulation (10 times for each filament of 60 and 80 mN) and high-intensity mechanical stimulation (10 times for each filament of 100, 200 and 300 mN). The increase in the number of responses observed with mechanical stimulation, and the reduction of latency to thermal stimulation were considered as a pronociceptive effect. All the data are presented as mean±s.e.m. Statistical significance was calculated using the one-way analysis of variance (ANOVA) with post hoc Dunnett's test (GraphPad Prism and GraphPad Instat for Windows). Differences were considered to be significant when the critical value reached a level of P<0.05.
Results
Effect of ATRA
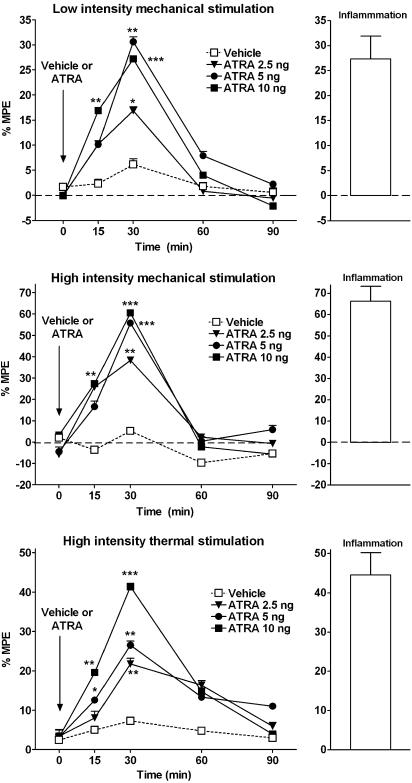
It. injections of ATRA (2.5 ng, n=12; 5 ng, n=12; and 10 ng, n=14) increased the number of responses to both low- and high-intensity mechanical stimulation, and reduced the latency to noxious thermal stimulation significantly (Figure 1). The effect peaked 30 min after administration, although it was significant at 15 min after injection in some tests (Figure 1), and recovered at 60 min post-injection. Maximal changes of responses observed were 31±3% (P<0.01) for low-intensity mechanical stimulation, 60±2% (P<0.001) for high-intensity mechanical stimulation and 41±2% (P<0.001) for noxious thermal stimulation. The effect was dose-dependent, although a plateau was observed with 5 ng of ATRA in response to mechanical stimulation. It. injection of ATRA vehicle (n=10) did not modify any of the responses studied. These results indicate that the it. administration of ATRA induces a significant increment of nociceptive responses to noxious mechanical and thermal stimulation, and evokes nociceptive responses with low-intensity mechanical stimulation. The results were compatible with the phenomena of allodynia and hyperalgesia observed in inflammation-induced sensitization. In order to compare if the magnitude of the responses were similar to that seen during inflammation, we carried out similar experiments in animals with carrageenan-induced soft-tissue inflammation (Figure 1). The volume of the paw before the induction of inflammation was 1.6±0.1 ml. The injection of carrageenan induced a significant increment of the volume of the paw, when measured after the tests: 2.6±0.1 ml (P<0.01). In these experiments, the maximal increment of responses was of 27±4% (vs 31±3% in ATRA treatment) for low-intensity mechanical stimulation, 66±7% (vs 60±2% in ATRA treatment) for high-intensity mechanical stimulation and 44±5% (vs 41±2% in ATRA treatment) for noxious thermal stimulation (Figure 1). As indicated, the increment of nociceptive responses observed in inflammation was very similar to that seen after the it. administration of ATRA in normal animals. A visual inspection of all animals was made throughout the tests, in order to check for any possible signs of toxicity or pain behaviour. An increase in aggressiveness or irritability, hair loss, hyperactivity, lethargy or signs of fatigue or hyperactivity or any other behaviour compatible with pain or discomfort was never observed, indicating that ATRA is devoid of a proalgesic action on its own when injected in the cord, and the effect observed is more compatible with sensitization, which is evoked by mechanical, thermal or electrical stimulation, but not as spontaneous pain.
Figure 1.
Effect of it. administration of ATRA or equivalent amounts of vehicle in responses to low-intensity (innocuous) and high-intensity (noxious) mechanical and noxious thermal stimulation. An increase in percentage of maximal possible effect (MPE; positive values) indicates an enhancement of nociceptive responses. Direct spinal cord administration of ATRA induced a significant enhancement of withdrawal responses to innocuous (allodynia) and noxious (hyperalgesia) stimulation. The effect peaked 30 min after administration and recovered 60 min post-administration. The results indicate an effect of ATRA in the spinal cord of normal rats similar to that observed in rats with carrageenan-induced inflammation (inflammation). P<0.05 (*), P<0.01 (**), P<0.001 (***), one-way analysis of variance (ANOVA) with post hoc Dunnett's test. Comparison of each drug time point vs that of vehicle.
Interaction with retinoic acid and retinoid X receptors
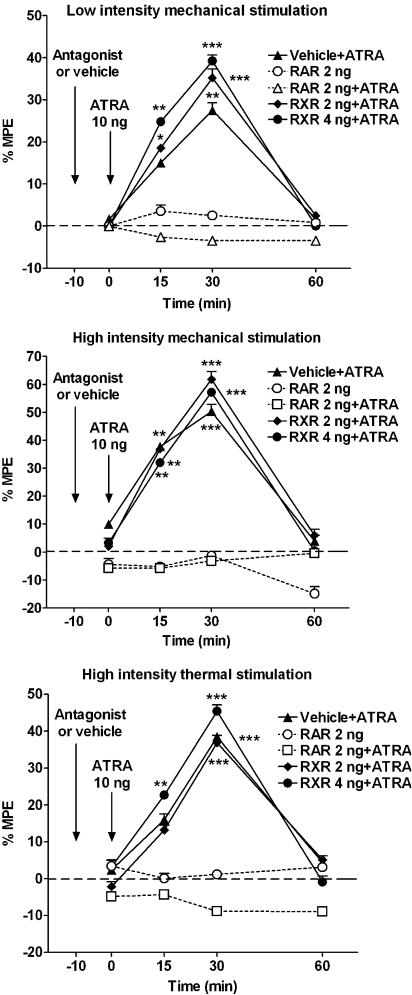
It is possible, however, that the effects observed after the administration of ATRA were not specific, that is, due to a simple chemical interference with cell membranes or neurotransmitters. In order to reject this possibility, we studied the effect of selective antagonists for RAR and RXR on ATRA effect. In these experiments, the it. injection of the RAR pan-antagonist LE540 (2 ng; n=6) did not modify any of the responses studied when injected alone (Figure 2). However, the same dose of LE540 injected it. 10 min before administration of ATRA (n=12) resulted in a complete inhibition of ATRA-induced sensitization effect in all tests (Figure 2). The effect of ATRA on RAR seems to be selective as the it. injection of 2 ng (n=6) or 4 ng (n=6) of the RXR pan-antagonist HX531 did not modify ATRA activity (Figure 2). Likewise, the effect of ATRA was not modified by the previous injection of the antagonists solvent (DMSO, n=6; Figure 2).
Figure 2.
Effect of it. RAR and RXR pan-antagonists on ATRA-mediated sensitization. The administration of it. 2 ng of the RAR pan-antagonist LE540 (RAR), 10 min before the administration of 10 ng of ATRA, fully blocked the effect of ATRA. The administration of 2 ng of LE540 alone did not modify the nociceptive responses. However, the it. administration of 2 or 4 ng of the RXR pan-antagonist HX531 (RXR) did not modify the effect of ATRA. The results indicate that the sensitization-like effect induced by ATRA was mediated by the interaction with spinal cord RA receptors. Statistical comparison and layout as for Figure 1.
Inhibition of COX enzyme activity
As the results seem to indicate that the sensitization-like effect induced by ATRA takes place within the spinal cord through an action on RARs, and is associated with an upregulation of COX enzymes (see part I of this study in the accompanying paper), we wondered if a commonly used COX inhibitor like dexketoprofen would inhibit its effect within the spinal cord. This would also demonstrate that upregulated COX-2 was functionally active.
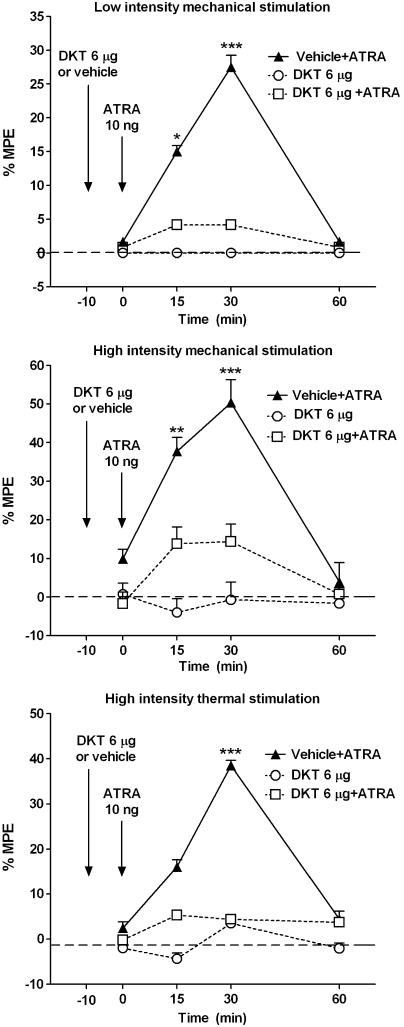
Preliminary studies with it. injection of the non-selective COX inhibitor DKT showed that a dose of 4 μg (n=4) did not modify the activity evoked by it. ATRA. A dose of 8 μg of DKT (n=6) fully blocked the activity of ATRA, although it also induced (n=6) a reduction in responses that might misinterpret a possible inhibition on ATRA-induced effect. However, the it. administration of 6 μg (n=6) of DKT did not show any significant activity on behavioural tests when injected alone (Table 1; Figure 3). The same dose injected 10 min before administration of ATRA (n=12) fully inhibited ATRA-induced sensitization effect (Figure 3), supporting an interaction of ATRA with the activity of COX enzymes. The effect of ATRA was not modified by equivalent amounts of the solvent used (DMSO, n=6).
Figure 3.
Effect of the COX inhibitor dexketoprofen (DKT) on ATRA-mediated sensitization. The it. administration of 6 μg of DKT 10 min before administration of 10 ng of ATRA fully blocked the effect induced by ATRA. The same dose of DKT did not modify the nociceptive responses when injected alone. The results confirm that the effect of ATRA is mediated by a modulation of the COX activity within the spinal cord. The administration of DKT vehicle did not modify ATRA activity. Statistical comparison and layout as for Figure 1.
Inhibition of IL-1 receptor
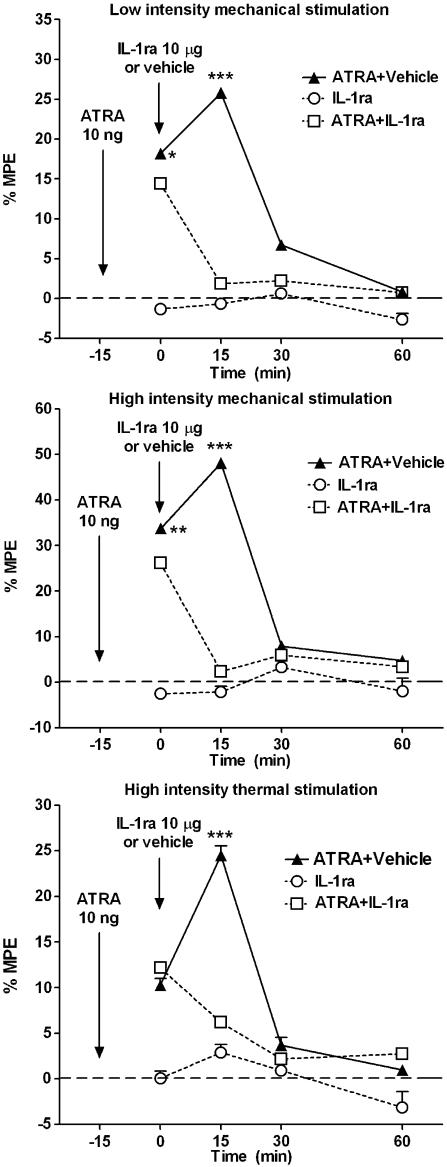
Finally, as the upregulation of COX enzymes, mainly COX-2, during central sensitization is followed by an upregulation of IL-1 expression in the spinal cord, as quoted in the introduction (Samad et al., 2001), we studied the effect of the it. IL-1 receptor antagonist IL-1ra (Amgen) on the ATRA-induced sensitization-like effect. IL-1ra was injected it. 15 min after the administration of ATRA. Preliminary experiments showed that a dose of 20 μg of IL-1ra (n=4) depressed the responses to both mechanical and thermal stimulation. However, a dose of it. 10 μg of IL-1ra (n=8) did not change the baseline data when injected alone (Figure 4). We therefore chose the dose of 10 μg of IL-1ra to challenge the effect of 10 ng of ATRA. This dose (n=12), when injected it. 15 min after ATRA, fully and quickly reversed ATRA-induced sensitization effect (Figure 4), indicating that the effect induced by ATRA is associated not only with the expression of COX enzymes but also with a modulation of the IL-1 receptor. Equivalent amounts of the IL-1ra solvent did not modify the activity of ATRA (n=12).
Figure 4.
Effect of IL-1ra on ATRA-mediated sensitization. The it. administration of 10 μg of the IL-1β antagonist (IL-1ra) 15 min after the administration of ATRA fully reversed the effect of ATRA, indicating that the sensitization induced by ATRA was not only mediated by COX activity but also by the IL-1 system. The same amount of the antagonist did not cause any effect on the withdrawal responses when injected alone. In addition, the administration of IL-1ra vehicle did not modify ATRA activity. Statistical comparison and layout as for Figure 1.
Discussion
The results from part I of the present study (see accompanying paper) showed that the administration of oral ATRA induces in the spinal cord neuronal activity changes identical to those seen in inflammation-induced sensitization. The effect of sensitization induced by ATRA in these experiments might be the explanation for the enhancement of allodynia and hyperalgesia observed in behavioural experiments performed in our lab (Romero-Sandoval et al., 2004). In addition, the experiments showed that the sensitization induced by ATRA was associated with a clear enhancement of the expression of COX-2, but not COX-1, in the spinal cord. Therefore, an enhancement of COX-2 expression in the spinal cord might explain the sensitization observed after the administration of ATRA in electrophysiological experiments, both in normal and inflamed animals.
The observations made in the present behavioural experiments support the findings described in part I of this study (see accompanying paper). In this case, the administration of it. ATRA induced nociceptive withdrawal responses to low-intensity (innocuous) mechanical stimulation, an effect similar to that observed during inflammation-induced sensitization and which is termed allodynia, defined in humans as pain due to a stimulus that does not normally provoke pain (IASP, 1994). Furthermore, withdrawal responses to noxious mechanical and thermal stimulation were significantly and greatly enhanced, in a similar way to that seen under inflammation-induced sensitization. The increment of responses to noxious stimulation, usually under an inflammation process, is termed hyperalgesia (IASP, 1994). These experiments correlate behaviourally the reduction of threshold intensity and the enlargement of receptive fields in spinal cord neurons observed in the electrophysiological experiments as does inflammation-induced sensitization in similar experiments (Woolf, 1983; McMahon and Wall, 1984; Schaible and Schmidt, 1985; Treede et al., 1992). In addition, our results demonstrate that the effect of ATRA was mainly located in the spinal cord and, therefore, the central actions of ATRA could substantially account for the sensitization effect. As this effect in the spinal cord was inhibited by blocking RARs but not RXRs, we may conclude that ATRA effect was selectively mediated by RA receptors located in the spinal cord.
It is important to note that the it. administration of ATRA was not followed by abnormal behaviour. The animals did not show any behaviour compatible with pain or discomfort, suggesting that ATRA on its own does not induce any proalgesic action or pain in the present experimental conditions. However, the fact that responses to either low- or high-intensity stimulation were enhanced, in a similar way to the increment observed in situations of central sensitization, indicates that ATRA might be related to some of the mechanisms involved in the initiation and/or maintenance of the process of central sensitization. Among these mechanisms are the positive modulation of the activity of COX enzymes and interleukins as mentioned in the introduction (Samad et al., 2001). The mechanisms of action involved in the effect of sensitization produced by ATRA seem to be similar to some of the mechanisms underlying inflammation-induced sensitization: an upregulation of the expression of COX-2 enzyme, which was functionally active as DKT, a well-known non-selective COX inhibitor, inhibited the effect, and an activation of IL-1β, as IL-1ra clearly inhibited the sensitization produced by ATRA. Retinoids enhance the expression of COX-2 and interleukin-1β gene in peripheral tissues (Nusing et al., 1995; Kanekura et al., 2000; Li et al., 2002; Liu and Gudas, 2002) but, to our knowledge, this is the first time that the two mechanisms of action of ATRA are described in the rat spinal cord.
In conclusion, the present findings support the assertion that the vitamin A metabolite ATRA induces changes in the spinal cord neurons and in behavioural experiments similar to and compatible with those observed in inflammation-induced sensitization. The effect of sensitization induced by ATRA is mediated by an interaction with RARs and associated with a modulation of COX-2 and IL-1 activities. ATRA might be involved in the mechanisms underlying the initiation and/or maintenance of sensitization in the spinal cord, responsible for the allodynia and hyperalgesia phenomena associated with inflammatory and neuropathic pains in humans.
Acknowledgments
We thank Dr H Kagechika; University of Tokyo for the kind donation of the RAR and RXR pan antagonists LE540 and HX531; to Amgen for the kind donation of the IL-1 receptor antagonist IL-1ra, and NicOx SA for the kind donation of dexketoprofen. This work was supported by grants from the Spanish Ministerio de Educacion y Ciencia (Grants SAF2005-06242-C03-01 and 03). We also thank Mr Lawrence Baron for English and scientific revisions of the manuscript. M Alique is a fellow of the Spanish Ministry of Science and Technology.
Abbreviations
- ATRA
all-trans retinoic acid
- COX
cyclooxygenase
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
Conflicts of interest
The authors state no conflict of interest.
References
- Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Ebisawa M, Umemiya H, Ohta K, Fukasawa H, Kawachi E, Christoffel G, et al. Retinoid X receptor-antagonistic diazepinylbenzoic acids. Chem Pharm Bull (Tokyo) 1999;47:1778–1786. doi: 10.1248/cpb.47.1778. [DOI] [PubMed] [Google Scholar]
- Gilchrist DH, Allard BL, Simone DA. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain. 1996;67:179–188. doi: 10.1016/0304-3959(96)03104-1. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Harris LS, Pierson AK. Some narcotic antagonists in the benzomorphan series. J Pharmacol Exp Ther. 1964;143:141–148. [PubMed] [Google Scholar]
- IASP (International Association for the Study of Pain) Classification of chronic pain IASP Task Force on Taxonomy 1994IASP Press: Seattle; 209–214.Merskey H., Bogduk N. (eds)2nd edn [Google Scholar]
- Kagechika H. Novel synthetic retinoids and separation of the pleiotropic retinoidal activities. Curr Med Chem. 2002;9:591–608. doi: 10.2174/0929867024606975. [DOI] [PubMed] [Google Scholar]
- Kanekura T, Higashi Y, kanzaki T. Inhibitory effects of 9-cis-retinoic acid and pyrrolidinedithiocarbamate on cyclooxygenase (COX)-2 expression and cell growth in human skin squamous carcinoma cells. Cancer Lett. 2000;161:177–183. doi: 10.1016/s0304-3835(00)00604-2. [DOI] [PubMed] [Google Scholar]
- Lahdesmaki J, Scheinin M, Pertovaara A, Mansikka H. The a2A-adrenoceptor subtype is not involved in inflammatory hyperalgesia or morphine-induced antinociception. Eur J Pharmacol. 2003;468:183–189. doi: 10.1016/s0014-2999(03)01677-7. [DOI] [PubMed] [Google Scholar]
- Laird JM, Cervero F. A comparative study of the changes in receptive-field properties of multireceptive and nocireceptive rat dorsal horn neurons following noxious mechanical stimulation. J Neurophysiol. 1989;62:854–863. doi: 10.1152/jn.1989.62.4.854. [DOI] [PubMed] [Google Scholar]
- Li M, Song S, Lippman SM, Zhang XK, Liu X, Lotan R, et al. Induction of retinoic acid receptor-beta suppresses cyclooxygenase-2 expression in esophageal cancer cells. Oncogene. 2002;21:411–418. doi: 10.1038/sj.onc.1205106. [DOI] [PubMed] [Google Scholar]
- Liu L, Gudas LJ. Retinoic acid induces expression of the interleukin-1beta gene in cultured normal human mammary epithelial cells and in human breast carcinoma lines. J Cell Physiol. 2002;193:244–252. doi: 10.1002/jcp.10173. [DOI] [PubMed] [Google Scholar]
- Maier JA, Hla T, Maciag T. Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. J Biol Chem. 1990;265:10805–10808. [PubMed] [Google Scholar]
- Mazario J, Gaitan G, Herrero JF. Cicloxygenase-1 versus cicloxygenase-2 inhibitors in the induction of antinociception in rodent withdrawal reflexes. Neuropharmacology. 2001;40:937–945. doi: 10.1016/s0028-3908(01)00020-x. [DOI] [PubMed] [Google Scholar]
- Mcmahon SB, Wall PD. Receptive fields of rat lamina 1 projection cells move to incorporate a nearby region of injury. Pain. 1984;19:235–247. doi: 10.1016/0304-3959(84)90002-2. [DOI] [PubMed] [Google Scholar]
- Molina C, Alique M, Romero-Sandoval A, Lucio J, Herrero JF.Retinoic acid enhances nociceptive responses after oral and intrathecal administration. Society for Neuroscience 2005. 35th Annual Meeting in Washington, DC, November. SFN Abstracts12: 16
- Nusing RM, Mohr S, Ullrich V. Activin A and retinoic acid synergize in cyclooxygenase-1 and thromboxane synthase induction during differentiation of J774.1 macrophages. Eur J Biochem. 1995;227:130–136. doi: 10.1111/j.1432-1033.1995.tb20368.x. [DOI] [PubMed] [Google Scholar]
- Ramos-Zepeda G, Schroder W, Rosenow S, Herrero JF. Spinal vs. supraspinal antinociceptive activity of the adenosine A(1) receptor agonist cyclopentyl-adenosine in rats with inflammation. Eur J Pharmacol. 2004;499:247–256. doi: 10.1016/j.ejphar.2004.07.083. [DOI] [PubMed] [Google Scholar]
- Romero-Sandoval EA, Alique M, Moreno-Manzano V, Molina C, Lucio FJ, Herrero JF. The oral administration of retinoic acid enhances nociceptive withdrawal reflexes in rats with soft-tissue inflammation. Inflamm Res. 2004;53:297–303. doi: 10.1007/s00011-004-1261-5. [DOI] [PubMed] [Google Scholar]
- Romero-Sandoval EA, Mccall C, Eisenach JC. Alpha2-adrenoceptor stimulation transforms immune responses in neuritis and blocks neuritis-induced pain. J Neurosci. 2005;25:8988–8994. doi: 10.1523/JNEUROSCI.2995-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol. 1995;115:1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. J Neurophysiol. 1985;54:1109–1122. doi: 10.1152/jn.1985.54.5.1109. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, King AE. Dynamic alterations in the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat spinal cord. J Neurosci. 1990;10:2717–2726. doi: 10.1523/JNEUROSCI.10-08-02717.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. An improved method for chronic catheterization of the rat spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]