Abstract
Background and purpose:
Trefoil factors (TFFs) secreted by mucus-producing cells are essential for the defence of the gastrointestinal mucosa. TFFs probably influence the viscoelastic properties of mucus, but this has not been demonstrated in vivo. We therefore studied the gastric secretion of systemically administered TFF2 and TFF3, and their influence on the viscosity of the secretions.
Experimental approach:
Mice and rats under general anaesthesia were injected intravenously with human (h) TFF2, hTFF3 (5 mg kg−1 to mice and 25 mg kg−1 to rats), murine (m) 125I-TFF3, or 125I-hTFF3 (300 000 cpm, mice only). The appearance of TFFs in the gastric mucosa and luminal secretions was analysed by autoradiography, gamma-counting, and ELISA, and the viscosity by rheometry.
Key results:
125I-mTFF3 and 125I-hTFF3 were taken up by secretory cells of the gastrointestinal tract and detected at the gastric mucosal surface 15 min after injection. Stressing the stomach by carbachol (3.5 μg kg−1) and pyloric ligation significantly increased the uptake. Injected hTFF2, hTFF3, and mTFF3 were retrieved from the gastric contents after 4 h. In rats, an approximately seven-fold increase in the viscosity was detected after injection of TFF2 compared to the controls, whereas TFF3 did not increase the viscosity. In mice, TFF2 increased the viscosity approximately 4-fold.
Conclusions:
These data indicate that systemically administered TFFs are transferred to the gastric lumen in a biologically active form.
Keywords: autoradiography, in situ hybridization, mucus, trefoil factor 2, trefoil factor 3, viscosity
Introduction
The trefoil factor family (TFF), TFF1, TFF2 and TFF3 comprise a group of polypeptides (7–12 kDa) secreted to mucosal surfaces by mucus-producing cells, most prominently in the gastrointestinal (GI) tract (Thim, 1989; Hoffmann et al., 2001). The peptides are important for maintaining the integrity of the GI mucosal lining (Mashimo et al., 1996; Farrell et al., 2002), and their expression is increased in areas of GI inflammation, ulceration and metaplasia in humans and in animal models of GI disease (Taupin and Podolsky, 2003). Beneficial effects of TFF2 and TFF3 on mucosal restitution in animal models of gastric ulceration and intestinal disease have been demonstrated following both luminal and systemic administration (Playford et al., 1995; Babyatsky et al., 1996; Poulsen et al., 2005). The mechanisms behind these effects have been investigated in vitro mainly by studying signalling pathways, cellular migration or by measuring proton permeation through the mucus layer (Taupin and Podolsky, 2003). Final conclusions have not been achieved however, and it is not clear whether the effects of the trefoil peptides are mediated via interactions with a possible cellular receptor or via direct interactions with gastric mucins. Detailed studies on TFF/receptor interactions have been hampered by the lack of relevant cell lines (resembling TFF-binding gastric cells) and unsuccessful attempts to identify, definitively, a trefoil receptor or binding molecule.
We have previously shown that the TFFs, especially TFF2, are able to increase the viscosity of gastric mucins in vitro (Thim et al., 2002). This may be the way endogenous TFFs protect the gastric mucosal surface and the way orally administered trefoil peptides exert their therapeutic effects in the stomach. Our previous finding that systemically administered 125I-TFFs specifically bind to mucus-producing cells in the rat stomach and that, by autoradiography, grains subsequently can be observed in the lumen of the gastric glands and the luminal mucus layer (Poulsen et al., 1999) render it possible that injected TFF is transported intact to the mucus layer and thereby acts similarly to endogenous TFF.
Identification of the TFF peptides in the gastric lumen after systemic administration and demonstration of a direct biological effect on mucus properties would bring strong in vivo evidence to support this hypothesis. In the present study, we have developed an in vivo model allowing us to (1) demonstrate the presence of injected TFF2 and 3 in the gastric contents of mice and rats, and (2) report a concomitant increase in the viscosity of the gastric secretion after TFF2 injection.
Methods
Animals
Female C57BL/6NTac mice (17–23 g) and female Wistar Hannover GALAS (HanTac:WH) rats (250–300 g) were purchased from Taconic M&B (RY, Denmark) and were housed in the conventional animal facility at The Panum Institute (University of Copenhagen, Denmark) in rooms with controlled temperature (21°C) and humidity (55%) and with a 12-h light/dark cycle. They were fed standard chow (Altromin, Lage, Germany) ad libitum and had free access to tap water. The animal studies were approved by the Danish National Committee for Animal Studies (i.e. the Animal Experiments Inspectorate). General anaesthesia was induced in mice with a mixture of fentanyl 0.15 mg kg−1, droperidol 9.8 mg kg−1 and midazolam 1 mg kg−1 administered subcutaneously (s.c.), and in rats with methohexital 50 mg kg−1 intraperotoneally. Surgical level of anaesthesia was confirmed by the absence of the toe-pinch reflex. Animals were supplied with half of the initial dose every 20–30 min for mice and 15–20 min for rats or sooner if the toe-pinch reflex recurred. For analgesia, buprenorphine 0.1 mg kg−1 s.c. was administered by the time of induction to all rats undergoing surgery, and after recovery to all mice, which regained consciousness before killing (i.e. following removal of the mandibular glands). A total of 124 mice and 44 rats was used in the experiments described here.
Binding of 125I-TFF3 in the tissues of control mice
A total of 32 mice in groups of eight were anaesthetized, opened by a midline incision and given 0.1 ml 125I-TFF3 (human or murine) into the inferior vena cava. The dose injected was 300 000 c.p.m. (approximately 0.04 μg kg−1 TFF3) in each animal. After closure of the incision, the mice were placed under a heating lamp, and remained anaesthetized until killed. After 15 or 180 min, they were perfused transcardially for 2 min with 20 ml 0.9% saline (room temperature), selected organs (including the GI tract) were excised and the radioactivity measured in a gamma-counter (Wallac Wizard 1740, PerkinElmer, Boston, MA, USA). The amount of tracer counted in each sample was expressed as the percentage of the total injected dose. Two mice were injected with 300 000 c.p.m. Na-125I and killed after 15 min by the same method.
Secretion of 125I-TFF3 into the gastric contents
A total of 36 mice had their mandibular glands removed 3–5 days before the experiments to avoid secretion of 125I and endogenous TFF3 from the glands to the gastric contents (Jagla et al., 1999). The mice were fasted overnight, injected with 30 mg kg−1 omeprazole intramuscularly (i.m.) 16 and 1 h before surgery (to reduce secretion from the oxyntic glands), anaesthetized and 0.1 ml 125I-TFF3 (300 000 c.p.m.) was injected into the inferior vena cava (16 mice received 125I-mTFF3 and eight received 125I-hTFF3). For collection of gastric contents, the pylorus was ligated to prevent gastric emptying. After surgery and closure of the abdominal incision, the mice remained anaesthetized until they were killed, 180 min after injection of tracer. Thirty minutes before death, 3.5 μg kg−1 of the mucus secretagogue carbachol was administered s.c to eight of the mice injected with 125I-mTFF3 and all mice receiving 125I-hTFF3. Immediately after death, the gastric contents and mucus were gently brushed off the mucosal surface with 0.3 ml 0.9% saline/0.1% Triton X-100 and collected in a 4.5 ml polyethylene tube. Histological samples of stomach tissue were stained with haematoxylin/eosin/periodic acid Schiff and examined by light microscopy to ensure that the surface epithelium was not damaged during sampling. The radioactivity was subsequently measured in 0.1 ml blood, the stomach tissue, gastric contents and mucus, one kidney and a sample of muscle tissue from the thigh.
As the stomach was rather distended following pyloric ligation, another set of experiments was performed where the pylorus was ligated but distension omitted by means of a soft polyethylene catheter (diameter 1.2 mm), inserted via the duodenum through the ligature into the stomach. The catheter was connected to a collection tube outside the animal where excess gastric secretions were collected. The remaining experimental procedure was performed as described above. Group one: 125I-mTFF3 and carbachol (n=6); group two: 125I-mTFF3 and saline (n=6).
Viscosity of stomach secretions after injection of hTFF2 and hTFF3
The initial studies were performed in mice, but rats were used subsequently to secure adequate amounts of sample material. Twenty mice and 44 rats had their submandibular glands removed 3–5 days before the experiments. The animals were fasted overnight and injected with 30 mg kg−1 omeprazole i.m. 16 and 1 h before surgery to reduce dilution of the gastric mucus by secretions from oxyntic glands. hTFF2, hTFF3 or 0.9% saline was administered i.v. or s.c. to anaesthetized animals (5 mg kg−1 to mice and 25 mg kg−1 to rats for each administration route). Thirteen mice received hTFF2 and seven received saline. Eighteen rats received hTFF2, six received hTFF3 and 20 were saline controls. The pylorus was ligated and about 40 μg kg−1 pepstatin was injected through the wall of the fore-stomach using a 27-gauge needle. Thirty minutes before death, 3.5 μg kg−1 carbachol was administered s.c. After 4 h, the animals were killed, the stomach removed, opened and its contents collected in a polyethylene tube and allowed to stand for 25 min before analysis for sedimentation of particles. The viscosity was measured in 500 μl samples from the mid-third of the contents in the tubes and compared to two viscosity standards (hydroxypropylmethylcellulose solutions) on a rheometer (Bohlin Reo Cue, Lund, Sweden). All values of viscosity were expressed in nm2 s−1.
Detection of hTFF in serum and gastric secretions by ELISA
hTFF2 and hTFF3 in the same doses as described above were injected i.v. and s.c. to mice and rats, and samples of gastric secretions and serum (rats only) were analysed for the contents of hTFF2 or hTFF3 by ELISA as described previously (Vestergaard et al., 2002, 2004). The gastric secretions were collected as for the viscosity measurements and were then sonicated, centrifuged and diluted with assay buffer before analysis. The lowest dilution that gave results within the calibration values (3–65 pM) was used to calculate the concentration of hTFF2 or hTFF3. As we wanted to discriminate the injected TFF from the endogenous, these studies were performed solely with human TFF. Analysis for hTFF2 was performed on samples from seven mice injected with hTFF2 and 11 with saline, 11 rats injected with hTFF2 and 11 with saline. hTFF3 contents were analysed in samples from nine mice injected with hTFF3 and nine with saline, six rats injected with hTFF3 and seven with saline. In serum, the hTFF2 content was analysed in samples from five rats injected with hTFF2 and five with saline. hTFF3 was measured in serum samples from five rats injected with hTFF3 and six with saline.
Autoradiography
For visualization of 125I-TFF3, given i.v., in the stomach and gastric secretions, four normal controls received 125I-mTFF3 and four received 125I-hTFF3 in a dose of 800 000 c.p.m. They were killed in groups of two for each peptide after 15 and 180 min. Two mice with stomach ligation received murine 125I-TFF3, and were killed after 180 min. Furthermore, two mice received Na125-I i.v. (800 000 c.p.m.) and were killed after 15 min. Blood was removed from the vascular system by transcardial perfusion with 20 ml of 0.9% saline (room temperature, 10 ml min−1), followed by 40 ml of 10% formalin (4°C, 20 ml min−1). Selected tissues including the GI tract were removed, processed, paraffin-embedded and histological sections (7 μm) prepared for autoradiography (Poulsen et al., 2003).
Immunohistochemistry
Immunohistochemistry on formalin-fixed paraffin-embedded tissue samples from mouse stomach and colon was performed as described previously (Poulsen et al., 2003).
In situ hybridization
In situ hybridization was performed on the same tissues as used for immunohistochemistry. Preparation of probes: Fragments of mouse TFF1-3 cDNA were generated by reverse transcriptase–polymerase chain reaction from mouse embryo 17 days polyA+ RNA and used to prepare the following subclones in pBluescript KS(+): mTFF1, base pairs 1–198; mTFF2, base pairs 21–312; mTFF3, base pairs 36–238 (EMBO database AQC numbers: mTFF1 NM_009362; mTFF2 NM_009363; mTFF3 NM_011575). All clones were confirmed by sequencing (MWG Biotech, Ebersberg, Germany). Generation of 35S-UTP labelled antisense and sense probes from these plasmids by in vitro transcription using the relevant polymerase (T3 or T7) was performed as described by Pyke et al. (1994), as was the in situ hybridization procedure (Pyke et al., 1994). Briefly, after initial preparation including acid and proteinase K treatment, the sections were incubated overnight in a hybridization solution containing 35S-UTP-labelled antisense or sense RNA probes. After hybridization and RNase A treatment, K5 autoradiographic emulsion was applied, and the sections were developed after 3 weeks of exposure.
Statistical analysis
Results are expressed as means with 95% confidence intervals (95% CIs). Results in the figures are shown as box (first and third quartiles) and whisker (range) plots with the median shown as a line in the box. A two-tailed Mann–Whitney U-test was used for statistical analysis. P<0.05 was considered statistically significant.
Materials
Drugs: fentanyl (Janssen-Cilag, Berchem, Belgium), droperidol (Nomeco, Copenhagen, Denmark), midazolam (Roche, Basel, Switzerland), methohexital (Lilly, Indianapolis, USA), buprenorphine (Schering-Plough, Brussels, Belgium), carbachol and hydroxypropylmethylcellulose (Sigma-Aldrich, St Louis, MO, USA), omeprazole (AstraZeneca, Soedertaelje, Sweden) and pepstatin (Roche, Switzerland). Methohexital was diluted in sterile water. Carbachol and pepstatin were diluted in 0.9% sterile saline. Omeprazole was diluted in the solvent supplied by the manufacturer.
Antibodies for immunohistochemistry: Polyclonal rabbit anti-human TFF1 (2239A) and anti-human TFF2 (2240A) (Vestergaard et al., 2002). Rabbit anti-mouse TFF3 (manufactured essentially as described previously (Vestergaard et al., 2002)).
Peptides and tracers: Human TFF3 dimer and human TFF2 were prepared at Novo Nordisk a/s (Thim et al., 1993, 1995). Murine TFF3 dimer was prepared at Novo Nordisk a/s essentially as described for the human TFF3, according to the published sequence (Tomita et al., 1995). Murine and human TFF3 dimers were labelled at Novo Nordisk a/s with sodium 125iodide (Na125I) using lactoperoxidase to a radiochemical purity of ∼98%. The specific activity was 2.2 μCi pmol−1 for both TFF tracers and for Na125I. All tracers were diluted with 0.9% saline to a final concentration of 300 000 c.p.m. (∼0.04 μg TFF3 kg−1) or 800 000 c.p.m. (∼0.11 μg TFF3 kg−1). In situ hybridization: Mouse embryo 17 days polyA+ RNA (P0480, Sigma-Aldrich, USA). pBluescript KS(+) and T3 and T7 polymerases (Stratagene, La Jolla, CA, USA), K5 autoradiographic emulsion (Ilford, Cheshire, UK).
Results
Distribution of 125I-TFF3 administered i.v. to the mouse stomach and gastric secretions
Binding of 125I-TFF3 administered i.v. in the tissues of control mice
The mean percentage of the injected dose of 125I-mTFF3 and 125I-hTFF3 per animal is shown in Table 1. Except for the GI tract, kidneys, mandibular glands and thyroids, the radioactivity in the remaining organs was low. Comparable results were obtained with 125I-hTFF3 and 125I-mTFF3 after 15 min, whereas after 180 min, a higher amount of radioactivity was measured in the blood and GI tract and less in the kidney following injection of 125I-hTFF3 than following injection of 125I-mTFF3.
Table 1.
Distribution of radioactivity from 125I-TFF3, given i.v., to the main target tissues 15 and 180 min after injection to mice
| Blood | Kidney | GI tract | Stomach | Gll. Man.a | Thyroids | |
|---|---|---|---|---|---|---|
|
125I-mTFF3 15 min |
35 (29, 41) |
18 (11, 26) |
14 (13, 15) |
4.7 (4.0, 5.0) |
0.5 (0.3, 0.6) |
0.2 (0.1, 0.2) |
|
125I-hTFF3 15 min |
34 (22, 45) |
20 (12, 27) |
15 (13, 18) |
4.9 (3.9, 5.9) |
0.5 (0.4, 0.6) |
0.2 (0.1, 0.2) |
|
125I-mTFF3 180 min |
16 (13, 19) |
8.1 (6.4, 10) |
7.8 (6.8, 8.8) |
4.2 (3.5, 4.8) |
4.2 (3.1, 5.2) |
2.6 (1.4, 3.8) |
| 125I-hTFF3 180 min | 24 (16, 32)* | 2.5 (1.6, 3.4)*** | 13 (9.8, 16)** | 6.9 (4.8, 8.9)* | 4.4 (3.1, 5.7) | 1.6 (0.9, 2.2) |
Abbreviations: CI, confidence intervals; GI, gastrointestinal; i.v., intravenously; TFF, trefoil factor family.
Mice (n=8 per group) were injected i.v. with either 125I-mTFF3 or 125I-hTFF3 (300 000 c.p.m.). After 15 or 180 min, the animals were killed and the radioactivity in a blood sample and in various tissues was measured in a gamma-counter. The values are presented as the mean percentage of injected dose with 95% CIs in parentheses. * mTFF3 vs hTFF3. *P<0.05. **P<0.01, ***P<0.0001.
Mandibular glands.
Detection of 125I-TFF3 in the surgically manipulated mouse stomach and its contents
Ligation of the pylorus (‘ligated') or ligation combined with catheterization of the stomach via the duodenum (‘catheterized') resulted in significantly increased amounts of 125I-TFF3 in the gastric tissue compared to non-manipulated controls as shown in Figure 1a. Carbachol did not affect the percentage of the injected dose found in the tissues.
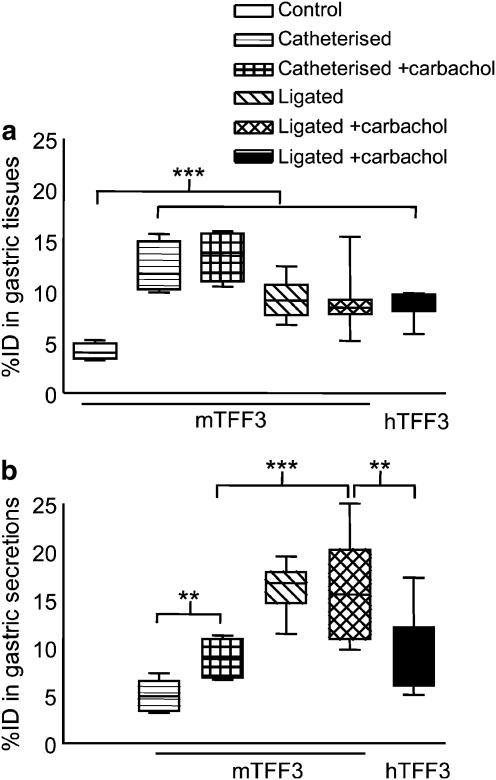
Figure 1.
Distribution of 125I-TFF3 to the stomach and gastric secretions of mice. Values shown are the percentage of injected dose (% ID) in a box and whisker plot. 125I-mTFF3 or 125I-hTFF3 was injected i.v. into control mice (n=8), mice that had the stomach ligated (n=16) or catheterized (n=12). Half of the mice in the surgically manipulated groups received 3.5 μg kg−1 carbachol s.c. (+carbachol) and half received saline. Specimens were sampled 180 min after injection and the radioactivity measured. (a) In the gastric tissue, all manipulations resulted in significantly increased % ID as compared to control mice. (b) In the gastric secretions, a significantly higher % ID was found after ligation than after catheterization. Carbachol caused a significantly increased accumulation of 125I-mTFF3 in catheterized but not ligated mice. In both (a) and (b), the last two values compare the distribution of mouse and human peptide. Although the tissue binding was comparable, significantly more 125I-mTFF3 than 125I-hTFF3 was detected in the secretions. **P<0.01, ***P<0.0001.
A considerable amount of the injected tracer could be detected also in the gastric secretions, with significantly more measured in the ligated animals than for the catheterized (Figure 1b). The total amount of tracer taken up and secreted by the stomach was thus approximately 29% of the injected dose for the ligated group and 13% of the injected dose in the catheterized group. Carbachol caused a further increase in the radioactivity found in the secretions from the catheterized animals, but not from the ligated animals. The species origin of the peptide influenced the percentage of the injected dose in secretions, but not in the tissue, as a higher percentage of the injected dose was found in the secretions after injection of murine 125I-TFF3 compared with that after injection of human 125I-TFF3 (see last two results in Figure 1b).
Histological analysis of stomach tissue samples showed no signs of surface epithelial damage to the gastric mucosa.
Viscosity of stomach secretions after injection of hTFF2 and hTFF3
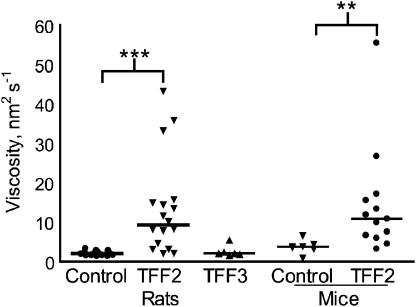
Administration of hTFF2 to mice and rats resulted in a significantly higher viscosity than injection of either saline or hTFF3. For rats, the viscosity was about six-fold greater in the hTFF2 group than that in either the hTFF3 group or the control group (Figure 2). For mice, the viscosity was similarly higher in the group injected with hTFF2 relative to that in the controls. No change in viscosity was detected in preliminary studies with hTFF3 in mice (data not shown).
Figure 2.
Viscosity of gastric secretions. Rats and mice were injected with hTFF2 (rats, n=18; mice, n=13), hTFF3 (rats, n=6) or 0.9% saline (control: rats, n=20; mice, n=7) i.v. and s.c. and the stomach was ligated. Four hours after injection, the stomach contents were collected and the viscosity measured. Viscosity is expressed in nm2 s−1 (individual values and median). **P<0.01, ***P<0.001.
Detection of unlabelled human TFF in gastric secretions and serum
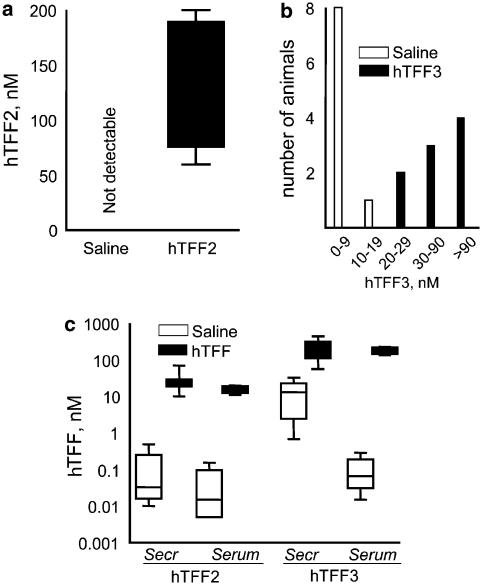
The concentrations of TFF both in serum and in gastric secretions were significantly higher in the groups of animals injected with TFFs when compared to the control groups. For TFF2 administered to mice, the mean hTFF2 concentration in gastric secretions from mice given the peptide i.v. was over 100 nM, whereas no hTFF2 reactivity was detected in control mice (Figure 3a). In rats, as shown in Figure 3c, the hTFF2 concentrations in gastric secretions and serum were raised well above the nonspecific reactivity for hTFF2 in the control group (Figure 3c).
Figure 3.
Retrieval of injected hTFF2 and hTFF3 in gastric secretions from mice (a–b) and in serum and gastric secretions from rats (c). hTFF2, hTFF3 or saline was injected i.v., and the stomach was ligated. Specimens were sampled after 4 h, and the TFF concentrations measured by ELISA for either hTFF2 or hTFF3. (a) hTFF2 in gastric secretions from mice. Box and whisker plots. (b) hTFF3 in gastric secretions of mice. Values shown are the number of samples in a specific concentration range. (c) hTFF2 or hTFF3 in gastric secretions (secr.) and serum from rats. Box and whisker plot. In all groups injected with hTFF2 or hTFF3, significantly higher concentrations of hTFF2 or hTFF3, respectively, were detected (P<0.001).
For mice injected with hTFF3, the concentrations of this peptide in the gastric secretions ranged from 20 to >90 nM, whereas the hTFF3 reactivity in the control group was lower, corresponding to 0.8–8 nM, with 18 nM recorded for one animal (Figure 3b). In rats, the hTFF3 concentrations in gastric secretions in the group injected with the peptide were the same as those in serum and higher than the corresponding values in the control group injected with saline (Figure 3c).
Visualization of injected 125I-mTFF3 and endogenous TFF1-3 in the stomach
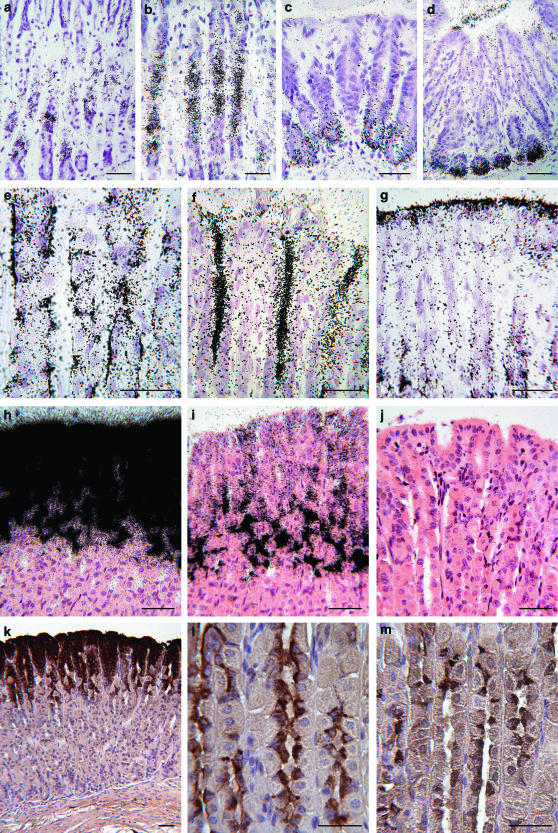
In stomach tissue from control mice injected with 125I-mTFF3, autoradiography showed accumulation of grains in the mucous neck cells and the pyloric glands (Figure 4a and c). The findings were similar after 15 and 180 min. Mice with stomach ligations had accumulation of grains in the same locations as the controls, but to a considerably higher extent (Figure 4b and d). In all mice, grains were also seen in the lumen of the glands, in the gastric pits and in the surface mucus layer (Figure 4e–g). Similar findings were made after injection of 125I-hTFF3, whereas no specific accumulation of grains was seen after injection of Na-125I.
Figure 4.
Localization of i.v. injected and endogenous TFF by autoradiography, in situ hybridization and immunohistochemistry on sections of stomach tissue from controls, and from ligated carbachol-treated mice. The accumulation of grains in the mucous neck cells and pyloric glands was considerably higher in ligated animals than in controls, as was the amount of grains in the lumen of the gastric glands and on the surface of the stomach. The localization of TFF mRNA and peptide correlated, except for TFF3, where peptide but not mRNA was found in the corpus fundus. (a) Accumulation of grains in neck cells, control, (b) higher amounts of grains in the ligated stomach. Comparable differences are seen between pyloric glands of controls (c) and ligated (d). (e) Binding of grains to the basal part of mucous neck cells 15 min after injection. (f) Marked accumulation of grains in the lumen of gastric pits and (g) in the surface mucus layer of the ligated stomach. (h) TFF1 mRNA in the gastric surface epithelial cells. (i) TFF2 mRNA in surface epithelial and mucous neck cells. (j) Negative in situ hybridization for TFF3 mRNA in the stomach. (k) TFF1 peptide in surface epithelia. (l) TFF2 peptide in mucous neck cells. (m) TFF3 peptide in mucous neck cells. Scale bars: 50 μm.
A strong signal for TFF1 mRNA and a weaker signal for TFF2 mRNA were detected in the epithelium lining, the gastric surface and pits, and high expression of TFF2 mRNA in the mucous neck cells and the pyloric glands (Figure 4h and i). No signal for TFF3 mRNA was found in the stomach (Figure 4j). The immunohistochemical findings mirrored the expression of the equivalent mRNA, except for TFF3, where a weak immunoreactivity was found in the mucous neck cells (Figure 4k–m).
Discussion
Several studies have demonstrated the effects of TFF1-3 after systemic administration in experimental models of GI disease (Chinery and Playford, 1995b; Playford et al., 1995; Marchbank et al., 1998). The effects of TFFs on cell motility and the pharmacological response to even low doses of systemically administered TFF2 (Playford et al., 1995) may suggest direct interactions between TFF and receptor-like molecules on the epithelial cells. On the other hand, the intimate colocalization of mucins and endogenous, as well as systemically administered, trefoil peptides, together with reports on mucin–TFF interplay (Dignass et al., 1994; Kindon et al., 1995; Tanaka et al., 1997; Newton et al., 2000; Tomasetto et al., 2000; Thim et al., 2002), suggest that TFFs could mediate some of their effects via interactions with mucin. Although direct TFF–mucin interaction offers a probable explanation for the effect after topical administration of the peptides, this seems less obvious after systemic administration, as this would require transport of the peptide across the mucosa and into the lumen, which never the less has been suggested from findings in our previous studies.
In the present study, we have demonstrated (1) the presence of murine and human 125I-TFF3 in stomach tissue and stomach secretions by autoradiography and gamma counting, (2) the appearance of hTFF2 and hTFF3 in gastric secretions as detected by ELISA and (3) an increased viscosity of gastric secretions after hTFF2 (but not hTFF3), all after i.v. administration of the peptides. These findings demonstrate for the first time by means of quantitative measurements that parenterally administered hTFF2 and TFF3 are secreted in forms that are biologically active through the stomach mucosa and into the lumen. This suggests that the pharmacological effects of systemically administered hTFF2 can be owing to direct interactions with mucins, resulting in increased viscosity of the luminal secretions. The cellular mechanism of this secretion has not been clarified, but the transport seems predominantly to occur via the mucus-secreting cells, as autoradiography of the stomach shows specific localization of the tracer to these cells and in the newly secreted mucus, possibly involving a receptor-like mechanism or a protein-transporting molecule. We have, so far, only demonstrated this mechanism in the stomach, but if the findings can be extrapolated to other TFF/mucus-secreting surfaces, this concept might be interesting from a therapeutic point of view, as it implies that TFF might be enabled to reach sites of the body that are less accessible by local treatment. A further advantage of the trans-mucosal distribution after systemic administration is that TFF not only reaches the luminal mucus layer, but may also interact with the mucins inside the cells before secretion or at the luminal surface of the cells just after secretion, which may be of importance when establishing the mucus–TFF network. This would probably not be possible with luminally administered TFF, as this becomes trapped in the outer mucus layer and is thus not accessible to interact with newly secreted mucus (Poulsen et al., 1999).
Carbachol enhanced the amounts of radioactivity detected in the gastric secretions. Cholinergic drugs have been shown to enhance secretion of endogenous TFF3 in the colon (Ogata and Podolsky, 1997), and it is possible that the secretion of injected TFF is regulated similarly to endogenous TFF3. The effect of carbachol may be owing to a direct stimulation of the mucus-secreting cells, but may also be a consequence of carbachol-induced severe contractions of the gastric muscle coat. The latter seems less likely, as autoradiography of stomach tissue from carbachol-treated mice showed increased binding of grains in the same cell types as in untreated tissue and the mucosal surface appeared intact, suggesting that nonspecific accumulation of TFF owing to tissue damage had not taken place. The tissue binding and gastric accumulation of TFF3 were also enhanced after surgical manipulation, indicating that distribution of the TFF3 to the target organs is increased during pathological conditions resulting in tissue distress. In accordance with this finding, crosslinking of TFF3 to a binding protein on a solubilised intestinal epithelial membrane preparation has previously been reported to increase 1.5-fold when the cells were wounded before assaying (Chinery and Cox, 1995a).
Administration of hTFF3 had no effect on the viscosity of the gastric secretions. This is in agreement with our earlier in vitro findings, which also showed that the morphology of TFF–mucus complexes was different, the TFF2–mucus complexes being large and confluent, whereas TFF3 made small, discrete complexes with mucus (Thim et al., 2002). TFF3 might change mucin characteristics apart from the viscosity, but this remains to be clarified.
The distribution and cellular localization of both human and murine injected TFF3 in mice was quite similar and in accordance with our findings in rats using human TFF3. Similar binding sites in the rat stomach and intestines by in vitro autoradiography using rat 125I-TFF3 have previously been reported (Chinery and Cox, 1995a), and collectively these findings may suggest that the binding and transport mechanism is preserved among different species. The cellular binding of injected TFF3 was compared to the localization of endogenous TFF1-3 mRNA and peptides in order to assess if there was any correlation between production and storage of endogenous TFFs and binding of injected TFF3. We found that injected TFF3 bound to the gastric mucous neck cells, which also expressed TFF2 mRNA and peptide. We did not detect TFF3 mRNA, but a limited amount of TFF3 immunoreactivity in these cells. TFF3 mRNA has been detected in the mouse stomach in minimal amounts by Northern blot (Mashimo et al., 1995), but studies of the cellular localization of both TFF3 peptide and mRNA in the mouse stomach have not been presented previously. It is uncertain whether the detected TFF3 immunoreactivity represents local production of the peptide or uptake of circulating peptide, but the fact that the same cells can take up injected TFF peptides renders the latter possible.
In conclusion, we have demonstrated that systemically administered TFF3 is taken up by mucous neck cells in the stomach and secreted to the gastric luminal surface. Furthermore, we found that systemically administered hTFF2 is secreted to the gastric lumen and, in contrast to hTFF3, increases the viscosity of the gastric mucus.
Conflict of interest
Stine Kjellev is the recipient of a co-financed PhD fellowship from the Danish Ministry of Science, Technology and Innovation and the Corporate Research Affairs at Novo Nordisk a/s. The TFFs were manufactured by Lars Thim, Novo Nordisk a/s.
Acknowledgments
We thank Charles Pyke, Axel K Hansen, Grazyna Poulsen, Inger Marie Jensen, Jette Mandelbaum, Jette Scousboe, Steen Kryger and Niels-Erik Viby Jensen. The study was supported by the Danish Research Council. Stine Kjellev is the recipient of a co-financed PhD fellowship from the Danish Ministry of Science, Technology and Innovation and the Corporate Research Affairs at Novo Nordisk a/s.
Abbreviations
- CI
confidence interval
- GI
gastrointestinal
- TFF
trefoil factor family
References
- Babyatsky MW, Debeaumont M, Thim L, Podolsky DK. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996;110:489–497. doi: 10.1053/gast.1996.v110.pm8566596. [DOI] [PubMed] [Google Scholar]
- Chinery R, Cox HM. Immunoprecipitation and characterization of a binding protein specific for the peptide, intestinal trefoil factor. Peptides. 1995a;16:749–755. doi: 10.1016/0196-9781(95)00045-l. [DOI] [PubMed] [Google Scholar]
- Chinery R, Playford RJ. Combined intestinal trefoil factor and epidermal growth factor is prophylactic against indomethacin-induced gastric damage in the rat. Clin Sci (Lond) 1995b;88:401–403. doi: 10.1042/cs0880401. [DOI] [PubMed] [Google Scholar]
- Dignass A, Lynch-Devaney K, Kindon H, Thim L, Podolsky DK. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994;94:376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell JJ, Taupin D, Koh TJ, Chen D, Zhao CM, Podolsky DK, et al. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest. 2002;109:193–204. doi: 10.1172/JCI12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann W, Jagla W, Wiede A. Molecular medicine of TFF-peptides: from gut to brain. Histol Histopathol. 2001;16:319–334. doi: 10.14670/HH-16.319. [DOI] [PubMed] [Google Scholar]
- Jagla W, Wiede A, Hinz M, Dietzmann K, Gulicher D, Gerlach KL. Secretion of TFF-peptides by human salivary glands. Cell Tissue Res. 1999;298:161–166. doi: 10.1007/s004419900087. [DOI] [PubMed] [Google Scholar]
- Kindon H, Pothoulakis C, Thim L, Lynch-devaney K, Podolsky DK. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology. 1995;109:516–523. doi: 10.1016/0016-5085(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Marchbank T, Westley BR, May FE, Calnan DP, Playford RJ. Dimerization of human pS2 (TFF1) plays a key role in its protective/healing effects. J Pathol. 1998;185:153–158. doi: 10.1002/(SICI)1096-9896(199806)185:2<153::AID-PATH87>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Mashimo H, Podolsky DK, Fishman MC. Structure and expression of murine intestinal trefoil factor: high evolutionary conservation and postnatal expression. Biochem Biophys Res Commun. 1995;210:31–37. doi: 10.1006/bbrc.1995.1623. [DOI] [PubMed] [Google Scholar]
- Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–265. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- Newton JL, Allen A, Westley BR, May FEB. The human trefoil peptide, TFF1, is present in different molecular forms that are intimately associated with mucus in normal stomach. Gut. 2000;46:312–320. doi: 10.1136/gut.46.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata H, Podolsky DK. Trefoil peptide expression and secretion is regulated by neuropeptides and acetylcholine. Am J Physiol. 1997;273:G348–G354. doi: 10.1152/ajpgi.1997.273.2.G348. [DOI] [PubMed] [Google Scholar]
- Playford RJ, Marchbank T, Chinery R, Evison R, Pignatelli M, Boulton RA, et al. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology. 1995;108:108–116. doi: 10.1016/0016-5085(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Poulsen SS, Kissow H, Hare K, Hartmann B, Thim L. Luminal and parenteral TFF2 and TFF3 dimer and monomer in two models of experimental colitis in the rat. Regul Pept. 2005;126:163–171. doi: 10.1016/j.regpep.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Poulsen SS, Thulesen J, Christensen L, Nexo E, Thim L. Metabolism of oral trefoil factor 2 (TFF2) and the effect of oral and parenteral TFF2 on gastric and duodenal ulcer healing in the rat. Gut. 1999;45:516–522. doi: 10.1136/gut.45.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen SS, Thulesen J, Hartmann B, Kissow HL, Nexo E, Thim L. Injected TFF1 and TFF3 bind to TFF2-immunoreactive cells in the gastrointestinal tract in rats. Regul Pept. 2003;115:91–99. doi: 10.1016/s0167-0115(03)00145-9. [DOI] [PubMed] [Google Scholar]
- Pyke C, Romer J, Kallunki P, Lund LR, Ralfkiaer E, Dano K, et al. The gamma 2 chain of kalinin/laminin 5 is preferentially expressed in invading malignant cells in human cancers. Am J Pathol. 1994;145:782–791. [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Podolsky DK, Engel E, Guth PH, Kaunitz JD. Human spasmolytic polypeptide decreases proton permeation through gastric mucus in vivo and in vitro. Am J Physiol. 1997;272:G1473–G1480. doi: 10.1152/ajpgi.1997.272.6.G1473. [DOI] [PubMed] [Google Scholar]
- Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–732. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- Thim L. A new family of growth factor-like peptides. ‘Trefoil' disulphide loop structures as a common feature in breast cancer associated peptide (pS2), pancreatic spasmolytic polypeptide (PSP), and frog skin peptides (spasmolysins) FEBS Lett. 1989;250:85–90. doi: 10.1016/0014-5793(89)80690-8. [DOI] [PubMed] [Google Scholar]
- Thim L, Madsen F, Poulsen SS. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur J Clin Invest. 2002;32:519–527. doi: 10.1046/j.1365-2362.2002.01014.x. [DOI] [PubMed] [Google Scholar]
- Thim L, Norris K, Norris F, Nielsen PF, Bjorn SE, Christensen M, et al. Purification and characterization of the trefoil peptide human spasmolytic polypeptide (hSP) produced in yeast. FEBS Lett. 1993;318:345–352. doi: 10.1016/0014-5793(93)80543-4. [DOI] [PubMed] [Google Scholar]
- Thim L, Woldike HF, Nielsen PF, Christensen M, Lynch-Devaney K, Podolsky DK. Characterization of human and rat intestinal trefoil factor produced in yeast. Biochemistry. 1995;34:4757–4764. doi: 10.1021/bi00014a033. [DOI] [PubMed] [Google Scholar]
- Tomasetto C, Masson R, Linares JL, Wendling C, Lefebvre O, Chenard MP, et al. pS2/TFF1 interacts directly with the VWFC cysteine-rich domains of mucins. Gastroenterology. 2000;118:70–80. doi: 10.1016/s0016-5085(00)70415-x. [DOI] [PubMed] [Google Scholar]
- Tomita M, Itoh H, Ishikawa N, Higa A, Ide H, Murakumo Y, et al. Molecular cloning of mouse intestinal trefoil factor and its expression during goblet cell changes Biochem J 1995311293–297.(Part 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard EM, Brynskov J, Ejskjaer K, Clausen JT, Thim L, Nexo E, et al. Immunoassays of human trefoil factors 1 and 2: measured on serum from patients with inflammatory bowel disease. Scand J Clin Lab Invest. 2004;64:146–156. doi: 10.1080/00365510410001176. [DOI] [PubMed] [Google Scholar]
- Vestergaard EM, Poulsen SS, Gronbaek H, Larsen R, Nielsen AM, Ejskjaer K, et al. Development and evaluation of an ELISA for human trefoil factor 3. Clin Chem. 2002;48:1689–1695. [PubMed] [Google Scholar]