Abstract
Background and purpose: Protection against ischaemia-reperfusion (I/R) injury involves PI3K-Akt and p44/42 MAPK activation. Leptin which regulates appetite and energy balance also promotes myocyte proliferation via PI3K-Akt and p44/42 MAPK activation. We, therefore, hypothesized that leptin may also exhibit cardioprotective activity.
Experimental approach: The influence of leptin on I/R injury was examined in perfused hearts from C57Bl/6 J mice that underwent 35 min global ischaemia and 35 min reperfusion, infarct size being assessed by triphenyltetrazolium chloride staining. The concomitant activation of cell-signalling pathways was investigated by Western blotting. The effect of leptin on mitochondrial permeability transition pore (MPTP) opening was studied in rat cardiomyocytes.
Key results: Leptin (10 nM) administered during reperfusion reduced infarct size significantly. Protection was blocked by either LY294002 or UO126, inhibitors of Akt and p44/42 MAPK, respectively. Western blotting confirmed that leptin stimulated p44/42 MAPK phosphorylation significantly. Akt phosphorylation was also enhanced but did not achieve statistical significance. Additionally, leptin treatment was associated with a significant increase in p38 phosphorylation. By contrast, leptin caused downregulation of phosphorylated and non-phosphorylated STAT3, and of total AMP-activated kinase. Cardiomyocytes responded to leptin with delayed opening of the MPTP and delayed time until contracture.
Conclusions and implications: Our data indicate for the first time that the adipocytokine, leptin, has direct cardioprotective properties which may involve the PI3-Akt and p44/42 MAPK pathways.
Keywords: leptin, myocardial ischaemia–reperfusion, cardiomyocytes, mitochondrial permeability transition pore, Akt, p44/42 MAPK
Introduction
Protection against myocardial ischaemia–reperfusion (I/R) injury is associated with the activation of the phosphatidylinositol 3-OH kinase (PI3K)-cellularAkt/protein kinase B (Akt) and p44/42 mitogen-activated protein kinase (MAPK) extracellular signal-regulated MAPK (Erk1/2) signalling cascades, collectively referred to as the reperfusion injury salvage kinase (RISK) pathway (Hausenloy and Yellon, 2004). Ischaemic preconditioning (IPC), that is, short periods of ischaemia interspersed with reperfusion before a sustained period of ischaemia, improves myocardial survival via the RISK pathway but is not an option clinically, as its beneficial effects are dependent on its institution before ischaemia. The search, therefore, continues for effective pharmacological alternatives to IPC that are cardioprotective via similar cellular mechanisms, but can be instituted at the moment of reperfusion. The identification of endogenous circulating substances that reduce I/R injury and could represent intrinsic cardioprotective agents may prove valuable in this search. Various chemically diverse agents, some of which exhibit mitogenic activity, have proved beneficial, as indicated by reduced infarct size, when added at reperfusion. Significantly, these agents have included endogenous factors such as insulin (Jonassen et al., 2001), insulin-like growth factor (Yellon and Baxter, 1999) and glucagon-like peptide 1 (Bose et al., 2005). The protection afforded by these factors involves receptor stimulation and, importantly, like IPC, activation of PI3K-Akt and p44/42 MAPK (Yellon and Baxter, 1999; Yellon and Downey, 2003; Hausenloy and Yellon, 2004): recent reviews from this laboratory provide detailed information regarding those substances and procedures which are protective via RISK pathway activation (Hausenloy and Yellon, 2005, 2006). It also involves inhibition of the opening of the mitochondrial permeability transition pore (MPTP), a process that may represent a key determining factor in protection against reperfusion injury (Hausenloy et al., 2004b).
Leptin, an adipocytokine hormone that has both central and peripheral actions and is implicated in obesity, has important roles in the regulation of appetite and energy balance (Harvey and Ashford, 2003; Ren, 2004). Its metabolic actions are mediated via the leptin receptor (Ob-R) (Zabeau et al., 2003) and involve the activation of AMP-activated protein kinase (AMPK) (Minokoshi et al., 2002). Leptin receptor stimulation is associated with janus kinase (JAK)/signal transducer and activator of transcription (STAT) signalling, and also with the activation of the PI3K-Akt and MAPK pathways (Harvey and Ashford, 2003; Zabeau et al., 2003), which, as outlined earlier, are implicated directly in cardioprotection (Hausenloy and Yellon, 2004). Leptin, administered chronically in high doses, increases mean arterial pressure and heart rate, the latter possibly involving cardiac leptin receptors (Shirasaka et al., 2003; Ren, 2004). Indeed, the heart expresses leptin receptors and, interestingly, synthesises leptin itself, releasing it into the coronary effluent, raising the possibility that leptin of cardiac origin feeds back onto the cardiomyocyte to exert physiological effects (Winnicki et al., 2001; Purdham et al., 2004). Leptin induces hypertrophy in rat cardiomyocytes via activation of the MAPK system, including p38 MAPK and p44/42 MAPK (Rajapurohitam et al., 2003), and it was reported that myocyte proliferation was blocked by inhibitors of PI3K-Akt and p44/42 MAPK signalling (Tajmir et al., 2004). To our knowledge, no information is currently available concerning leptin and cardioprotection. The fact, however, that leptin is mitogenic, activating mechanisms similar to those implicated in protection against I/R injury (Yellon and Baxter, 1999) lead us to speculate that leptin may represent an endogenous substance that affords protection against myocardial infarction. The aim of this study, therefore, was to investigate if leptin reduced myocardial I/R injury focusing on infarct size, cell-signalling pathways implicated in cardioprotection and the MPTP. The data presented in this paper demonstrate that leptin protects against myocardial infarction when given at reperfusion in an isolated mouse heart model. In addition, data obtained using isolated rat cardiomyocytes suggest that this adipocytokine, acting via prosurvival kinases, causes a delay in MPTP opening.
Materials and methods
Animals
Male C57Bl/6J mice (20–30 g; Charles River, Wilmington, USA; total 51) and Sprague–Dawley rats (300–400 g; Charles River; total 6) were used in these experiments. The animals were kept under conditions and the experiments were conducted, in accordance with the Animals (Scientific Procedures) Act 1986 published by the UK Home Office and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Langendorff isolated perfused mouse heart
Mice were given 100 U of heparin by intraperitoneal injection before killing by cervical dislocation. Hearts were then excised and perfused retrogradely via the aorta at a constant pressure of 100 mm Hg with oxygenated Krebs–Henseleit buffer containing NaCl 118 mM, NaHCO3 24 mM, KCl 4 mM, NaH2PO4 1 mM, CaCl2 1.8 mM, MgCl2 1.2 mM and glucose 10 mM (Sumeray and Yellon, 1998; Efthymiou et al., 2005). Heart rate was monitored using an intra-ventricular balloon inserted into the left ventricle. Myocardial temperature was monitored with a temperature probe inserted into the right ventricle and maintained at 37±0.5°C. Hearts underwent a stabilization period of 30 min followed by 35 min global ischaemia and 35 min reperfusion. Krebs buffer containing 10 nM leptin, which is at the upper end of the physiological range and represents a level of concentration that has been reported to activate cell-signalling pathways in ventricular myocytes implicated in cardioprotection (Rajapurohitam et al., 2003; Tajmir et al., 2004), and/or 15 μM LY294002 or 10 μM UO126, was substituted for normal Krebs buffer at reperfusion, in some experiments and was present in the reperfusate for the entire reperfusion period. Following reperfusion the hearts were injected with 1% triphenyltetrazolium chloride (TTC) and incubated in TTC at 37°C for 10 min before being frozen at −20°C. Subsequently, hearts were sliced (<1 mm slices), destained in formalin, photographed and planimetered using the NIH Image 1.63 software package (National Institutes of Health, Bethesda, MD, USA), and infarct size calculated as the percentage of risk volume (%I/R).
It should be noted that it has been demonstrated previously in this laboratory that infarct size does not differ significantly between mouse hearts that have been reperfused for 30 and 120 min (Marber et al., 1995; Sumeray and Yellon, 1998). This indicates that 30 min reperfusion is sufficient to ensure effective washout of dehydrogenase enzymes and cofactors from infarcted tissue, and accurate determination of infarct size by TTC staining. In addition, Langendorff experiments were conducted with mouse hearts because our ultimate aim is to investigate cardiac function in mice that have been genetically engineered with respect to the circulating levels of leptin and Ob-R tissue density.
Western blot analysis
Mouse hearts were perfused for 30 min to allow them to stabilize followed by 35 min global ischaemia and 10 min reperfusion (Efthymiou et al., 2005). At the end of the reperfusion period, the hearts were snap-frozen and stored at −80°C until the time of analysis, which occurred shortly after sampling. Proteins were extracted by homogenizing the samples on ice in a suspension buffer consisting of 100 mM NaCl, 10 mM Tris (pH 7.6), 1 mM ethylenediaminetetraacetic acid (pH 8.0), 2 mM sodium pyrophosphate, 2 mM sodium fluoride, 2 mM β-glycerophosphate and a protease inhibitor cocktail, followed by high-speed centrifugation. The supernatants were then assayed for protein content using a bicinchoninic acid (BCA) assay and proteins separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), 30–60 μg total protein being loaded per well for each sample. After separation the proteins were transferred to Hybond enhanced chemiluminescence (ECL) nitrocellulose membranes. Primary and secondary antibodies and an ECL Western blotting reagent were then used to detect the total and phosphorylated forms of Akt, p44/42 MAPK, endothelial nitric oxide synthase (eNOS), AMPK, p38 MAPK and STAT3. The nitrocellulose membranes were then exposed to photographic film, which was scanned and the intensities of the protein bands, which were expressed as arbitrary units (a.u.), determined by computerized densitometry (NIH Image 1.63). The relative changes for the proteins of interest were then calculated correcting for differences in protein loading as established by probing for β-actin.
Rat cardiomyocytes
Ventricular cardiomyocytes were isolated by treating hearts from adult male Sprague–Dawley rats (Charles River) with collagenase (Hausenloy and Yellon, 2004), 1.5–3 million cells being routinely obtained per heart. The induction of cardiomyocyte MPTP opening was monitored, in a model of oxidative stress (Jacobson and Duchen, 2002; Hausenloy et al., 2003, 2004c). Cardiomyocytes were loaded with the fluorescent dye, tetra-methyl rhodamine methyl ester (TMRM), for 15 min and the buffer changed. The cells were then subjected to laser stimulation, resulting in mitochondrial reactive oxygen species (ROS) generation, simulating ROS production during reperfusion. MPTP opening was indicated by mitochondrial depolarization and dequenching of TMRM fluorescence as the dye traversed the mitochondrial wall into the cytoplasm (Jacobson and Duchen, 2002; Hausenloy et al., 2003). The time until depolarization and the subsequent rigor contracture of the myocytes were recorded. In experiments with leptin (10 nM), washed cardiomyocytes were preincubated for 10 min with saline, LY294002 (15 μM) or mitogen-induced extracellular kinase (MEK) inhibitor 1 (1 μM), an inhibitor of MAPK activation (Wityak et al., 2004), before incubation (15 min) with the adipocytokine and subsequent laser stimulation. Experiments involving the addition of 100 μM Nω-nitro-L-arginine methyl ester (L-NAME) before leptin were also conducted. In addition, a positive control involving the addition of 200 nM cyclosporin A (CSA) (a recognized inhibitor of MPTP opening) to cardiomyocytes was included in the study.
Adult rat cardiomyocytes were used in preference to mouse cells for several reasons. First, obtaining cells with the required degree of integrity can prove difficult with hearts from adult mice; to circumvent this problem, cardiomyocytes are frequently prepared from neonatal or embryonic animals, although these may need a period of culture before attaining functional normality. Second, the yield of cardiomyocytes from adult mouse hearts is generally poor.
Materials
Leptin, L-NAME and the protease inhibitor cocktail were all from Sigma Chemical Co. (Poole, UK). LY294002 and UO126 were obtained from Tocris Bioscience (Avonmouth, UK) and the MEK inhibitor 1 from Merck Biosciences Ltd (Nottingham, UK). Protein content was measured with the BCA assay from Pierce (Lutterworth, UK). Primary and secondary antibodies to the total and phosphorylated forms of Akt, p44/42 MAPK, eNOS, AMPK, p38 MAPK and STAT3 were obtained from Cell Signaling Technology (Hitchin, UK) and antibody to β-actin was from Abcam (Cambridge, UK). The Hybond ECL nitrocellulose membranes and the ECL Western blotting reagent were from Amersham Biosciences (Little Chalfont, UK).
Statistical analysis
Data are expressed as mean±s.e.m. For comparisons between more than two groups factorial, one-way analysis of variance (ANOVA) was employed. Where a significant F-value was obtained, the Fisher's protected least significant difference post hoc test was applied for between group comparisons. Where there were only two groups to be compared, the Student's t-test was used. Differences were considered to be statistically significant if a value of P<0.05 was obtained.
Results
Effect of leptin administered at reperfusion upon myocardial infarct size
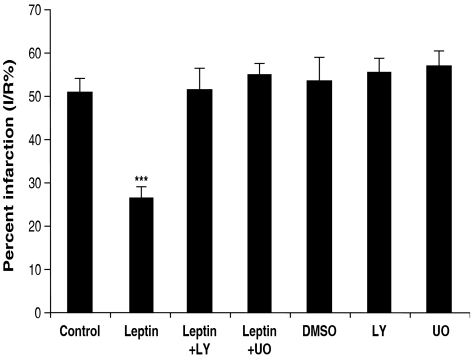
Langendorff experiments revealed that leptin, when administered at reperfusion, protected against I/R injury (Figure 1). Thus, infarct size in hearts perfused with 10 nM leptin was about half that in untreated (control) hearts. This adipocytokine concentration is comparable to that seen in plasma in some obese individuals (Considine et al., 1996). When LY294002, the PI3K inhibitor, was administered together with leptin, the protection was blocked. Similarly, the p44/42 MAPK inhibitor UO126 also blocked the protective effects of leptin. The inhibitors LY294002 and UO126, and their solvent (dimethyl sulphoxide) did not influence infarct size when administered by themselves.
Figure 1.
Infarct size, as a percentage of the risk zone (% I/R), in isolated mouse hearts perfused with or without leptin (10 nM) during reperfusion (35 min) in the presence or absence of LY294002 or UO126, inhibitors of PI3K and p44/42 MAPK, respectively. Values are expressed as means±s.e.m. of 4–12 experiments (***P<0.01 vs control).
Effect of leptin, administered at reperfusion, upon the phosphorylation of Akt, p44/42 MAPK, p38 MAPK, eNOS, AMPK and STAT3
Employing Western blot analysis, the actions of leptin on the phosphorylation states of the so-called prosurvival kinases were examined. Thus, the phosphorylation of Akt and p44/42 MAPK was measured under control conditions and after the administration of leptin in the presence and absence of specific inhibitors of these enzymes. In addition, the influence of leptin on the phosphorylation states of p38 MAPK, eNOS, AMPK and STAT3 was investigated.
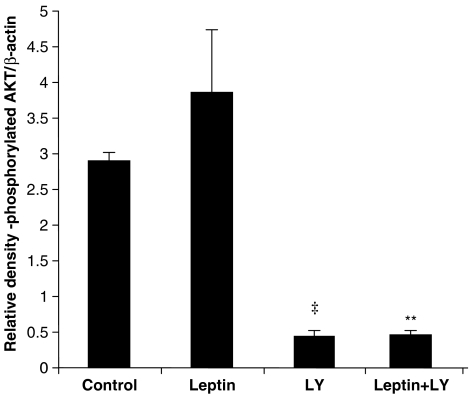
Densitometric analysis revealed that while leptin produced an increase in Akt phosphorylation, this did not achieve statistical significance (Figure 2). The phosphorylation of Akt was inhibited by LY294002 in both control and leptin-treated samples. The total levels for Akt did not differ markedly between the various treatment groups (data not shown).
Figure 2.
Total and phosphorylated Akt in tissue extracts from hearts subjected to ischaemia–reperfusion. Data are presented as relative densitometry (RD) values in a.u. normalized for β-actin. Values are expressed as mean±s.e.m. (‡P<0.001, LY vs control and **P<0.02, leptin+LY vs leptin; n=5).
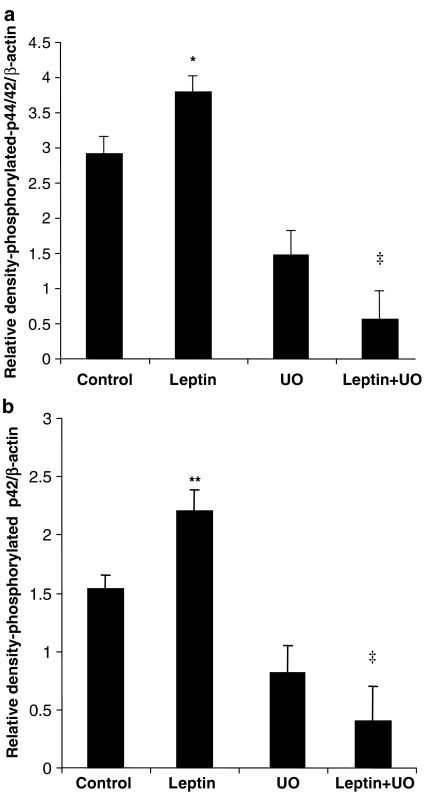
Reperfusion with leptin resulted in a significant, almost 30%, increase in p44/42 MAPK phosphorylation, which was attenuated by UO126 (leptin vs leptin/UO126; Figure 3a). Interestingly, this increase appeared to reside predominantly with the p42 isoform, which showed about 40% increase in phosphorylation after leptin. As seen for p44/42 MAPK, UO126 blocked p42 phosphorylation under leptin-stimulated conditions (Figure 3b). Total p44/42 MAPK levels were unaltered by any of the treatments (data not shown).
Figure 3.
Total and phosphorylated p44/42 MAPK in tissue extracts from hearts subjected to ischaemia–reperfusion. Data are shown as RD values (a.u.) normalized for β-actin loading and show that leptin stimulates p44/42 MAPK (a) and p42 (b) phosphorylation, which is blocked by UO126. Values are presented as mean±s.e.m. (*P<0.05 and **P<0.02, leptin vs control and ‡P<0.001, leptin+UO vs leptin; n=4–5).
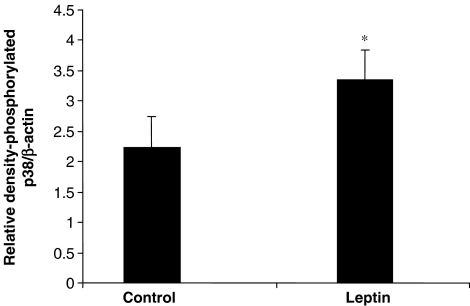
The phosphorylation of p38 MAPK was also increased after leptin treatment (Figure 4), whereas total p38 MAPK levels were unaltered (data not shown).
Figure 4.
Total and phosphorylated p38 MAPK in tissue extracts from hearts subjected to ischaemia–reperfusion. Data are shown as RD values (a.u.) normalized for β-actin loading and show that leptin stimulates p38 MAPK phosphorylation. Values are presented as mean±s.e.m. (*P<0.05 vs control; n=5).
Leptin was not found to influence eNOS phosphorylation, although, as expected, LY294002 inhibited eNOS phosphorylation occurring as a consequence of reperfusion (data not shown).
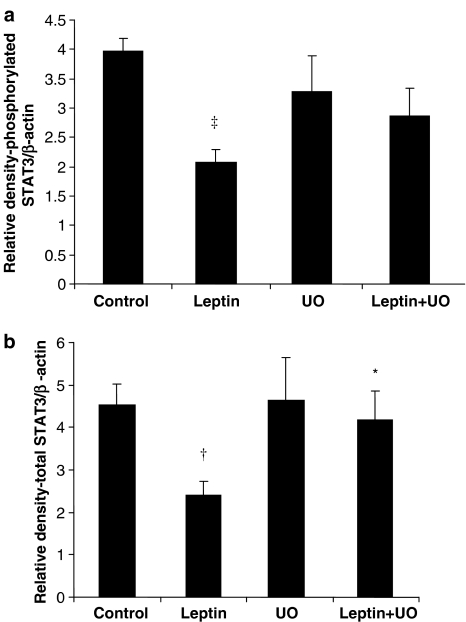
STAT3 phosphorylation with leptin contrasted with that observed for p44/42 MAPK and p38 MAPK, with leptin decreasing phosphorylation to half that of control levels (Figure 5a). Total STAT3 levels were also reduced by a similar proportion following leptin treatment (Figure 5b). The inclusion of UO126 in the perfusion buffer, when leptin was present, resulted in the restoration to control levels of pSTAT3 (Figure 5a) and total STAT3 (Figure 5b). UO126 on its own produced no effect (Figure 5a and b).
Figure 5.
Total and phosphorylated STAT3 in tissue extracts from hearts subjected to ischaemia–reperfusion. Data are shown as RD values (a.u.) normalized for β-actin loading and indicate that leptin induces downregulation of both phosphorylated (a) and total STAT3 (b), which is reversed by UO126. Values are presented as mean± s.e.m. (†P<0.01 and ‡P<0.001 vs control, and *P<0.05, leptin+UO vs leptin; n=4–5).
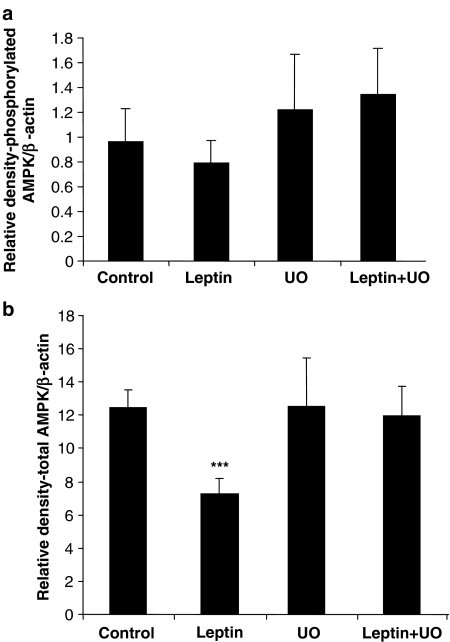
With respect to AMPK, although pAMPK levels were not affected by leptin treatment, total AMPK was decreased significantly. As observed for STAT3, UO126 blocked this reduction (Figure 6b). Again UO126 on its own had no effect.
Figure 6.
Total and phosphorylated AMPK in tissue extracts following ischaemia–reperfusion. Data are shown as RD values (a.u.) normalized for β-actin loading and indicate that leptin induces downregulation of both phosphorylated (a) and total AMPK (b), which is reversed by UO126. Values are presented as mean±s.e.m. (***P<0.01 vs control; n=4–5).
Effect of leptin, administered to isolated cardiomyocytes, upon MPTP opening following an oxidative stress
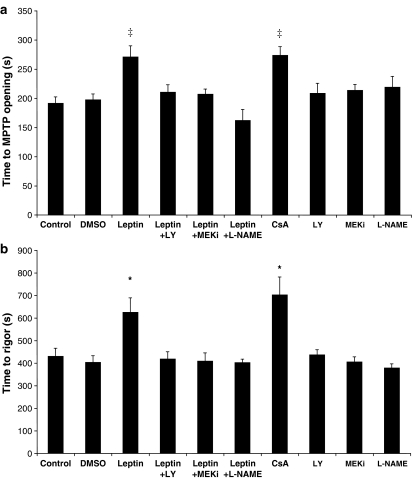
Cardioprotection, whether induced by IPC, postconditioning or pharmacological agents involves the activation of the RISK pathway and appears, ultimately, to be mediated through inhibition of MPTP opening (Hausenloy et al., 2004b). Leptin (10 nM) delayed the times until MPTP opening and rigor contracture significantly (Figure 7a and b). LY294002 blocked the delaying effects of leptin on MPTP opening, providing further evidence that the actions of leptin involved PI3K-Akt signalling (Figure 7a and b). Experiments with UO126 were not carried out as it is highly light sensitive and prone to degradation as a consequence of laser stimulation, and we have demonstrated that the use of this compound leads to artefactual results. As a consequence, a selective inhibitor of MEK (MEK inhibitor 1), which is upstream of p44/42 MAPK and chemically related to UO126 but more stable, was used (Wityak et al., 2004). This was found to block the effects of leptin (Figure 7a and b). L-NAME also abolished the effects of leptin (Figures 7a and b). None of the inhibitors by themselves influenced MPTP opening or time to contracture, as compared to control (Figures 7a and b). CsA (200 nM), a recognized inhibitor of MPTP opening and used here as a positive control, delayed the times to MPTP opening and time to contracture, to the same extent as leptin (10 nM) (Figures 7a and b).
Figure 7.
The times until the initiation of mitochondrial depolarization, that is, MPTP opening (a) and cardiomyocyte contracture (b) in the presence and absence of leptin with or without LY294002 or MEK inhibitor 1 (MEKi), inhibitors of PI3K and p44/42 MAPK, respectively. Data are presented as means±s.e.m. (*P<0.05 and ‡P<0.001 vs control) and were obtained with a total of 19–51 cells from at least three hearts.
Discussion
Previously, it has been reported that leptin can protect against I/R injury in the gut (Brzozowski et al., 2001) and kidney (Erkasap et al., 2004), occurring via ROS production and increased NO synthesis. In the present study, we have demonstrated for the first time that leptin, the obesity-associated hormone, protects the mouse myocardium against I/R injury through a direct action on the heart. This was associated with the modulation of various cell-signalling pathways, some of which are recognized as playing pivotal roles in cardioprotection.
The so-called RISK pathway, which incorporates the PI3K-Akt and p44/42 MAPK cascades, appears to be essential in mediating cardioprotection induced by IPC and drugs administered at reperfusion through the upregulation of downstream antiapoptotic pathways linked to cellular survival (Hausenloy and Yellon, 2004). Several hormones have also been shown to activate these cascades, including insulin (Jonassen et al., 2001), glucagon-like peptide (Bose et al., 2005) and erythropoietin (Bullard et al., 2005), and the novel data obtained in the current study would indicate that leptin, apart from its crucial endocrine role in the control of metabolism, may also serve as an important mediator of cell death in the heart and could represent an intrinsic cardioprotective agent. We hypothesized that as leptin has been shown in other experimental systems to cause cellular proliferation and activation of PI3K-Akt and p44/42 MAPK, properties proposed as being prerequisites for tissue preserving agents, it may exhibit cardioprotective activity. Indeed, not only were we able to demonstrate that leptin reduced infarct size substantially, we also showed that its protective effect appeared to involve the PI3K-Akt and p44/42 MAPK pathways. Specific blockers of PI3K-Akt and p44/42 MAPK, that is, LY294002 and UO126, respectively, were found to attenuate the cardioprotective effects of leptin.
The involvement of p44/42 was borne out by the data obtained when cardiac extracts were subjected to Western blot analysis. However, while infarct size studies supported a role for PI3K in leptin-induced protection, this was not corroborated by Western blotting. The 33% increase in Akt phosphorylation recorded did not achieve statistical significance and, therefore, only limited importance can be assigned to this observation. Nevertheless, one could argue that these data merely indicate that the window of maximal activation was missed because the Akt signal was in the process of being downregulated as a result of phosphatase action. Additionally, it has been reported that in hepatocytes, leptin stimulation results in a weak or undetectable phosphorylation of Akt (Zhao et al., 2000), raising the possibility that this may also be the case in the heart. Clearly, however, further studies will need to be carried out in order to establish if Akt plays a role in cardioprotection induced by leptin. The data obtained for p44/42 MAPK were intriguing because the major proportion of the activation signal appeared to be represented by p42. The biological significance of this finding is uncertain as little information is available concerning the relative contributions made by the two isoforms to the normal activity of the enzyme. Apoptosis is an important component of the mechanisms contributing to cell death, including in the heart (Fiers et al., 1999; Gottlieb and Engler, 1999). Interestingly, leptin receptor activation has been shown to be associated with the inhibition of apoptotic pathways, including in neutrophils (Bruno et al., 2005). Further studies, however, are needed in order to establish if leptin reduces apoptosis in the heart.
Adding further weight to the proposal that leptin is cardioprotective via activation of the RISK pathway were the observations made concerning the MPTP. The MPTP may constitute an integral component of the machinery of the cell for reducing damage and promoting protection (Hausenloy et al., 2004b). Hence, apart from demonstrating that leptin delayed MPTP opening and the time to cardiomyocyte contracture, we also found that these actions were inhibited by LY294002 and MEK inhibitor 1, which acts upstream of p44/42 MAPK (Wityak et al., 2004). Recently, we reported that delayed opening of the MPTP induced by insulin was blocked by Wortmannin and LY-294002, providing evidence that signalling via the RISK pathway is linked to closure of the MPTP (Davidson et al., 2006). Interestingly, in the present study L-NAME was also found to inhibit the effects of leptin indicating that it acts via NOS activation. Leptin has been reported to cause activation of NOS in a number of tissues, including the heart (Nickola et al., 2000). We, however, were unable to detect significant leptin-stimulated changes in eNOS phosphorylation in cardiac extracts from Langendorff experiments. However, as suggested for Akt, it is possible that the eNOS signal may have been dissipated by the time the hearts had been removed from the Langendorff apparatus and a stronger signal would have been obtained if sampling had occurred earlier in the reperfusion period.
To produce its regulatory actions on energy balance and satiety, leptin acts via leptin receptor (Ob-R) activation (Zabeau et al., 2003). This results in the phosphorylation of STAT3 (Harvey and Ashford, 2003; Zabeau et al., 2003). Leptin-induced phosphorylation of STAT3 has been demonstrated in the heart, and has been observed following I/R (Stephanou, 2004). In the current study, however, STAT3 phosphorylation was found to be suppressed by leptin treatment. Additionally, the total tissue levels of STAT3 were found to be significantly reduced. These observations may be explained by a number of mechanisms. Under our conditions, it is possible that ischaemia had induced maximal phosphorylation of STAT3 that was subsequently downregulated on reperfusion as a result of the activation of other signalling pathways. It has, for example, been shown that upregulation of p44/42 MAPK may be associated with downregulation of STAT3 phosphorylation (Li et al., 2004). In a study carried out in rat cardiomyocytes treatment with the cytokine, cardiotrophin-1 (CT-1) was found to be associated with the activation of STAT3 (Li et al., 2004). Full activation, as induced by a maximal concentration of CT-1, however, was found to be coupled with simultaneous activation of p44/42 MAPK, which when blocked by UO126 resulted in the activity of STAT3 being increased further, indicating that p44/42 MAPK downregulated STAT3 activity: in other words, cross-talk was occurring between p44/42 MAPK and STAT3. Cross-talk between the cell survival kinases occurs in the heart and has been suggested as playing a role in the mediation of IPC-induced protection (Hausenloy et al., 2004a). In the present study, although leptin treatment was not found to be associated with increased STAT3 phosphorylation, confirmation that cross-talk had occurred between p44/42 MAPK and STAT3 was provided by the finding that UO126 inhibited the leptin-stimulated decrease in STAT3 phosphorylation and also the observed decrease in total STAT3 levels. The latter could be interpreted as being indicative of leptin-induced STAT3 degradation, possibly as a result of proteasome-mediated proteolysis (Daino et al., 2000; Johnston, 2004).
AMPK is another signalling enzyme through which leptin produces its regulatory actions on lipid synthesis and metabolism (Minokoshi et al., 2002). Whereas leptin failed to produce significant effects on AMPK phosphorylation, significant decreases in total AMPK levels were observed that, interestingly, were reversed when UO126 was present. So, as seen for STAT3, cross-talk between AMPK and p44/42 MAPK appeared to have occurred, together with downregulation of total AMPK. Indeed, a body of evidence for cross-talk between AMPK and various cell-signalling pathways has accumulated. Thus, adiponectin, another adipocytokine, was shown to stimulate angiogenesis by promoting cross-talk between AMPK and Akt (Ouchi et al., 2004). Meanwhile in the myocardium, Akt activity was found to negatively regulate AMPK phosphorylation (Kovacic et al., 2003). Evidence for cross-talk between p44/42 MAPK and AMPK was obtained in vascular smooth muscle (Rubin et al., 2005). In addition, adiponectin-mediated modulation of hypertrophic signals in the heart was recently reported to involve communication between AMPK and p44/42 MAPK (Shibata et al., 2004).
Leptin has been reported to stimulate the activation of various intracellular signal-transduction pathways apart from Akt, p44/42 MAPK, eNOS, AMPK and STAT3. For example, leptin induces p38 MAPK activation in skeletal muscle and C2C12 cells (Maroni et al., 2003, 2005). Leptin-induced hypertrophy in ventricular myocytes and smooth muscle cells also involves p38 MAPK phosphorylation (Rajapurohitam et al., 2003; Shin et al., 2005). Protection against I/R injury induced by atorvastatin was found to be associated with p38 MAPK phosphorylation, which was abolished by SB203580, a p38 MAPK inhibitor (Efthymiou et al., 2005). It has to be said, however, that the significance of p38 phosphorylation with regard to cardioprotection is uncertain. Contradictory results that may reflect the various experimental procedures used have been reported. Indeed, as discussed by Schulz et al. (2003), differential activation/downregulation of p38MAPKα and p38MAPKβ isoforms can occur as a consequence of different experimental treatments and could be responsible for the variability seen. We found that leptin caused a significant increase in p38 MAPK activation, but further studies are necessary in order to establish if p38 MAPK plays a pivotal role in leptin-induced cardioprotection.
With regard to the Western blot data, we recognize that these must be approached with caution. It was derived from entire hearts, that is, hearts in which no distinction between viable and infarcted tissue was made. Thus, assuming, for example, that RISK activation is only associated with viable tissue, the possibility that Akt or p44/42 phosphorylation might merely reflect that proportion of the tissue in a heart that is viable and not increased Akt or p44/42 phosphorylation per se must be considered.
Thus, the key findings of our investigation were that leptin administered at the time of reperfusion reduced infarct size significantly and delayed the opening of the MPTP. These data indicate that leptin reduces reperfusion-induced cell death and that the protection afforded by this agent is mediated via upregulation of RISK pathway components. As leptin is produced by the heart and the long form of the leptin receptor, Ob-Rb, which contains all the elements necessary for functional leptin signalling, is present on cardiomyocytes, one could make a case for leptin representing an ‘inbuilt' cardioprotective factor (Purdham et al., 2004). Hence, leptin could function in an autocrine manner, being released by the heart during I/R and feeding back onto the myocardium to regulate cardiac activity and limit damage. This is, however, pure speculation and considerably more work is needed before this theory can be confirmed or rejected. In future studies, hearts from animals that have been genetically modified, that is, mice that are leptin-deficient (ob/ob) or leptin receptor-deficient (db/db), should be examined. This would allow for a more detailed examination of the mechanisms by which leptin protects the myocardium against I/R injury. Although this is the first study to demonstrate that leptin possesses cardioprotective properties, a recent report shows that the adipocytokine, adiponectin, also exhibits similar properties (Shibata et al., 2005). It is therefore interesting to speculate that adipocytokines as a whole may offer a new area of investigation in our efforts to find new ways of protecting the ischaemia and reperfused myocardium.
Acknowledgments
This project was supported by a Programme Grant from the British Heart Foundation.
Abbreviations
- Akt
cellularAkt/protein kinase B
- AMPK
AMP-activated kinase
- BCA
bicinchoninic acid
- CsA
cyclosporin A
- CT-1
cardiotrophin 1
- ECL
enhanced chemiluminescence
- eNOS
endothelial nitric oxide synthase
- ERK
extracellular signal-regulated MAPK
- IPC
ischaemic preconditioning
- I/R
ischaemia–reperfusion
- JAK
janus kinase
- MAPK
mitogen-activated protein kinase
- MPTP
mitochondrial permeability transition pore
- PI3K
phosphatidylinositol 3-OH kinase
- RISK
reperfusion injury salvage kinase
- SDS-PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- STAT
signal transducer and activator of transcription
- TMRM
tetra-methyl rhodamine methyl ester
- TTC
triphenyltetrazolium chloride
Conflict of interest
The authors state no conflict of interest.
References
- Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischaemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- Bruno A, Conus S, Schmid I, Simon HU. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J Immunol. 2005;174:8090–8096. doi: 10.4049/jimmunol.174.12.8090. [DOI] [PubMed] [Google Scholar]
- Brzozowski T, Konturek PC, Pajdo R, Kwicien S, Ptak A, Sliwowski Z, et al. Brain–gut axis in gastroprotection by leptin and cholecystokinin against ischaemia–reperfusion induced gastric lesions. J Physiol Pharmacol. 2001;52:583–602. [PubMed] [Google Scholar]
- Bullard AJ, Govewalla P, Yellon DM. Erythropoietin protects the myocardium against reperfusion injury in vitro and in vivo. Basic Res Cardiol. 2005;100:397–403. doi: 10.1007/s00395-005-0537-4. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Daino H, Matsumura I, Takada K, Odajima J, Tanaka H, Ueda S, et al. Induction of apoptosis by extracellular ubiquitin in human hematopoietic cells: possible involvement of STAT3 degradation by proteasome pathway in interleukin 6-dependent hematopoietic cells. Blood. 2000;95:2577–2585. [PubMed] [Google Scholar]
- Davidson SM, Hausenloy D, Duchen MR, Yellon DM. Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int J Biochem Cell Biol. 2006;38:414–419. doi: 10.1016/j.biocel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Efthymiou CA, Mocanu MM, Yellon DM. Atorvastatin and myocardial reperfusion injury: new pleiotropic effect implicating multiple prosurvival signaling. J Cardiovasc Pharmacol. 2005;45:247–252. doi: 10.1097/01.fjc.0000154376.82445.06. [DOI] [PubMed] [Google Scholar]
- Erkasap S, Erkasap N, Koken T, Kahraman A, Uzuner K, Yazihan N, et al. Effect of leptin on renal ischaemia–reperfusion damage in rats. J Physiol Biochem. 2004;60:79–84. doi: 10.1007/BF03168443. [DOI] [PubMed] [Google Scholar]
- Fiers W, Beyaert R, Declerq W, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–7730. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- Gottlieb RA, Engler RL. Apoptosis in myocardial ischaemia–reperfusion. Ann NY Acad Sci. 1999;874:412–426. doi: 10.1111/j.1749-6632.1999.tb09255.x. [DOI] [PubMed] [Google Scholar]
- Harvey J, Ashford ML. Leptin in the CNS: much more than a satiety signal. Neuropharmacology. 2003;44:845–854. doi: 10.1016/s0028-3908(03)00076-5. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia–reperfusion injury. Cardiovasc Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Mocanu MM, Yellon DM. Cross-talk between the survival kinases during early reperfusion: its contribution to ischaemic preconditioning. Cardiovasc Res. 2004a;63:305–312. doi: 10.1016/j.cardiores.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Wynne A, Duchen M, Yellon DM. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004b;109:1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia–reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. Realizing the clinical potential of ischemic preconditioning and postconditioning. Nat Clin Pract. 2005;2:568–575. doi: 10.1038/ncpcardio0346. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–253. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM, Mani-Babu S, Duchen MR. Preconditioning protects by inhibiting the mitochondrial permeability transition. Am J Physiol Heart Circ Physiol. 2004c;287:H841–H849. doi: 10.1152/ajpheart.00678.2003. [DOI] [PubMed] [Google Scholar]
- Jacobson J, Duchen MR. Mitochondrial oxidative stress and cell death in astrocytes – requirement for stored Ca2+ and sustained opening of the permeability transition pore. J Cell Sci. 2002;115:1175–1188. doi: 10.1242/jcs.115.6.1175. [DOI] [PubMed] [Google Scholar]
- Johnston JA. Are SOCS suppressors, regulators, and degraders. J Leukocyte Biol. 2004;75:743–748. doi: 10.1189/jlb.1003507. [DOI] [PubMed] [Google Scholar]
- Jonassen AK, Sack MN, Mjos OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res. 2001;89:1191–1198. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- Li YJ, Cui W, Tian ZJ, Hao YM, Du J, Liu F, et al. Crosstalk between ERK1/2 and STAT3 in the modulation of cardiomyocyte hypertrophy induced by cardiotrophin-1. Chin Med J (England) 2004;117:1135–1142. [PubMed] [Google Scholar]
- Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischaemic injury. J Clin Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroni P, Bendinelli P, Piccoletti R. Early intracellular events induced by in vivo leptin treatment in mouse skeletal muscle. Mol Cell Endocrinol. 2003;201:109–121. doi: 10.1016/s0303-7207(02)00427-6. [DOI] [PubMed] [Google Scholar]
- Maroni P, Bendinelli P, Piccoletti R. Intracellular signal transduction pathways induced by leptin in C2C12 cells. Cell Biol Int. 2005;29:542–550. doi: 10.1016/j.cellbi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Nickola MW, Wold LE, Colligan PB, Wang GJ, Samson WK, Ren J. Leptin attenuates cardiac contraction in rat ventricular myocytes. Role of NO. Hypertension. 2000;36:501–505. doi: 10.1161/01.hyp.36.4.501. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdham DM, Zou MX, Rajapurohitam V, Karmazyn M. Rat heart is a site of leptin production and action. Am J Physiol Heart Circ Physiol. 2004;287:H2877–H2884. doi: 10.1152/ajpheart.00499.2004. [DOI] [PubMed] [Google Scholar]
- Rajapurohitam V, Gan XT, Kirshenbaum LA, Karmazyn M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res. 2003;93:277–279. doi: 10.1161/01.RES.0000089255.37804.72. [DOI] [PubMed] [Google Scholar]
- Ren J. Leptin and hyperleptinemia – from friend to foe for cardiovascular function. J Endocrinol. 2004;181:1–10. doi: 10.1677/joe.0.1810001. [DOI] [PubMed] [Google Scholar]
- Rubin LJ, Magliola L, Feng X, Jones AW, Hale CC. Metabolic activation of AMP kinase in vascular smooth muscle. J Appl Physiol. 2005;98:296–306. doi: 10.1152/japplphysiol.00075.2004. [DOI] [PubMed] [Google Scholar]
- Schulz R, Gres P, Skyschally A, Duschin A, Belosjorow S, Konietzka I, et al. Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. FASEB. 2003;17:1355–1357. doi: 10.1096/fj.02-0975fje. [DOI] [PubMed] [Google Scholar]
- Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Sato K, Pimenetel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischaemia–reperfusion injury through AMPK- and COX-2 dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HJ, Oh J, Kang SM, Lee JH, Shin MJ, Hwang KC, et al. Leptin induces hypertrophy via p38 mitogen-activated protein kinase in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 2005;329:18–24. doi: 10.1016/j.bbrc.2004.12.195. [DOI] [PubMed] [Google Scholar]
- Shirasaka T, Takasaki M, Kannan H. Cardiovascular effects of leptin and orexins. Am J Physiol Regul Integr Comp Physiol. 2003;284:R639–R651. doi: 10.1152/ajpregu.00359.2002. [DOI] [PubMed] [Google Scholar]
- Stephanou A. Role of STAT-1 and STAT-3 in ischaemia/reperfusion injury. J Cell Mol Med. 2004;8:519–525. doi: 10.1111/j.1582-4934.2004.tb00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumeray MS, Yellon DM. Characterisation and validation of a murine model of global ischaemia–reperfusion injury. Mol Cell Biochem. 1998;186:61–68. [PubMed] [Google Scholar]
- Tajmir P, Ceddia RB, Li RK, Coe IR, Sweeney G. Leptin increases cardiomyocyte hyperplasia via extracellular signal-regulated kinase- and phosphatidylinositol 3-kinase-dependent signaling pathways. Endocrinology. 2004;145:1550–1555. doi: 10.1210/en.2003-1128. [DOI] [PubMed] [Google Scholar]
- Winnicki M, Phillips BG, Accurso V, Van De BP, Shamsuzzaman A, Patil K, et al. Independent association between plasma leptin levels and heart rate in heart transplant recipients. Circulation. 2001;104:384–386. doi: 10.1161/hc2901.094150. [DOI] [PubMed] [Google Scholar]
- Wityak J, Hobbs FW, Gardner DS, Santella JB, Petraitis JJ, Sun JH, et al. Beyond U0126. Dianion chemistry leading to the rapid synthesis of a series of potent MEK inhibitors. Bioorg Med Chem Lett. 2004;14:1483–1486. doi: 10.1016/j.bmcl.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Baxter G. Reperfusion injury revisited: is there a role for growth factor signaling in limiting lethal reperfusion injury. Trends Cardiovasc Med. 1999;9:245–249. doi: 10.1016/s1050-1738(00)00029-3. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- Zabeau L, Lavens D, Peelman F, Eyckerman S, Vandekerckhove J, Tavernier J. The ins and outs of leptin receptor activation. FEBS Lett. 2003;546:45–50. doi: 10.1016/s0014-5793(03)00440-x. [DOI] [PubMed] [Google Scholar]
- Zhao AZ, Shinohara MM, Huang D, Shimizu M, Eldar-Finkelman H, Krebs EG, et al. Leptin induces insulin-like signaling that antagonizes cAMP elevation by glucagon in hepatocytes. J Biol Chem. 2000;275:11348–11354. doi: 10.1074/jbc.275.15.11348. [DOI] [PubMed] [Google Scholar]