Abstract
Activation of JAK2 by chromosomal translocation or point mutation is a recurrent event in hematopoietic malignancies, including acute leukemias and myeloproliferative disorders. Although the effects of activated JAK2 signaling have been examined in cell lines and murine models, the functional consequences of deregulated JAK2 in the context of human hematopoietic cells are currently unknown. Here we report that expression of TEL-JAK2, a constitutively active variant of the JAK2 kinase, in lineage-depleted human umbilical cord blood cells results in erythropoietin-independent erythroid differentiation in vitro and induces the rapid development of myelofibrosis in an in vivo NOD/SCID xenotransplantation assay. These studies provide functional evidence that activated JAK2 signaling in primitive human hematopoietic cells is sufficient to drive key processes implicated in the pathophysiology of polycythemia vera and idiopathic myelofibrosis. Furthermore, they describe an in vivo model of myelofibrosis initiated with primary cells, highlighting the utility of the NOD/SCID xenotransplant system for the development of experimental models of human hematopoietic malignancies.
Keywords: myeloproliferative disorders, NOD/SCID, cord blood
The JAK family of tyrosine kinases are key mediators of cytokine signal transduction in hematopoietic cells and consequently play a central role in the immune response and in the regulation of blood cell production (1). Multiple lines of evidence indicate that these kinases, in particular JAK2, are frequently deregulated in hematologic malignancies. Chromosomal translocations involving fusion of the Ets-family transcription factor TEL to JAK2 have been identified in patients with atypical chronic myelogenous leukemia (aCML) as well as T and B cell acute lymphoblastic leukemia (2, 3). These translocations juxtapose the oligomerization domain of TEL and the catalytic JH1 domain of JAK2, resulting in constitutive activation of the tyrosine kinase and its downstream targets, including STAT5, ERK1/2, and AKT (4–8). Recent studies have identified comparable fusion genes in which the coiled-coil domains of pericentriolar material 1 (PCM1) and breakpoint cluster region (BCR) are joined to the catalytic domain of JAK2 in a number of patients with acute leukemias, aCML and myeloproliferative disorders (9–14). Furthermore, a recurrent somatic mutation in the JAK2 gene, resulting in a valine-to-phenylalanine substitution at amino acid 617 (V617F), is found in the majority of patients with polycythemia vera (PV) and approximately half of essential thrombocythemia (ET) and idiopathic myelofibrosis (IMF) patients (15–18). The V617F mutation occurs in the JH2 pseudokinase domain of JAK2 and is thought to decrease negative autoregulation of the kinase, thereby leading to constitutive activation of STAT5 as well as the ERK and PI-3 kinase pathways (18). Given the widespread involvement of deregulated JAK2 signaling in hematologic diseases, an understanding of its effects on the developmental program of hematopoietic cells has the potential to provide important mechanistic insights into the pathophysiology of these malignancies.
To date, the majority of oncogenes associated with acute leukemias and myeloproliferative disorders, including activated JAK2 variants, have predominantly been studied by using leukemic cell lines or mouse models. Expression of TEL-JAK2 in IL-3-dependent Ba/F3 cells confers factor independence, whereas JAK2V617F, which does not constitutively dimerize in the cytoplasm, requires coexpression of a cytokine receptor to effect signaling and transform this cell line (3–5, 17–19). In transplantation studies of retrovirally transduced murine bone marrow (BM) cells, TEL-JAK2 induced a mixed myelo- and lymphoproliferative disorder (5), whereas JAK2V617F expression resulted in an initial erythrocytosis that progressed to myelofibrosis, mimicking disease evolution in PV patients (20, 21). Although these approaches have provided significant insights, important limitations exist. Studies in cell lines are confounded by uncharacterized genetic alterations, including those specific to the establishment of in vitro growth, and cannot provide information regarding the temporal sequence of changes required for transformation. Additionally, there are inherent differences in the molecular mechanisms underlying neoplastic transformation in human and mouse cells (reviewed in ref. 22). Together, these observations underscore the importance of studying the effects of activated JAK2 signaling in the most relevant cellular context, that of primary human hematopoietic cells.
To this end, we have established a system to functionally assess the effects of activated JAK2 signaling in a lineage-depleted fraction of human umbilical cord blood enriched for stem and progenitor cells (Lin-CB). We show that expression of TEL-JAK2 in these cells drives erythropoietin (EPO)-independent erythropoiesis in vitro and the rapid development of myelofibrosis in NOD/SCID mice. Together, these findings establish that activated JAK2 signaling in primary human hematopoietic cells is sufficient to induce several of the distinct pathophysiological features of the Philadelphia-negative myeloproliferative disorders and demonstrate the utility of xenotransplantation systems to develop in vivo models of human hematopoietic malignancies.
Results
Expression of TEL-JAK2 in Primary Human Hematopoietic Cells Drives EPO-Independent Erythropoiesis.
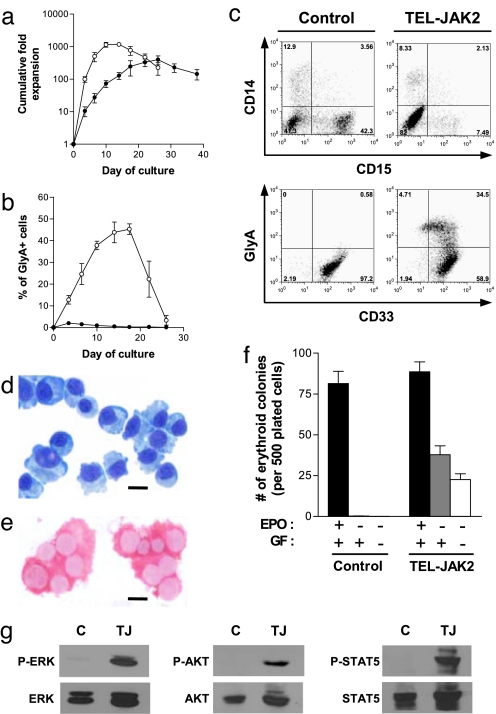
To determine the biological effects of activated JAK2 signaling in primary human hematopoietic cells, Lin-CB cells were transduced with lentiviral vectors encoding either TEL-JAK2 or EGFP cDNAs and seeded into suspension cultures under conditions that promote myeloid differentiation. Control (EGFP) cells expanded steadily in culture, yielding populations of CD14+ monocyte-macrophages and CD15+ granulocytes. In contrast, cells expressing TEL-JAK2 underwent a proliferative burst over the first 14 days of culture (Fig. 1a), coincident with the expansion of myeloid lineage cells, as well as a population of cells that expressed the erythroid-specific marker glycophorin A (GlyA), displayed erythroblastic morphology and produced hemoglobin A (Fig. 1 b–e). These cells acquired CD36 and GlyA in a temporal manner consistent with normal erythroid differentiation (Fig. 5, which is published as supporting information on the PNAS web site; ref. 23) but also expressed the myeloid antigen CD33, normally present on only a small percentage of control GlyA+ erythroblasts generated in vitro (Fig. 1c and Fig. 6, which is published as supporting information on the PNAS web site). Notably, in myeloid-promoting culture conditions (Fig. 1 a–e), TEL-JAK2 supported erythroid expansion in the absence of exogenous EPO. In fact, cells expressing TEL-JAK2 were capable of extensive growth and erythroid differentiation in cultures that were not supplemented with any cytokines (Fig. 7, which is published as supporting information on the PNAS web site). Consistent with these findings from suspension culture, TEL-JAK2-expressing progenitors, but not control cells, were capable of generating erythroid colonies in methylcellulose assays in the absence not only of EPO but also of any exogenous cytokines (Fig. 1f). Studies in the Ba/F3 cell line have indicated that TEL-JAK2 confers factor independence through activation of STAT5, ERK, and PI-3 kinase (6–8). Of note, these same signaling pathways were constitutively activated in cultured cord blood cells expressing TEL-JAK2 (Fig. 1g), providing a potential mechanism for the observed factor independence and establishing a parallel with the JAK2V617F point mutant (18). Together, these data demonstrate that the expression of TEL-JAK2 confers EPO independence to human erythroid progenitors, a hallmark feature of PV (24).
Fig. 1.
Expression of TEL-JAK2 induces EPO-independent erythropoiesis. (a) Growth of control (●) and TEL-JAK2-transduced (○) cells in myeloid-promoting suspension cultures. (b) Percentage of GlyA+ cells generated in cultures of control and TEL-JAK2-transduced cells. (c) Flow cytometric analysis of cells after 12 days of culture. (d and e) Cytospin preparation of sorted GlyA+ TEL-JAK2-expressing cells from day 12 of culture assessed by May–Grünwald–Giemsa staining (d) and immunostained for hemoglobin A (e). (Scale bars, 10 μm.) (f) Erythroid colony formation in methylcellulose cultures supplemented with varying combinations of myeloid growth factors (GF) and EPO. (g) Western blot analysis showing constitutive phosphorylation of ERK, AKT, and STAT5 in TEL-JAK2 cells. Control (C) or TEL-JAK2-expressing (TJ) cells were grown for 13 days in suspension culture, then cytokine-depleted for 6 h before harvest. Error bars in a, b, and f indicate SEM from five experiments.
Expression of TEL-JAK2 Alters the Lineage Distribution of Human Cells Generated in Transplanted NOD/SCID Mice.
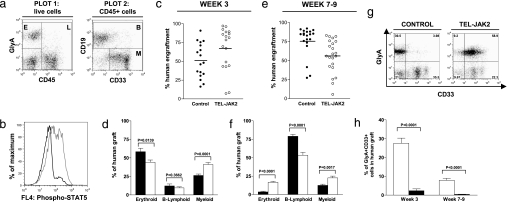
We next investigated the effects of activated JAK2 signaling in human multipotent repopulating cells using the NOD/SCID xenotransplantation assay. Lin-CB cells were transduced with either TEL-JAK2 or control EGFP lentiviruses, then intrafemorally injected into recipients, a transplantation protocol that supports high levels of engraftment by human erythroid and myeloid cells (25). Recipients of control and TEL-JAK2-expressing cells (hereafter referred to as control and TEL-JAK2 mice, respectively) displayed high levels of human engraftment at 3 weeks posttransplant (Fig. 2a and c). Furthermore, an increase in the level of STAT5 phosphorylation was observed in human cells within the BM of TEL-JAK2 mice (Fig. 2b), confirming successful repopulation by cells with activated JAK2 signaling. Interestingly, the composition of the human graft varied between the two groups of mice at 3 weeks posttransplant (Fig. 2d and Table 1, which is published as supporting information on the PNAS web site). Compared with controls, the percentage and absolute number of myeloid (CD33+CD19–CD45+) cells within the human graft was significantly higher in the BM of TEL-JAK2 mice. Mice killed between 7 and 9 weeks posttransplant also displayed high levels of human engraftment in the BM (Fig. 2e). As expected for this time point of analysis (26), the human graft in control recipients was predominantly B lymphoid (CD19+CD45+), with minor contributions from the erythroid (GlyA+CD45−) and myeloid lineages. In comparison, as shown in Fig. 2f and Table 1, the level of B lymphoid cells in the BM of TEL-JAK2 mice was markedly decreased, whereas the percentages of erythroid and myeloid cells were significantly increased, although this did not correspond to a higher absolute number of human cells in either lineage because of the low BM cellularity in these mice (see below). Taken together, these findings indicate that expression of TEL-JAK2 in Lin-CB results in the generation of a different spectrum of progeny upon transplantation into NOD/SCID mice, with a decreased output of B lymphoid cells and a greater proportion of myeloid cells. However, the extension of these differences in lineage distribution to human patients with deregulated JAK2 signaling should be cautious, because the myeloid/B cell ratio in repopulated NOD/SCID mice differs significantly from that observed in human BM (26).
Fig. 2.
Characterization of the human graft in transplanted NOD/SCID mice. (a) Flow cytometric analysis of the BM of a representative TEL-JAK2 mouse at 3 weeks posttransplant (wpt). Plot 1, GlyA vs. CD45 analysis showing human erythroid cells (E, GlyA+CD45− cells) and human leukocytes (L, CD45+ cells). Plot 2, CD45+ cells analyzed for CD33 and CD19 expression, illustrating human B lymphoid cells (B, CD19+CD45+) and myeloid cells (M, CD33+CD19−CD45+). (b) Intracellular flow cytometry showing activation of STAT5 within the BM of a representative TEL-JAK2 mouse (gray line) vs. a control mouse (black line), at 3 wpt. Plot is gated on human CD45+ cells. (c and e) The percentage of human cells (sum of E and L from a) in the BM of control and TEL-JAK2 mice at 3 (c) and 7–9 (e) wpt. Each circle represents an individual mouse, and the mean is indicated by a line. (d and f) The percentages of erythroid, B lymphoid, and myeloid cells (as defined in a) within the human graft of control (black bars) and TEL-JAK2 (white bars) mice at 3 (d) and 7–9 (f) wpt. (g) Expression of GlyA and CD33 on human cells in the BM of transplanted mice at 3 wpt. (h) Proportion of cells coexpressing GlyA and CD33 (corresponding to the top right quadrant of g) within the human graft of control and TEL-JAK2 mice. Errors bars in d, f, and h indicate SEM; for 3 wpt, n = 17 for control, n = 18 for TEL-JAK2; for 7–9 wpt, n = 20 for control, n = 21 for TEL-JAK2.
Interestingly, despite the striking in vitro effects of TEL-JAK2 upon erythropoiesis, transplanted mice did not develop erythrocytosis in vivo. At both time points of analysis, human GlyA+ cells were undetectable in the peripheral blood (data not shown), and the absolute number of erythroid cells in the BM of TEL-JAK2 mice was not significantly greater than in control mice (Table 1). However, expression of TEL-JAK2 did enhance the output of GlyA+ cells coexpressing CD33 in the BM of transplanted mice (Fig. 2 g and h), drawing a parallel to our in vitro findings. Furthermore, BM cells from TEL-JAK2 mice were capable of generating erythroid colonies in the absence of EPO (data not shown). These findings illustrate that TEL-JAK2 expression does affect the emerging erythroid lineage in vivo and suggest that the lack of human erythrocytosis in recipient mice could be a consequence of the xenograft environment. In both control and TEL-JAK2 mice, high levels of erythroid engraftment in the BM are limited to the early posttransplant period. However, even at this time, mature human erythrocytes cannot be detected in the peripheral blood despite the high levels of erythroid progenitors in the BM, possibly due to the shortened half-life of mature human red cells in murine circulation (27).
NOD/SCID Mice Transplanted with TEL-JAK2-Expressing Cells Rapidly Develop Myelofibrosis.
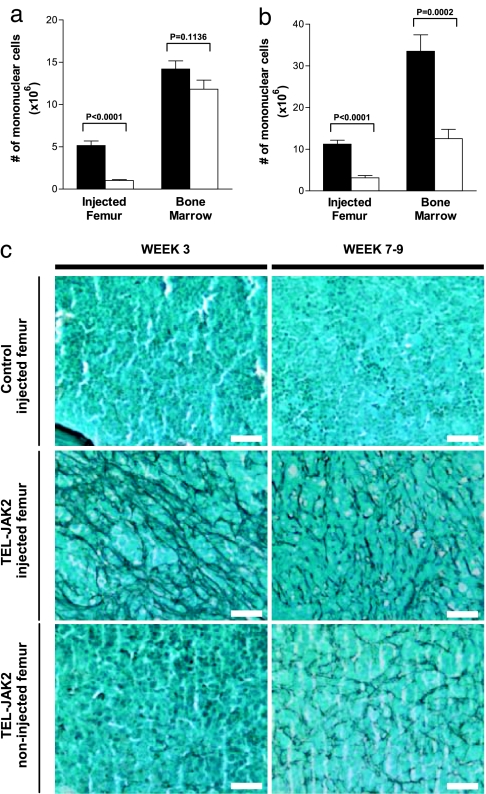
Given the recent association of activated JAK2 signaling with IMF, we assessed the effects of TEL-JAK2 expression on BM cellularity and architecture in transplanted mice. At 3 weeks posttransplantation, a striking reduction in mononuclear cell numbers (both murine and human) was noted in the injected femur of TEL-JAK2 mice compared with controls (Fig. 3a). Histological examination of these marrows confirmed hypocellularity and showed patent sinusoids and a dense network of reticulin fibers, all key histopathological findings in myelofibrosis (Figs. 3c and 4c). These myelofibrotic changes were predominantly localized to the injected femur; the remainder of the BM showed a slight nonsignificant decrease in cellularity compared with controls and was predominantly reticulin negative (Fig. 3 a and c).
Fig. 3.
Development of myelofibrosis in the BM of TEL-JAK2 mice. (a and b) The number of mononuclear cells in the injected femur and remaining BM (noninjected femur, tibiae, and pelvis) at 3 wpt (a, n = 11 for control; n = 10 for TEL-JAK2) and 7–9 wpt (b, n = 12 for control; n = 11 for TEL-JAK2). Error bars indicate SEM. (c) Representative reticulin staining of longitudinal sections of femurs from mice at 3 (left column) and 7–9 weeks (right column) posttransplant. (Scale bars, 25 μm.)
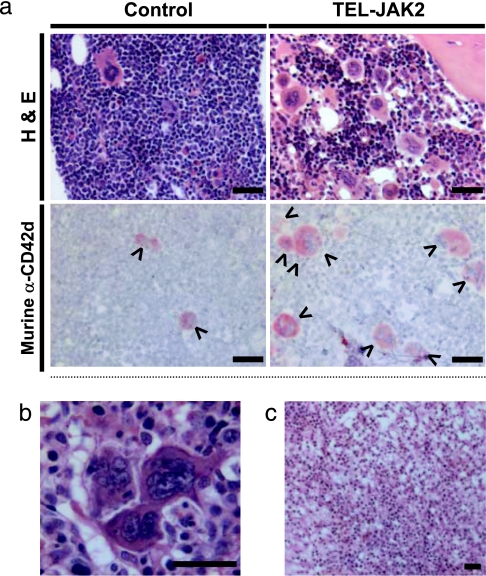
Fig. 4.
Murine megakaryocyte hyperplasia and atypia in the BM of TEL-JAK2 mice. (a) Representative H&E staining and mouse-specific CD42d immunostaining of femur sections from mice at 7–9 wpt. Murine (arrowheads, CD42d+) megakaryocytes are highlighted. (b) High-magnification image of the BM of a TEL-JAK2 mouse at 9 wpt showing a cluster of megakaryocytes with a dispersed chromatin pattern. (c) Representative H&E section from the femur of a TEL-JAK2 mouse at 3 wpt illustrating the absence of overt megakaryocytic hyperplasia. (Scale bars, 25 μm.)
Interestingly, TEL-JAK2 mice killed 7–9 weeks posttransplant exhibited progression from the localized myelofibrosis observed in mice at the 3-week time point. Cellularity was reduced not only in the injected femur but also in the remainder of the BM (Fig. 3b). Extensive networks of reticulin fibers were present in all bones examined histologically (Fig. 3c). Consistent with this extensive fibrosis, TEL-JAK2 mice killed at 7–9 weeks posttransplant had a normochromic normocytic anemia, with reduced hematocrit [0.31 ± 0.026 (n = 9) vs. 0.43 ± 0.021 in controls (n = 11); P = 0.0022] but no overt leukocytosis or alterations in platelet count. Furthermore, the BM of TEL-JAK2 mice, in contrast to controls, exhibited patent sinusoids and increased numbers of megakaryocytes displaying cytologic and architectural atypia such as aggregation in clusters, nuclear hyperlobulation, and a dispersed chromatin pattern (Fig. 4a and b). Interestingly, these atypical megakaryocytes were murine in origin, as evidenced by positive staining for murine-specific CD42d (Fig. 4a), negative staining for both human-specific Alu repeats and human-specific CD61 (Fig. 8, which is published as supporting information on the PNAS web site), and FISH analysis, which showed positive hybridization for murine centromeric DNA sequences in this cell type (Fig. 9, which is published as supporting information on the PNAS web site). These findings suggest that TEL-JAK2 expression in human hematopoietic cells has cell nonautonomous effects on the murine megakaryocytic lineage in repopulated NOD/SCID mice. Notably, despite the extensive fibrosis observed in the injected femur of TEL-JAK2 mice 3 weeks posttransplant, overt megakaryocytic hyperplasia was not evident at this time point (Fig. 4c). Thus, these observations suggest that the expanded megakaryocyte population need not be part of the malignant clone in myelofibrosis, and that extensive fibrosis can be initiated without involvement from this lineage.
A defining feature of this xenotransplant model is the rapid onset and progression of myelofibrosis. Initially localized to the injected femur at 3 weeks, fibrosis eventually progressed to involve distant bones with time, a pattern consistent with the migration of transduced cells from the initial injection site (25). Peripheral blood films did not show marked reticulocytosis, poikylocytosis, or anisocytosis, and the spleens of these animals were not enlarged compared with control mice (data not shown). Consistent with the lack of overt splenomegaly in TEL-JAK2 mice, flow cytometric analysis, murine colony-forming assays, and histological examination of spleens did not demonstrate significant extramedullary murine hematopoiesis above the level routinely noted in NOD/SCID recipients with high levels of human cell engraftment (data not shown). Thus, the myelofibrotic changes in TEL-JAK2 mice occurred rapidly and in the absence of compensatory changes in murine hematopoiesis, features seen in patients with the rare clinical entity of acute myelofibrosis (28).
To rule out the possibility that the observed in vivo phenotype was due to cotransplantation of TEL-JAK2-expressing mesenchymal stem/progenitor cells in the small CD45− population present in Lin-CB preparations, CD45+CD34+ hematopoietic cells were sorted, transduced, and injected into NOD/SCID mice (Fig. 10, which is published as supporting information on the PNAS web site). CFU-F assays detected no mesenchymal stem/progenitor cells in the sorted cell fractions either before or after viral transduction (data not shown). At 3 weeks posttransplant, mice injected with CD34+CD45+ cells expressing TEL-JAK2 developed extensive networks of reticulin fibers in the injected femur, affirming that transduced hematopoietic cells played a causative role in the development of marrow fibrosis.
Discussion
In this study, we have used lentiviral transduction of Lin-CB to investigate the effects of activated JAK2 signaling in primary human hematopoietic cells. TEL-JAK2 expression supports EPO-independent erythroid differentiation and proliferation in vitro and the rapid onset of extensive marrow fibrosis in NOD/SCID mice in vivo. Our findings are particularly notable in light of the recent identification of the activating JAK2V617F mutation in patients with PV, ET, and IMF (15–18). EPO-independent growth is a defining characteristic of erythroid progenitors in PV and, to a lesser extent, IMF and ET, whereas marrow fibrosis is the hallmark feature of IMF, also seen in late-stage PV and ET. Although TEL-JAK2 differs from the JAK2V617F mutant in that it constitutively dimerizes in the cytoplasm and therefore does not require a cytokine receptor scaffold for its signaling activities (19), both JAK2 variants activate similar downstream pathways (refs. 4, 6–8, and 18; see also Fig. 1g). Thus, our studies establish that, in the context of primary human cells, activated JAK2 signaling, likely propagated by STAT5, ERK1/2, and AKT, is sufficient to induce several of the distinct pathophysiological features of the Philadelphia-negative myeloproliferative disorders.
Our data showing that TEL-JAK2 expression drives EPO-independent erythropoiesis are consistent with previous work showing that JAK2 signaling is required for the formation of endogenous erythroid colonies in PV (18, 29). In addition to TEL-JAK2, the expression of BCR-ABL, STAT5(1*6) or BCL-XL has similarly been shown to induce EPO independence in human progenitor cells (30–32). Together, these studies suggest that the constitutive activation of STAT5 and up-regulation of its targets including BCL-XL play a central role in this process.
Notably, this work provides a direct association between TEL-JAK2 and myelofibrosis. Myelofibrotic changes have not been noted in the three patients in whom this translocation has been identified (2, 3) or in recipients of murine BM expressing TEL-JAK2 (5). However, our finding that activated JAK2 signaling can induce myelofibrosis is supported by a number of observations. First, murine BM transplant studies have shown that overexpression of constitutively active STAT5a or oncostatin M, downstream targets of JAK2, results in the development of marrow fibrosis and has cell nonautonomous effects (33). Second, myelofibrosis has been noted in the BM of 40% of patients with the highly related PCM1-JAK2 translocation. Similar to TEL, PCM1 is thought to mediate cytoplasmic dimerization of the fusion protein, resulting in constitutive activation of the JAK2 kinase domain (10–14). Last, as previously discussed, the activating JAK2V617F mutation has been identified in ≈50% of IMF patients (15–18). Our study extends these clinical associations by demonstrating that activated JAK2 signaling in primary human hematopoietic cells plays a causal role in marrow fibrosis, likely by inducing the secretion of fibrogenic cytokines that can activate mesenchymal cells, such as oncostatin M and/or TGF-β (33, 34). Furthermore, our study indicates that the megakaryocytic hyperplasia observed in myelofibrosis patients could be a reactive event that is not absolutely required for the initiation of BM stromal changes. This observation has important implications regarding the pathogenesis of this disease process. Investigation of the cytokines secreted by cells expressing TEL-JAK2 may provide further insight into the cell nonautonomous effects on the murine megakaryocytic lineage and BM stromal elements that we observed in vivo.
NOD/SCID mice transplanted with Lin-CB cells expressing TEL-JAK2 develop a hematologic disease bearing similarity to acute myelofibrosis, with rapidly progressive BM fibrosis and anemia arising in the absence of concomitant splenomegaly. These findings are distinct from transplantation studies of murine BM cells expressing Jak2V617F in which mice develop an initial polycythemia that progresses to myelofibrosis over time (20, 21). The differing kinetics of marrow fibrosis are likely in part because of differences in the signaling properties of JAK2V617F and TEL-JAK2. In addition to its requirement for a Type I cytokine receptor for its signaling and transforming activities, it is likely that JAK2V617F, which has decreased negative autoregulation of its kinase domain, is a relatively weak allele compared with TEL-JAK2, which is activated by pointed domain-mediated oligomerization (17, 19, 35). Thus, strong TEL-JAK2-mediated signaling appears to be sufficient to drive the secretion of fibrogenic cytokines in vivo and induce the rapid onset of myelofibrosis, whereas JAK2V617F requires the acquisition of additional genetic/epigenetic changes to initiate this process. Characterization of differences in the signaling profiles and downstream effects of JAK2V617F vs. TEL-JAK2 will facilitate identification of events that cooperate with JAK2V617F in patients to moderate the initiation and progression of myelofibrosis.
To date, there are only a limited number of studies where primary human cells expressing oncogenes have been assayed in an in vivo xenotransplant setting; however, the resulting growth disruptions were generally limited to shifts in the lineage distribution of transduced cells (31, 36–38). In contrast, in this report, the targeted genetic modification of human hematopoietic cells has resulted in the initiation of an in vivomyelofibrosis. Thus, our study demonstrates the feasibility of using a xenograft system to develop models of hematopoietic malignancies that have the potential to enhance our understanding of the processes underlying malignant transformation in human cells.
Methods
Sample Collection and Purification.
Cord blood samples were obtained according to procedures approved by the institutional review boards of the University Health Network and Trillium Hospital and were collected, processed, and stored as described (26). Briefly, Lin-CB cells were purified by negative selection by using the StemSep Human Progenitor Cell Enrichment Kit according to the manufacturer's protocol (Stem Cell Technologies, Vancouver, BC, Canada). Cells expressing GlyA, CD2, CD3, CD14, CD16, CD19, CD24, CD41, CD56, and CD66b were removed in this procedure.
Lentivirus Production.
TEL-JAK2 (5–19) cDNA was cloned into a third-generation lentiviral vector containing an IRES-pac selection cassette (39). A control vector contained the EGFP gene in place of TEL-JAK2. Viral particles pseudotyped with vesicular stomatitis virus G protein were generated as described and titered on 293T cells (39).
Suspension Culture and Methylcellulose Assays.
Lentiviral infection of Lin-CB cells was performed over a period of 48 h, as described (39). Gene transfer efficiency was determined by methylcellulose plating in the presence or absence of 300 ng/ml of puromycin and ranged from 20% to 57% for EGFP and from 6% to 37% for TEL-JAK2. Cells were seeded after infection into myeloid-promoting culture conditions [Iscove's Modified Dulbecco's Medium containing 15% FCS, 20 ng/ml stem cell factor, and 2 ng/ml IL-3 (Amgen, Thousand Oaks, CA)] with 250–300 ng/ml puromycin for selection. Western blotting was performed as described (6, 8) by using antibodies reactive to PKB and phospho-PKB (Cell Signaling Technology, Beverly, MA), ERK1/2 (Upstate Biotechnology, Lake Placid, NY), phospho-ERK1/2 (Santa Cruz Biotechnology, Santa Cruz, CA), STAT5A/B (BD Biosciences, Franklin Lakes, NJ), and phospho-STAT5A/B (Zymed, Carlsbad, CA). Methylcellulose cultures were performed as described (26), supplemented with a full complement of human growth factors with or without 2 units/ml human EPO (Amgen) or in the absence of human cytokines. Details for erythroid differentiation cultures are provided in Supporting Text, which is published on the PNAS web site.
NOD/SCID Xenotransplantation.
Lentiviral transduction of Lin-CB cells for in vivo experiments was performed as described above but limited to 24 h in the presence of 15 ng/ml thrombopoietin and 100 ng/ml stem cell factor (Amgen). Gene transfer efficiencies ranged from 17% to 50% for EGFP and from 14% to 50% for TEL-JAK2. Twenty-four hours before transplant, mice were sublethally irradiated (3.5 Gy) and injected i.p. with 200 μg of anti-CD122 antibody produced as described (40). Eight- to 10-week-old NOD/LtSz-scid/scid (NOD/SCID) mice were transplanted by intrafemoral injection (25) by using 1.25 × 105 Lin-CB cells (corresponding to ≈1 × 105 CD34+ cells) or, for sorting experiments, 1 × 105 CD34+CD45+ cells. Mice were killed at 3 or 7–9 weeks posttransplant and analyzed as described (26). Briefly, the marrow of collected bones was pooled and human engraftment levels were determined by the sum of the human GlyA+CD45− (erythroid) and human CD45+ (leukocyte) populations as assessed by flow cytometry. Peripheral blood was collected from the femoral artery of anesthetized mice, and automated complete blood cell counts were performed on a Beckman Coulter (Fullerton, CA) analyzer. For histopathology, bones were fixed in 10% neutral buffered formalin for 48 h, decalcified in 6% formic acid for 2–3 days, then embedded in paraffin. H&E and reticulin staining were performed on 3- and 5-μm sections, respectively, by using standard methodology.
Immunohistochemistry and in Situ Hybridization.
Cytospin preparations of cultured cells were fixed in acetone, then incubated with a rabbit anti-human hemoglobin A antibody (A118, Dako, Carpenteria, CA) for 2 h. After incubation with a biotinylated goat anti-rabbit secondary, sections were developed by using an alkaline phosphatase–streptavidin-labeling reagent and Vector red (Vector Laboratories, Burlingame, CA). For CD42d immunostaining, 10-μm cryostat sections of mouse BM were fixed in acetone, blocked with 10% horse serum (Vector Laboratories), then incubated overnight with a purified hamster anti-mouse and -rat CD42d antibody (Becton Dickinson, Franklin Lakes, NJ). After incubation with a biotinylated mouse anti-hamster mixture (Becton Dickinson), sections were developed as above. Details pertaining to CD61 immunostaining, Alu in situhybridization, and fluorescence in situhybridization are provided in Supporting Text.
Flow Cytometry.
Analysis and sorting were performed on a Becton Dickinson FACSCalibur and FACSVantage. Cells were stained with antibodies specific for human antigens, including phycoerythrin-conjugated anti-GlyA, CD45, CD14, and CD19 (Becton Dickinson); phycoerythrin cyanin 5-conjugated anti-CD33 and CD15; and allophycocyanin-conjugated anti-CD45 and CD36 (Beckman Coulter). Intracellular flow cytometry was performed by using Alexa Fluor 647-conjugated anti-phospho-STAT5 (Y694) (Becton Dickinson) with slight modification of the manufacturer's protocols. After fixation (Becton Dickinson Cytofix Buffer) and permeabilization with ice-cold 100% methanol, cells were incubated with 4% BSA for 30 min, then simultaneously stained for phospho-STAT5 and human CD45.
Statistical Analysis.
Data are presented as the mean ± SEM. Statistical analysis was performed by using unpaired two-tailed Student's t tests.
Supplementary Material
Acknowledgments
We thank M. Doedens and K. Hope for assistance with intrafemoral injections, R. Nayyar for flow sorting, Dr. T. Tanaka (Osaka University Medical Centre, Osaka, Japan) for supplying the TM-β1 hybridoma cell line, and the University Health Network Pathology Research Program for tissue sectioning and immunohistochemistry. This work was supported by a Canadian Institutes of Health Research (CIHR) MD/PhD studentship (to J.A.K.); a CIHR clinician-scientist award (to F.B.); grants from the CIHR (to J.E.D. and D.L.B.) and Genome Canada through the Ontario Genomics Institute (to J.E.D.); the Ontario Cancer Research Network with funds from the Province of Ontario (to J.E.D.); the Leukemia and Lymphoma Society (to J.E.D.); the National Cancer Institute of Canada (NCIC) with funds from the Canadian Cancer Society (to J.E.D.); the Terry Fox Foundation (to J.E.D.), an NCIC Research Scientist award (to D.L.B.); and a Canada Research Chair (to J.E.D.).
Abbreviations
- PV
polycythemia vera
- ET
essential thrombocythemia
- IMF
idiopathic myelofibrosis
- EPO
erythropoietin
- GlyA
glycophorin A
- BM
bone marrow
- Lin-CB
human umbilical cord blood enriched for stem and progenitor cells.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Yamaoka K, Saharinen P, Pesu M, Holt VE III, Silvennoinen O, O'Shea JJ. Genome Biol. 2004;5:253. doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peeters P, Raynaud SD, Cools J, Wlodarska I, Grosgeorge J, Philip P, Monpoux F, Van Rompaey L, Baens M, Van den BH, et al. Blood. 1997;90:2535–2540. [PubMed] [Google Scholar]
- 3.Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffe M, Berthou C, Lessard M, Berger R, Ghysdael J, et al. Science. 1997;278:1309–1312. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 4.Ho JM, Beattie BK, Squire JA, Frank DA, Barber DL. Blood. 1999;93:4354–4364. [PubMed] [Google Scholar]
- 5.Schwaller J, Frantsve J, Aster J, Williams IR, Tomasson MH, Ross TS, Peeters P, Van Rompaey L, Van Etten RA, Ilaria R, Jr, et al. EMBO J. 1998;17:5321–5333. doi: 10.1093/emboj/17.18.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho JM, Nguyen MH, Dierov JK, Badger KM, Beattie BK, Tartaro P, Haq R, Zanke BW, Carroll MP, Barber DL. Blood. 2002;100:1438–1448. [PubMed] [Google Scholar]
- 7.Lacronique V, Boureux A, Monni R, Dumon S, Mauchauffe M, Mayeux P, Gouilleux F, Berger R, Gisselbrecht S, Ghysdael J, et al. Blood. 2000;95:2076–2083. [PubMed] [Google Scholar]
- 8.Nguyen MH, Ho JM, Beattie BK, Barber DL. J Biol Chem. 2001;276:32704–32713. doi: 10.1074/jbc.M103100200. [DOI] [PubMed] [Google Scholar]
- 9.Griesinger F, Hennig H, Hillmer F, Podleschny M, Steffens R, Pies A, Wormann B, Haase D, Bohlander SK. Genes Chromosomes Cancer. 2005;44:329–333. doi: 10.1002/gcc.20235. [DOI] [PubMed] [Google Scholar]
- 10.Adelaide J, Perot C, Gelsi-Boyer V, Pautas C, Murati A, Copie-Bergman C, Imbert M, Chaffanet M, Birnbaum D, Mozziconacci MJ. Leukemia. 2006;20:536–537. doi: 10.1038/sj.leu.2404104. [DOI] [PubMed] [Google Scholar]
- 11.Bousquet M, Quelen C, De MV, Duchayne E, Roquefeuil B, Delsol G, Laurent G, Dastugue N, Brousset P. Oncogene. 2005;24:7248–7252. doi: 10.1038/sj.onc.1208850. [DOI] [PubMed] [Google Scholar]
- 12.Heiss S, Erdel M, Gunsilius E, Nachbaur D, Tzankov A. Hum Pathol. 2005;36:1148–1151. doi: 10.1016/j.humpath.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Murati A, Gelsi-Boyer V, Adelaide J, Perot C, Talmant P, Giraudier S, Lode L, Letessier A, Delaval B, Brunel V, et al. Leukemia. 2005;19:1692–1696. doi: 10.1038/sj.leu.2403879. [DOI] [PubMed] [Google Scholar]
- 14.Reiter A, Walz C, Watmore A, Schoch C, Blau I, Schlegelberger B, Berger U, Telford N, Aruliah S, Yin JA, et al. Cancer Res. 2005;65:2662–2667. doi: 10.1158/0008-5472.CAN-04-4263. [DOI] [PubMed] [Google Scholar]
- 15.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, et al. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, et al. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 17.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 18.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, et al. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 19.Lu X, Levine R, Tong W, Wernig G, Pikman Y, Zarnegar S, Gilliland DG, Lodish H. Proc Natl Acad Sci USA. 2005;102:18962–18967. doi: 10.1073/pnas.0509714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wernig G, Mercher T, Okabe R, Levine RL, Lee BH, Gilliland DG. Blood. 2006;107:4274–4281. doi: 10.1182/blood-2005-12-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacout C, Pisani DF, Tulliez M, Moreau GF, Vainchenker W, Villeval JL. Blood. 2006;108:1652–1660. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- 22.Rangarajan A, Weinberg RA. Nat Rev Cancer. 2003;3:952–959. doi: 10.1038/nrc1235. [DOI] [PubMed] [Google Scholar]
- 23.Pereira DS, Dorrell C, Ito CY, Gan OI, Murdoch B, Rao VN, Zou JP, Reddy ES, Dick JE. Proc Natl Acad Sci USA. 1998;95:8239–8244. doi: 10.1073/pnas.95.14.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prchal JF, Axelrad AA. N Engl J Med. 1974;290:1382. doi: 10.1056/nejm197406132902419. [DOI] [PubMed] [Google Scholar]
- 25.Mazurier F, Doedens M, Gan OI, Dick JE. Nat Med. 2003;9:959–963. doi: 10.1038/nm886. [DOI] [PubMed] [Google Scholar]
- 26.Guenechea G, Gan OI, Dorrell C, Dick JE. Nat Immunol. 2001;2:75–82. doi: 10.1038/83199. [DOI] [PubMed] [Google Scholar]
- 27.Larochelle A, Vormoor J, Lapidot T, Sher G, Furukawa T, Li Q, Shultz LD, Olivieri NF, Stamatoyannopoulos G, Dick JE. Hum Mol Genet. 1995;4:163–172. doi: 10.1093/hmg/4.2.163. [DOI] [PubMed] [Google Scholar]
- 28.Brunning RD, Matutes E, Flandrin G, Vardiman JW, Bennett J, Harris NL. In: World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Lyon, France: IARC; 2001. pp. 103–104. [Google Scholar]
- 29.Ugo V, Marzac C, Teyssandier I, Larbret F, Lecluse Y, Debili N, Vainchenker W, Casadevall N. Exp Hematol. 2004;32:179–187. doi: 10.1016/j.exphem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Chalandon Y, Jiang X, Hazlewood G, Loutet S, Conneally E, Eaves A, Eaves C. Blood. 2002;99:3197–3204. doi: 10.1182/blood.v99.9.3197. [DOI] [PubMed] [Google Scholar]
- 31.Schuringa JJ, Chung KY, Morrone G, Moore MA. J Exp Med. 2004;200:623–635. doi: 10.1084/jem.20041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcon L, Rivat C, James C, Lacout C, Camara-Clayette V, Ugo V, Lecluse Y, Bennaceur-Griscelli A, Vainchenker W. Blood. 2006;108:1551–1554. doi: 10.1182/blood-2005-10-009514. [DOI] [PubMed] [Google Scholar]
- 33.Schwaller J, Parganas E, Wang D, Cain D, Aster JC, Williams IR, Lee CK, Gerthner R, Kitamura T, Frantsve J, et al. Mol Cell. 2000;6:693–704. doi: 10.1016/s1097-2765(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 34.Chagraoui H, Komura E, Tulliez M, Giraudier S, Vainchenker W, Wendling F. Blood. 2002;100:3495–3503. doi: 10.1182/blood-2002-04-1133. [DOI] [PubMed] [Google Scholar]
- 35.Tzankov A, Heiss A. Hum Pathol. 2006;37:500–502. [Google Scholar]
- 36.Basecke J, Schwieger M, Griesinger F, Schiedlmeier B, Wulf G, Trumper L, Stocking C. Leuk Lymphoma. 2005;46:265–272. doi: 10.1080/10428190400010767. [DOI] [PubMed] [Google Scholar]
- 37.Buske C, Feuring-Buske M, Antonchuk J, Rosten P, Hogge DE, Eaves CJ, Humphries RK. Blood. 2001;97:2286–2292. doi: 10.1182/blood.v97.8.2286. [DOI] [PubMed] [Google Scholar]
- 38.Chalandon Y, Jiang X, Christ O, Loutet S, Thanopoulou E, Eaves A, Eaves C. Leukemia. 2005;19:442–448. doi: 10.1038/sj.leu.2403650. [DOI] [PubMed] [Google Scholar]
- 39.Wang JC, Warner JK, Erdmann N, Lansdorp PM, Harrington L, Dick JE. Proc Natl Acad Sci USA. 2005;102:14398–14403. doi: 10.1073/pnas.0504161102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKenzie JL, Gan OI, Doedens M, Dick JE. Blood. 2005;106:1259–1261. doi: 10.1182/blood-2005-03-1081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.