Abstract
Synaptotagmin, a synaptic vesicle protein involved in Ca2+-regulated exocytosis, displayed direct high affinity interaction with neuronal sodium channels. Monoclonal antibodies directed against synaptotagmins I and II adsorbed in a concentration-dependent and -specific manner [3H]saxitoxin prelabeled sodium channels extracted with detergent from nerve endings. Conversely, co-immunoprecipitation of synaptotagmin was achieved by antibodies against sodium channel subunits. Consistent with the co-immunoprecipitation assays, solubilized [3H]saxitoxin-prelabeled sodium channels were trapped on immobilized maltose binding protein (MBP)-synaptotagmin I. In vitro recombinant protein assays were employed to identify the interaction site of synaptotagmin I, which was located on the cytoplasmic loop between domains I and II of the sodium channel αIIA subunit. The co-immunoprecipitated synaptotagmin-sodium channel complexes were found to be Ca2+-dependent; this effect was mimicked by Ba2+ and Sr2+ but not Mg2+. Finally the complex was shown to be distinct from the synaptotagmin-SNARE protein complex that can selectively interact with presynaptic calcium channels (N and P/Q types). Thus, our findings demonstrate an unexpected and direct interaction between sodium channels and synaptotagmin. The Ca2+-regulated association between sodium channels and a protein implicated in vesicular fusion may have intriguing consequences for the establishment and regulation of neuronal excitability.
One of the major physiological roles of voltage-gated sodium channels is to initiate and propagate action potentials in excitable cells. In neurons, after the generation of a large transient Na+ current at the axon hillock/initial segment or at the first of node Ranvier (reviewed in ref. 1), sodium channels ensure conduction along myelinated or unmyelinated fibers to nerve terminals. Sodium channels also participate in the integration of synaptic input and the modulation of firing properties and mediate the backpropagation of action potentials into the dendritic arborization, in certain types of neurons (reviewed in ref. 1). In addition, sodium channels can produce a non-inactivating Na+ current that only constitutes a small fraction of the total Na+ current but strongly affects neuronal firing properties (for a review, see ref. 2).
At the molecular level, sodium channels purified from rat brain nerve endings are composed of a heterotrimeric complex. The highly glycosylated α subunit (260 kDa), which is the pore-forming protein, is associated noncovalently with the β1 subunit (36 kDa), and with the β2 subunit (33 kDa) via disulfide bonds (for a review, see ref. 3). At least four genes encoding distinct α subtypes that are mainly expressed in the central nervous system have been identified: αI and αII/αIIA (4, 5), αIII (6), and α6 (7). In contrast, each auxiliary subunit is encoded by a single gene (8, 9).
In nerve terminals, the arrival of the depolarizing wave triggers the opening of presynaptic N- and P/Q type calcium channels, producing the calcium influx that induces the fusion of docked synaptic vesicles at the active zones. Multiple pharmacological and biochemical studies have shown that sodium channels are significantly expressed in nerve endings. Recently, electrophysiological recordings in identified mammalian terminals further confirmed the presence of sodium channels. A fast inactivating and tetrodotoxin (TTX)-sensitive Na+ current has been characterized in presynaptic terminals of the giant glutamatergic calyx of Held (10) as well as in cerebellar basket cell terminals (11). The physiological role of presynaptic sodium channels may be to ensure action potential propagation in close proximity to active zones in which regulated Ca2+ exo-endocytosis takes place. However, the possibility that their role in nerve terminals is not restricted to action potential propagation cannot be excluded and they could also be implicated in the complex machinery that controls neurotransmitter release.
In the present study, we investigated whether sodium channels associate with proteins involved in exo-endocytosis. Synaptotagmin, a synaptic vesicle protein involved in Ca2+-regulated exocytosis (reviewed in refs. 12 and 13) was found to display a direct high affinity interaction with sodium channels, demonstrated by co-immunoprecipitation and in vitro recombinant protein binding assays. A binding site was identified on the cytoplasmic loop between domains I and II of the sodium channel αIIA subunit. The synaptotagmin-sodium channel complex was shown to be distinct from the synaptotagmin-SNARE protein complex that associates with voltage-sensitive calcium channels.
Experimental Procedure
Reagents.
mAbs directed against synaptotagmins I and II (mAb 1D12), synaptotagmin II (mAb 8G2b), syntaxin 1 (mAb 10H5), and SNAP-25 (mAb BR05), polyclonal antibodies against synaptotagmin I (Pu 58K) and VAMP 2, and a polyclonal antibody that recognizes both calcium channel α1A and α1B subunits (B1Nt) were generous gifts of M. Takahashi (Mitsubishi Kasei Institute of Life Sciences, Tokyo). The specificity of the distinct synaptotagmin antibodies was confirmed by immunoblotting of recombinant synaptotagmin isoforms I- III and IX (M. Takahashi, personal communication). Antibodies against the sodium channel α subunit used for Western blotting were from Upstate Biotechnology (Lake Placid, NY). Immunoprecipitation experiments were performed either with an antibody directed against a sequence conserved in neuronal sodium channel subtypes αI-III and 6 (561–575) of αII (14) or with an antibody directed against a peptide from the C-terminal domain of the β1 subunit (15). The latter immunoprecipitated a fraction of ≈70% of sodium channel β1 subunits initially present in solubilized rat brain nerve endings. Complementary DNA of rat brain sodium channel αIIA subunit was a gift of H. Lester (California Institute of Technology, Pasadena, CA); the αSkM1 clone was donated by P. Backx (University of Toronto, Toronto), and pCDNA3-synaptotagmin I was provided by M. De Waard (Institut National de la Santé et de la Recherche Médicale, U464, Marseille, France). [3H]saxitoxin (STX) was purchased from Amersham. The specific radioactivity of 3H[STX] was 35–65 dpm/fmol of STX. TTX was from Latoxan (Valence, France), ωGVIA was from the Peptide Institute (Osaka, Japan), and its iodinated derivative (125I-ωGVIA) was prepared as described (16).
Solubilization and Immunoprecipitation Assays of [3H]STX Receptors.
Nerve endings from adult rat forebrain or from rat cerebellum were prepared as described (16). Membranes were resuspended in buffer A (100 mM KCl/10 mM Hepes, pH 7.4) containing the protease inhibitors (1 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride, 2 mM pepstatin, and 1 mM iodoacetamide). Membranes were prelabeled with 20 nM [3H]STX at 4°C for 2 h (15), were washed by centrifugation, and were solubilized (5–6 mg/ml protein) for 30 min at 4°C in buffer B (1.5% Triton X-100/100 mM KCl/10 mM Hepes, adjusted to pH 7.4 with Tris-base) containing the protease inhibitors. Aliquots of 100,000 × g supernatants were subjected to immunoprecipitation assays, and the immunoprecipitated radioactivity was determined by scintillation counting (15). In parallel, total ligand-receptor complex was determined (15). Typically, a 45–50% fraction of solubilized [3H]STX-receptor complexes was immunoprecipitated by the anti-sodium channel β1 subunit antibodies.
Determination of the Stoichiometry Between Co-Immunoprecipitated Sodium Channels and Synaptotagmin.
The calculations were carried out with the average specific radioactivity of 3H[STX] (50 dpm/fmol). An equimolar ratio between 3H[STX] and sodium channel was used for calculations because it has been previously shown that specific saxitoxin binding to purified rat brain sodium channel (mol/mol) is equal to 0.9, and that there is an equimolar association between α and β1 subunits (3). Membranes were prelabeled with 3H[STX] and were solubilized as described above. Each immunoprecipitation input (95 μl) contained 1,636 ± 300 fmol of 3H[STX]receptor complex that was a 43% fraction of the total radioactivity (unbound and bound 3H[STX]). Immune complexes were recovered as described above, and the immunoprecipitated radioactivity was counted. In parallel, identical samples were analyzed by Western blotting and were compared to a recombinant maltose binding protein (MBP)-synaptotagmin I standard curve.
Immunoprecipitation of 125I-ωGVIA Receptors.
P2 membranes were prelabeled overnight with 0.2 nM 125I-ωGVIA as described (16) and were solubilized (5–6 mg/ml protein) in buffer B. Immunoprecipitation assays were carried out as for [3H]STX receptors.
Effect of Calcium on Synaptotagmin/Sodium Channel Interactions.
Rat forebrain P2L membranes were extracted in buffer B with protease inhibitors, but EDTA was omitted. Aliquots of extracts were supplemented with either 1 mM EDTA, 1 mM CaCl2, 1 mM MgCl2, 1 mM SrCl2, or 1 mM BaCl2 and were incubated with antibodies. To determine the Ca2+ dependency, lysed P2 membranes were extracted in buffer B containing 2 mM EDTA (Sigma). Aliquots were supplemented with sufficient EDTA (Sigma) and CaCl2 (Fluka) to yield the indicated free Ca2+ concentrations calculated by using cabuf software (G. Droogmans, Katoholieke Universiteit, Leuven, Belgium). Immune pellets were recovered and analyzed by SDS/PAGE and immunoblotting.
Recombinant Proteins.
MBP-synaptotagmin I fusion protein was prepared as described (17). Glutathione S-transferase (GST)-fusion proteins containing intracellular domains of rat brain or skeletal muscle sodium channel were constructed by PCR amplification and cloning in pGEX-2T, 2TK, or 4T (Pharmacia). All recombinant proteins were expressed in Escherichia coli strain BL21 and were purified according to standard procedure; concentrations were determined by SDS/PAGE and Coomassie blue staining with a BSA standard scale.
Binding of [3H]STX Receptors to MBP Fusion Proteins.
Recombinant MBP-synaptotagmin I fusion protein or MBP alone were immobilized on amylose resin (New England Biolabs) in 0.2% Triton X-100, 100 mM KCl, 0.1% BSA, and 10 mM Hepes (pH 7.4). After 1 h at 4°C, the beads were extensively washed to remove unbound proteins and were incubated with 200 μl of Triton X-100 extract containing [3H]STX receptors. After 3 h incubation at 4°C, the beads were washed, and the amount of bound receptors was assessed by scintillation counting.
GST Fusion Protein Binding Assays.
[35S]-labeled synaptotagmin I was obtained by using a pCDNA3-synaptotagmin I construction by in vitro coupled transcription and translation in the presence of [35S]l-methionine (Dupont) by using the TNT system (Promega). GST fusion proteins were bound to 40 μl of glutathione-agarose beads (50%) for 1 h in TBS and 0.1% Triton X-100; supernatants were removed, and the beads were incubated with [ 35S]-labeled protein in 200 μl of TBS and 0.1% Triton X-100 for 4–5 h at 4°C under constant agitation; finally, the beads were washed 4 times with 1 ml of TBS and 0.1% Triton X-100, and the bound proteins were analyzed either by SDS/PAGE and autoradiography or by scintillation counting. SDS/PAGE and Coomassie blue staining confirmed that equivalent amounts of GST fusion proteins were used in the assays.
Electrophoresis and Immunoblotting.
SDS/PAGE (9% or 4.5%) was performed as described (15). Detection was carried out with anti-IgG peroxidase conjugated antibodies and ECL detection (Amersham).
Results
Co-Immunoprecipitation of Neuronal Sodium Channels and Synaptotagmin.
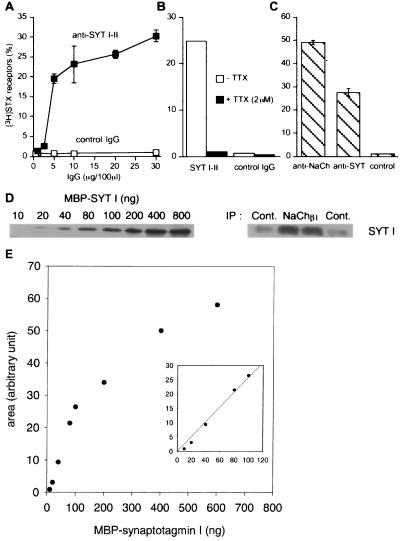
A quantitative and specific assay (15) was used to investigate whether sodium channels are associated with synaptotagmin. Rat cerebellar membranes prelabeled with tritiated saxitoxin ([3H]STX) were solubilized with Triton X-100 and were subjected to immunoprecipitation. Fig. 1A shows that a monoclonal antibody (mAb 1D12) directed against the synaptotagmin isoforms I and II adsorbed in a concentration-dependent manner solubilized [3H]STX-receptor complexes. At the plateau, 25–30% of receptors were specifically immunoprecipitated by the anti-synaptotagmin antibodies. The amount of radioactivity detected in the immune pellet was reduced to the background level (typically <1%) when TTX was added during the immunoprecipitation step (Fig. 1B), indicating that immunoprecipitated [3H]STX was associated with sodium channels. When prelabeled sodium channels were extracted from lysed nerve endings from rat forebrain, 48% of [3H]STX-receptor complexes were trapped by antibodies against the sodium channel β1 subunit and 28% by anti-synaptotagmin antibodies (Fig. 1C). Under these conditions, a fraction of ≈60% of synaptotagmin initially present in the TritonX-100 extract was typically recovered by the mAb1D12 antibody (not shown). To determine the stoichiometry between co-immunoprecipitated sodium channels and synaptotagmin, the immunoprecipitated [3H]STX-receptor complexes and the non-immune samples were analyzed by Western blotting and compared to a recombinant MBP-synaptotagmin I standard curve (Fig. 1 D and E). Determination of the immunoprecipitated radioactivity indicated that 864 ± 28 and 14.3 ± 2 fmol of 3H[STX]receptor complex were immunoprecipitated by anti-β1 subunit antibody and by non-immune serum respectively (n = 4). The non-immune value was subtracted, giving 850 fmol of sodium channel immunoprecipitated. The immunoreactive bands for synaptotagmin I were quantified by using molecular analyst software (BioRad); the values corresponded to anti-sodium channel and non-immune immunoprecipitates were 34.6 ± 1.3 and 10 ± 1.5, respectively (expressed in arbitrary units). After subtraction of the non-immune signal, the value was found to be in the linear range of the MBP-synaptotagmin standard curve (Fig. 1E) and indicated that 946 fmol of synaptotagmin co-immunoprecipitated with 850 fmol of 3H[STX]receptor complex, giving a stoichiometry between co-immunoprecipitated sodium channels and synaptotagmin of 0.9:1. Taking into account that we used the average specific radioactivity of saxitoxin for calculations (50 dpm/fmol), the stoichiometry could be between 0.7:1 and 1.3:1, depending on the specific radioactivity of 3H[STX] saxitoxin. Finally, comparison of the immunoreactive bands for synaptotagmin in the immunoprecipitation input and in sodium channel immunoprecipitates indicated that a very low fraction of synaptotagmin (0.6–1%) was recovered with sodium channels (not shown).
Figure 1.
Specific co-immunoprecipitation of synaptotagmin and sodium channels from detergent extract of rat brain. (A) Rat cerebellar membranes were labeled with [3H]STX, were extracted with Triton X-100, and were incubated with increasing amounts of anti-synaptotagmin antibodies (mAb 1D12) or non-immune IgG. (B) Triton X-100 extracts containing [3H]STX receptors were incubated with an anti-synaptotagmin antibody (mAb 1D12) or non-immune IgG, in the absence or presence of TTX (2 μM). (C) [3H]STX-prelabeled receptors were incubated with an anti-sodium channel β1 subunit or mAb1D12. The radioactivity recovered in immune complexes was determined by scintillation counting (mean ± SD, n = 3; three independent experiments). Results were expressed as the percentage of [3H]STX-receptors immunoprecipitated versus total amount of complexes in the initial detergent extract. (D) Increasing amounts of recombinant MBP-synaptotagmin I and immunoprecipitated [3H]STX-prelabeled receptors were separated by SDS/PAGE, and immunoblotting for synaptotagmin (mAb1D12) was performed. (E) Recombinant MBP-synaptotagmin standard curve. The bands immunoreactive for synaptotagmin I were quantified by using molecular analyst software and were plotted as function of MBP-synaptotagmin I concentration. Some SD values were too small to be seen on the graphs.
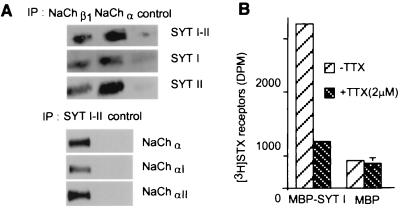
Fig. 2A shows that synaptotagmin I and II (18, 19) were detected after immunoprecipitation of solubilized cerebellar membrane proteins with sodium channel antibodies. Among the sodium channel α subunits expressed in the central nervous system, αII is predominant in the forebrain whereas αI is more strongly expressed in the cerebellum and spinal cord (20); both types were associated with synaptotagmins (Fig. 2A).
Figure 2.
Synaptotagmin I and II associate with sodium channel type I and II. (A) Sodium channels solubilized from rat cerebellar membranes were immunoprecipitated with antibodies against sodium channel α subunit, β1 subunit, with mAb 1D12, or non-immune serum. The immunoprecipitates (IP) were separated by SDS/PAGE and were analyzed by immunoblotting for the indicated proteins. (B) Equal amounts of solubilized [3H]STX receptors from P2L rat brain membranes were incubated with MBP-synaptotagmin I or MBP bound to amylose resin in the presence or absence of 2 μM TTX. The beads were washed, and the amount of [3H]STX receptors bound was assessed by scintillation counting (mean ± SD, n = 3). Some SD values were too small to be seen on the graphs.
Binding of Solubilized [3H]STX Receptors to MBP-Synaptotagmin I.
Solubilized [3H]STX receptors from P2L forebrain membranes were incubated with MBP-synaptotagmin I or with MBP immobilized on amylose beads. After 3 h of incubation at 4°C, the amount of radioactivity retained on MBP-synaptotagmin I was 2,570 ± 115 dpm whereas only 420 ± 44 dpm were retained on MBP (Fig. 2B). The radioactivity bound to MBP-synaptotagmin I was reduced to the background level as determined with MBP alone when an excess of TTX was added during the incubation step. The amount of radioactivity bound to MBP-synaptotagmin I was found to depend on the amount of solubilized [3H]STX receptors loaded (data not shown).
The Intracellular Loop Between Domains I and II of the Sodium Channel αIIA Subunit Interacts with Synaptotagmin I.
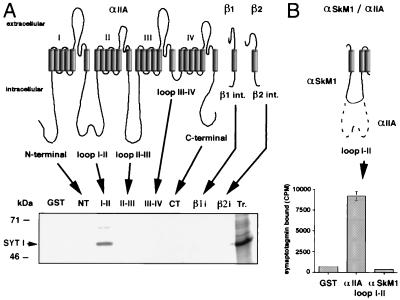
To examine whether synaptotagmin binds directly to sodium channels, GST fusion proteins containing all intracellular domains of αIIA, β1, and β2 channel subunits were constructed. These proteins are αIIA N-terminal (amino acids 1–128), αIIA loop I-II (430–704), αIIA loop II-III (988–1,208), αIIA loop III-IV (1,477–1,529), αIIA C-terminal (1,780–2,005), β1 (180–218), and β2 intracellular domains (182–215). The GST-αIIA loop I-II was not complete as the entire loop (428–753) was difficult to express in bacteria. Binding assays were performed by using GST fusion proteins immobilized on glutathione-agarose beads and synaptotagmin I synthesized in vitro and labeled with [35S]methionine. In vitro translated synaptotagmin only bound specifically to GST-αIIA loop I-II (Fig. 3A) as adsorption to the other intracellular domains of the sodium channel was equivalent to that observed with GST alone. Because loop I-II is partially deleted in the skeletal muscle α subunit (SkM1), we constructed a GST fusion protein of the αSkM1 loop I-II (446–559); no specific interaction with synaptotagmin I was detected (Fig. 3B), indicating that the domain that is deleted in SkM1 is essential for synaptotagmin binding.
Figure 3.
Binding of [35S]synaptotagmin I to GST fusion proteins containing intracellular sodium channel domains. GST fusion proteins (1 μM) containing cytoplasmic domains of αIIA sodium channel subunit, β1 and β2 subunits (A), and loop I-II of αSkM1 sodium channel (B) were immobilized on glutathione-agarose beads and were incubated at 4°C for 4–5 h with in vitro translated [35S]methionine-labeled synaptotagmin I (SYT I); bound proteins were eluted in sample buffer and were analyzed on SDS/PAGE by autoradiography (A), or bound radioactivity was measured by scintillation counting (B) (mean ± SD, n = 3). The last lane of the autoradiogram contains 1 μl of [35S]synaptotagmin I (Tr.).
Characterization of the Interaction Between Synaptotagmin I and GST-αIIA Loop I-II.
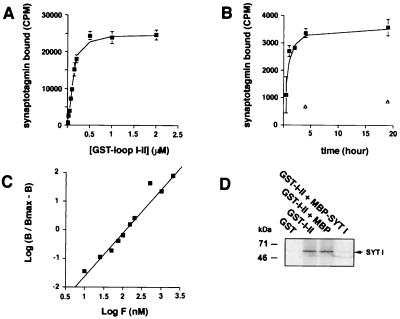
Direct in vitro interaction between [35S]methionine-labeled synaptotagmin and GST-αIIA loop I-II was further characterized by incubating increasing concentrations of GST fusion protein with the same amount of synaptotagmin I (Fig. 4A); the curve revealed saturable binding. A kinetic study of the binding showed that a 4-h incubation was sufficient for 90% of the binding to occur even at low concentrations of GST fusion protein (Fig. 4B). From the Hill plot (Fig. 4C), the equilibrium dissociation constant (KD) was 100 nM and the Hill coefficient was 1.6, indicating positive cooperativity. Competition experiments revealed that an excess of MBP-synaptotagmin I but not of MBP alone inhibited the binding of [35S]synaptotagmin I to GST-αIIA loop I-II (Fig. 4D).
Figure 4.
Characterization of the interaction between synaptotagmin I and αIIA loop I-II. (A) Saturable specific binding of [35S]synaptotagmin I to GST-αIIA. Increasing concentrations of GST-αIIA loop I-II immobilized on glutathione-agarose beads were incubated for 4–5 h at 4°C with a constant amount of [35S]synaptotagmin I. Bound radioactivity was measured by scintillation counting (mean ± SD, n = 3), and nonspecific binding to GST alone was subtracted. Half maximum saturation gave an approximate KD = 100–150 nM. (B) Association kinetics of [35S]synaptotagmin I to GST-αIIA loop I-II (0.05 μM) at 4°C. Maximum binding occurred at 4 h of incubation. Open triangles show nonspecific binding (mean ±SD, n = 3). (C) Hill plot. B, specific binding; Bmax, maximum specific binding; F, GST fusion protein concentration. The slope of the linear regression was 1.6, and the calculated KD was 100nM. (D) [35S]synaptotagmin I (SYT I) was incubated with GST alone or GST-αIIA loop I-II (1 μM) or GST-αIIA loop I-II with MBP-synaptotagmin I or MBP in excess (10 μM). Bound proteins were analyzed by autoradiography.
Localization of the Interaction Site on the αIIA Loop I-II.
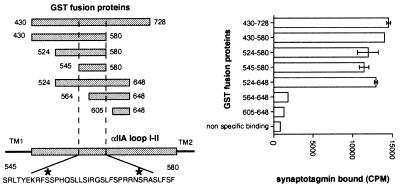
We next constructed several GST fusion proteins containing restricted domains of αIIA loop I-II (Fig. 5). Data show that a small region of the αIIA loop I-II between the amino acids 545 and 580 and all proteins that contain this fragment were able to bind synaptotagmin I whereas proteins that do not contain the fragment (605–648) or only a part of it (564–648) did not interact with synaptotagmin I. When the peptide corresponding to the amino acids 545–568 (1.75 μM final concentration) was added to detergent extract, a decrease of 55% in the synatotagmin co-immunoprecipitated with sodium channels was observed (data not shown).
Figure 5.
Localization of the binding site for synaptotagmin I in αIIA loop I-II. GST fusion proteins containing segments of the αIIA loop I-II are represented in alignment with the full length loop I-II; TM are transmembrane segments delimiting intracellular αIIA loop I-II. Numbers indicate amino acids located at their extremities. The sequence of the αIIA loop I-II between amino acids 545 and 580 is shown, with asterisks indicating consensus protein kinase A phosphorylation sites. [35S]synaptotagmin I was incubated with the different GST fusion proteins from αIIA loop I-II (1 μM). Bound synaptotagmin was analyzed by scintillation counting (mean ± SD, n = 3).
Effect of Ca2+ on the Interaction of Sodium Channels with Synaptotagmin.
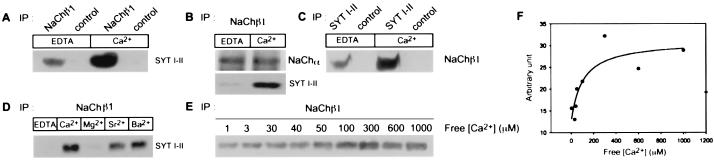
Synaptotagmins I and II contain in their cytoplasmic domain two regulatory C2 domains that bind calcium and mediate Ca2+-dependent processes (reviewed in ref. 12). When Triton X-100 extracts of P2L membranes were supplemented with either 1 mM Ca2+ or 1 mM EDTA, the amount of synaptotagmin immunoprecipitated by sodium channel β1 subunit antibodies was enhanced in the presence of Ca2+ (Fig. 6A). As expected, the amount of sodium channel α subunit immunoprecipitated by anti-β1 subunit antibodies did not depend on the Ca2+ concentration (Fig. 6B). The amount of sodium channel β1 subunit immunoprecipitated by anti-synaptotagmin antibodies (mAb 1D12) was higher in the presence of Ca2+ (Fig. 6C). We next compared the effect of various divalent ions such as Mg2+, Sr2+, Ba2+, and Ca2+. The amount of co-immunoprecipitated synaptotagmin detected by Western blotting was increased by Ba2+ or Sr2+ ions and was similar to that detected in the presence of Ca2+ ions (Fig. 6D). In contrast, a weak signal was observed in the presence of Mg2+or EDTA. When co-immunoprecipitation experiments were carried out with increasing concentrations of free Ca2+, the amount of synaptotagmin trapped with sodium channel anti-β1 antibodies was enhanced with an EC50 of ≈50 μM (Fig. 6 E and F).
Figure 6.
Effect of Ca2+ on sodium channel-synaptotagmin association. (A, B, and C) Triton X-100 extracts of rat brain P2L membranes were incubated with 1 mM CaCl2 or 1 mM EDTA and were immunoprecipitated with antibodies against sodium channel β1 subunit (A and B), synaptotagmin (mAb 1D12) (C), or non-immune serum. The immunoprecipitates were resolved by SDS/PAGE, and blots were probed with antibodies against the indicated proteins. (D and E) Co-immunoprecipitation of sodium channels and synaptotagmin was performed in the presence of 1 mM EDTA or 1 mM CaCl2, MgCl2, BaCl2, or SrCl2 (D) or in the presence of 3 or 4 mM EDTA and sufficient Ca2+ to yield the indicated free Ca2+ concentrations (E). After Western blotting of the immune pellets, the bands immunoreactive for synaptotagmin (mAb 1D12) were quantified by using molecular analyst software. The data were plotted, and curves were fitted by using sigma plot software (F).
The Synaptotagmin-Sodium Channel Complex Is Not Associated with SNARE Proteins or Presynaptic Calcium Channels.
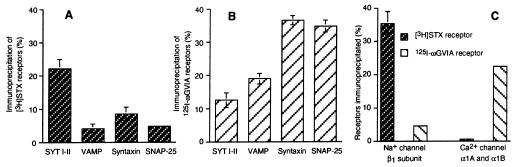
Co-immunoprecipitation and in vitro recombinant protein assays have shown direct interactions between the presynaptic P/Q- (α1A) and N-type (α1B) calcium channels, the SNARE complex, and synaptotagmin (16, 17, 21–23). To determine whether the SNARE complex interacts with sodium channels, the levels of immunoprecipitation of both [3H]STX receptors and 125I-ωGVIA receptors by antibodies against synaptotagmin, syntaxin 1, SNAP-25, or VAMP 2 were compared in the same conditions of detergent solubilization (i.e., Triton X-100). Significant recovery of 125I-ωGVIA receptors was detected with antibodies against syntaxin 1 (37 ± 1%), SNAP-25 (35 ± 2%), and VAMP 2 (20 ± 2%) (Fig. 7B). In contrast, these antibodies trapped a low fraction of solubilized [3H]STX receptors (<8%) unlike that observed with anti-synaptotagmin antibodies (Fig. 7A). Recovery of 3H[STX]receptor complexes by an antibody against synaptophysin was similar to that obtained with non-immune serum, although this antibody did immunoprecipitate its antigen (data not shown). These observations suggest that the SNARE complex proteins are not detectably associated with sodium channels. Fig. 7C shows that [3H]STX-prelabeled sodium channels solubilized from P2L forebrain membranes were not significantly immunoprecipitated by an antibody that recognizes both the α1A and α1B subunits of P/Q- and N-type calcium channels respectively (2%). Conversely, an anti-sodium channel antibody failed to immunoprecipate N-type calcium channels prelabeled with 125I-ωGVIA (4.5 ± 0.5%).
Figure 7.
Interaction of N-type calcium channels but not sodium channels with SNARE proteins. Rat brain membranes (P2L) were labeled with [3H]STX (A) and 125I-ωGVIA (B), were extracted with Triton X-100, and were incubated with antibodies against synaptotagmin (mAb 1D12), VAMP 2, syntaxin 1, SNAP-25, and non-immune IgG. The radioactivity recovered in immune complexes was determined by scintillation counting (mean ± SD, n = 6) and was expressed as a percentage of total labeled channels. Nonspecific radioactivity detected by immunoprecipitation with control IgG was subtracted. (C) Sodium channels and N-type calcium channels were labeled with [3H]STX and 125I-ωGVIA, respectively. Detergent extracts were incubated with an antibody against the sodium channel β1 subunit, an antibody that recognizes the α1A and α1B subunits of P/Q- and N-type calcium channels (B1Nt) or non-immune IgG. The radioactivity recovered in immune complexes was counted and expressed as described above (mean ± SD, n = 6). Some SD values were too small to be seen on graphs.
Discussion
Neuronal Sodium Channels Associate with Synaptotagmins I and II.
Synaptotagmins belong to a large family including 12 isoforms that display distinct expression patterns, both in neuronal and non-neuronal tissues (for a recent review, see ref. 13). The expression of synaptotagmins I and II is restricted to the nervous system exhibiting distinct developmental patterns (18, 19). Synaptotagmin I, so far the best studied isoform, is a multifunctional protein, acting both in a Ca2+-dependent and independent manner (for reviews, see refs. 12 and 13). On the basis of biochemical and genetic evidence, synaptotagmin is thought to be a Ca2+ sensor involved in Ca2+-regulated exocytosis in nerve terminals (for a review, see ref. 25).
We report here that neuronal sodium channels can specifically interact with synaptotagmin, as demonstrated by co-immunoprecipitation, Western blotting analysis, and in vitro assays using recombinant proteins. Furthermore, the synaptotagmin interaction domain was identified in a region comprising 35 residues located in the cytoplasmic loop between domains I and II of the sodium channel αIIA subunit (residues 545–580). Among the distinct sodium channel subtypes expressed in the central nervous system, the synaptotagmin interaction domain characterized on αIIA subtype displays 97% homology with αI. This is in good agreement with our observations showing that both subtypes, αII and αI, are trapped by anti-synaptotagmin antibodies. Interestingly, a more restricted homology was found in the loop I-II of αIII and of α6 (78% and 57%, respectively), suggesting that synaptotagmin binding might display different affinities for distinct sodium channel subtypes. Thus, synaptotagmin can interact with N- and P/Q-type calcium channels (17, 23) and/or sodium channels through specific binding sites located on distinct cytoplasmic loops of the pore-forming protein. The majority of the synaptotagmin complexed with sodium channels was not associated with SNARE proteins, in contrast to calcium channels (16, 22). Our results imply that synaptotagmin can associate with voltage-gated ion channels dependent on and independently of SNARE protein interactions.
Synaptotagmins I and II contain two C2 domains that are involved in Ca2+-dependent interactions with different partners (reviewed in ref. 12). For example, the C2A domain binds to negatively charged phospholipids with an EC50 of 5–10 μM and to syntaxin but in a higher range of Ca2+ concentrations (EC50 = 200–400 μM) (27). Strikingly, the co-immunoprecipitation of sodium channel with synaptotagmin was found to be enhanced by Ca2+ with an EC50 of 50 μM; this effect was mimicked by Sr2+ and Ba2+ but not by Mg2+. It has been previously reported that the C2B domain promotes Ca2+-dependent self-association of synaptotagmin with an EC50 of 5–30 μM (28) or >100 μM (29). In the latter case, the divalent cation specificity was similar to that reported here. These observations suggest that self-association of synaptotagmin mediated by Ca2+ may positively modulate the interaction with sodium channels, implying that synaptotagmin homo- or heterodimers possess a higher affinity for sodium channels.
Where Does the Interaction Take Place in Vivo?
Our results demonstrate that sodium channel associates with synaptotagmins I and II but do not indicate in which subcellular compartment this interaction takes place in vivo. However, it seems unlikely that the association occurs in myelinated fibers because immunoblotting of sciatic and optic nerve preparations failed to detect synaptotagmin (B.S., unpublished results). Therefore, the simplest interpretation is that the interaction occurs in nerve terminals in which both partners are significantly expressed. In nerve endings, synaptotagmin is predominantly localized in synaptic vesicles (30) but also present in the presynaptic plasma membrane (31). Thus, the interaction can occur when each partner is located in distinct membrane compartments: i.e., between docked synaptic vesicles and the presynaptic membrane. Synaptotagmin was found to co-sediment with a fraction of solubilized [3H]STX receptors extracted either from P2 or from P2L membrane preparations, each of which contains docked vesicles at the plasma membrane. Another possibility is that association takes place when both partners are located in the presynaptic plasma membrane.
Physiological Significance.
We can only speculate as to the physiological role of the interaction reported here as very little is known about the precise locations of voltage-gated sodium channels in central nervous system nerve endings, particularly in relation to the active zones. Recent studies (32, 33) have shown that NSF, a critical component of the general machinery implicated in vesicle trafficking (34) including Ca2+ regulated exocytosis, can interact directly with glutamate receptors (GluR2) that are responsible for excitatory synaptic transmission in the central nervous system. Moreover, α and β SNAPs were found to associate with NSF and GluR2 (33), implying that factors interacting with SNARE proteins participate in the trafficking of postsynaptic receptors. By analogy, synaptotagmin might therefore be involved in the trafficking and insertion of sodium channels in neurons. Consistent with this hypothesis, elevation of the intracellular Ca2+ concentration in growth cones induced insertion of sodium channels at the cell surface (35).
We have previously shown that sodium channels undergo activity-induced internalization in cultured neurons (36, 37). As synaptotagmin associates with clathrin adaptor AP2 (27, 38), it might be implicated in a regulatory endocytosis of sodium channels that is favored by an increase in intracellular Ca2+ concentration. Such a process could be restricted to nerve terminals where it could be involved in the recycling of synaptic vesicles, particularly in the pathway that is activated by high frequency stimulation and that originates away from active zones (39). Intense exocytosis may result in a increase of synaptotagmin on both sides of active zones, allowing association with sodium channels that would be followed by an regulatory endocytosis. The existence of an activity-dependent endocytotic pathway in neurons has been reported for the transcytosis of transferrin receptor (40), and, on the other hand, the β amyloid precursor protein is internalized in vesicles containing synaptotagmin (41).
The sodium channel-synaptotagmin complex could be involved in Na+-dependent exocytosis. It has been reported that the release of vasopressin and oxytocin from neurohypophyseal nerve endings is Na+-dependent (42). In motor nerve terminals, brevetoxin-3, a selective activator of sodium channels, induced a large increase in the quantal secretion of acetylcholine (43). However, this hypothesis would predict that the majority of synaptotagmin complexed with sodium channels are associated with SNARE proteins that are responsible for the fusion process.
Finally, sodium channel activity could be modulated by synaptotagmin binding. Sodium channel activity is affected by association with the auxiliary β subunits (8, 9) and by direct interaction with G proteins (44). On the other hand, synaptotagmin can modulate the activity of N-type calcium channels (45). Therefore, we cannot exclude the possibility that the functional properties of sodium channels are affected by synaptotagmin binding. As the principal phosphorylation site involved in the inhibition of voltage sensitive sodium channels by protein kinase A (46) is located inside the synaptotagmin interaction domain, synaptotagmin might modulate channel opening by controlling the level of channel phosphorylation. Further investigation is required to determine the physiological significance of the interaction of synaptotagmin with sodium channels, which may have important consequences for the regulation of neuronal excitability.
Acknowledgments
We thank Dr. M. Takahashi and Dr. G. Alcaraz for the generous gift of antibodies, Dr. P. Backx, Dr. M De Waard, Dr. H Lester, Dr. B. Marquèze, and Dr. D. Walker, who gave cDNAs, and Cécile Raymond for preparing 125I-ωGVIA.
Abbreviations
- STX
saxitoxin
- MBP
maltose binding protein
- TTX
tetrodotoxin
- GST
glutathione S-transferase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Stuart G, Spruston N, Sakmann B, Hausser M. Trends Neurosci. 1997;20:125–131. doi: 10.1016/s0166-2236(96)10075-8. [DOI] [PubMed] [Google Scholar]
- 2.Crill W E. Annu Rev Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- 3.Catterall W A. Physiol Rev. 1992;72,Suppl.:15–48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- 4.Auld V J, Goldin A L, Krafte D S, Marshall J, Dunn J M, Catterall W A, Lester H A, Davidson N, Dunn R J. Neuron. 1988;1:449–461. doi: 10.1016/0896-6273(88)90176-6. [DOI] [PubMed] [Google Scholar]
- 5.Noda M, Ikeda T, Suzuki H, Takeshima H, Takahashi T, Kuno M, Numa S. Nature (London) 1986;322:826–828. doi: 10.1038/322826a0. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki H, Beckh S, Kubo H, Yahagi N, Ishida H, Kayano T, Noda M, Numa S. FEBS Lett. 1988;228:195–200. doi: 10.1016/0014-5793(88)80615-x. [DOI] [PubMed] [Google Scholar]
- 7.Schaller K L, Krzemien D M, Yarowsky P J, Krueger B K, Caldwell J H. J Neurosci. 1995;15:3231–3242. doi: 10.1523/JNEUROSCI.15-05-03231.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isom L L, De Jongh K S, Patton D E, Reber B F, Offord J, Charbonneau H, Walsh K, Goldin A L, Catterall W A. Science. 1992;256:839–842. doi: 10.1126/science.1375395. [DOI] [PubMed] [Google Scholar]
- 9.Isom L L, Ragsdale D S, De Jongh K S, Westenbroek R E, Reber B F, Scheuer T, Catterall W A. Cell. 1995;83:433–442. doi: 10.1016/0092-8674(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 10.Forsythe I D. J Physiol (London) 1994;479:381–387. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Southan A P, Robertson B. J Neurosci. 1998;18:948–955. doi: 10.1523/JNEUROSCI.18-03-00948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudhof T C, Rizo J. Neuron. 1996;17:379–388. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- 13.Schiavo G, Osborne S L, Sgouros J G. Biochem Biophys Res Commun. 1998;248:1–8. doi: 10.1006/bbrc.1998.8527. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama H, Shikano H, Kanaoka Y. Biochim Biophys Acta. 1992;1175:67–72. doi: 10.1016/0167-4889(92)90010-9. [DOI] [PubMed] [Google Scholar]
- 15.Alcaraz G, Sampo B, Tricaud N, Giraud P, Martin-Eauclaire M F, Couraud F, Dargent B. Brain Res Mol Brain Res. 1997;51:143–153. doi: 10.1016/s0169-328x(97)00232-5. [DOI] [PubMed] [Google Scholar]
- 16.Leveque C, el Far O, Martin-Moutot N, Sato K, Kato R, Takahashi M, Seagar M J. J Biol Chem. 1994;269:6306–6312. [PubMed] [Google Scholar]
- 17.Charvin N, L'Eveque C, Walker D, Berton F, Raymond C, Kataoka M, Shoji-Kasai Y, Takahashi M, De Waard M, Seagar M J. EMBO J. 1997;16:4591–4596. doi: 10.1093/emboj/16.15.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marqueze B, Boudier J A, Mizuta M, Inagaki N, Seino S, Seagar M. J Neurosci. 1995;15:4906–4917. doi: 10.1523/JNEUROSCI.15-07-04906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ullrich B, Li C, Zhang J Z, McMahon H, Anderson R G, Geppert M, Sudhof T C. Neuron. 1994;13:1281–1291. doi: 10.1016/0896-6273(94)90415-4. [DOI] [PubMed] [Google Scholar]
- 20.Gordon D, Merrick D, Auld V, Dunn R, Goldin A L, Davidson N, Catterall W A. Proc Natl Acad Sci USA. 1987;84:8682–8686. doi: 10.1073/pnas.84.23.8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D K, Catterall W A. Proc Natl Acad Sci USA. 1997;94:14782–14786. doi: 10.1073/pnas.94.26.14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin-Moutot N, Charvin N, Leveque C, Sato K, Nishiki T, Kozaki S, Takahashi M, Seagar M. J Biol Chem. 1996;271:6567–6570. doi: 10.1074/jbc.271.12.6567. [DOI] [PubMed] [Google Scholar]
- 23.Sheng Z H, Yokoyama C T, Catterall W A. Proc Natl Acad Sci USA. 1997;94:5405–5410. doi: 10.1073/pnas.94.10.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.el Far O, Charvin N, Leveque C, Martin-Moutot N, Takahashi M, Seagar M J. FEBS Lett. 1995;361:101–105. doi: 10.1016/0014-5793(95)00156-4. [DOI] [PubMed] [Google Scholar]
- 25.Littleton J T, Bellen H J. Trends Neurosci. 1995;18:177–183. doi: 10.1016/0166-2236(95)93898-8. [DOI] [PubMed] [Google Scholar]
- 26.Sakurai T, Westenbroek R E, Rettig J, Hell J, Catterall W A. J Cell Biol. 1996;134:511–528. doi: 10.1083/jcb.134.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C, Ullrich B, Zhang J Z, Anderson R G, Brose N, Sudhof T C. Nature (London) 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 28.Chapman E R, An S, Edwardson J M, Jahn R. J Biol Chem. 1996;271:5844–5849. doi: 10.1074/jbc.271.10.5844. [DOI] [PubMed] [Google Scholar]
- 29.Sugita S, Hata Y, Sudhof T C. J Biol Chem. 1996;271:1262–1265. doi: 10.1074/jbc.271.3.1262. [DOI] [PubMed] [Google Scholar]
- 30.Matthew W D, Tsavaler L, Reichardt L F. J Cell Biol. 1981;91:257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi M, Arimatsu Y, Fujita S, Fujimoto Y, Kondo S, Hama T, Miyamoto E. Brain Res. 1991;551:279–292. doi: 10.1016/0006-8993(91)90942-o. [DOI] [PubMed] [Google Scholar]
- 32.Nishimune A, Isaac J T, Molnar E, Noel J, Nash S R, Tagaya M, Collingridge G L, Nakanishi S, Henley J M. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- 33.Osten P, Srivastava S, Inman G J, Vilim F S, Khatri L, Lee L M, States B A, Einheber S, Milner T A, Hanson P I, Ziff E B. Neuron. 1998;21:99–110. doi: 10.1016/s0896-6273(00)80518-8. [DOI] [PubMed] [Google Scholar]
- 34.Sollner T, Whiteheart S W, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman J E. Nature (London) 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 35.Wood M R, DeBin J, Strichartz G R, Pfenninger K H. J Neurosci. 1992;12:2948–2959. doi: 10.1523/JNEUROSCI.12-08-02948.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dargent B, Paillart C, Carlier E, Alcaraz G, Martin-Eauclaire M F, Couraud F. Neuron. 1994;13:683–690. doi: 10.1016/0896-6273(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 37.Paillart C, Boudier J L, Boudier J A, Rochat H, Couraud F, Dargent B. J Cell Biol. 1996;134:499–509. doi: 10.1083/jcb.134.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J Z, Davletov B A, Sudhof T C, Anderson R G. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 39.Koenig J H, Ikeda K. J Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemar A, Olivo J C, Williamson E, Saffrich R, Dotti C G. J Neurosci. 1997;17:9026–9034. doi: 10.1523/JNEUROSCI.17-23-09026.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marquez-Sterling N R, Lo A C Y, Sisodia S S, Koo E H. J Neurosci. 1997;17:140–151. doi: 10.1523/JNEUROSCI.17-01-00140.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stuenkel E L, Nordmann J J. J Physiol (London) 1993;468:357–378. doi: 10.1113/jphysiol.1993.sp019776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meunier F A, Colasante C, Molgo J. Neuroscience. 1997;78:883–893. doi: 10.1016/s0306-4522(96)00568-4. [DOI] [PubMed] [Google Scholar]
- 44.Ma J Y, Catterall W A, Scheuer T. Neuron. 1997;19:443–452. doi: 10.1016/s0896-6273(00)80952-6. [DOI] [PubMed] [Google Scholar]
- 45.Wiser O, Tobi D, Trus M, Atlas D. FEBS Lett. 1997;404:203–207. doi: 10.1016/s0014-5793(97)00130-0. [DOI] [PubMed] [Google Scholar]
- 46.Smith R D, Goldin A L. J Neurosci. 1997;17:6086–6093. doi: 10.1523/JNEUROSCI.17-16-06086.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]