Abstract
Protease-activated receptor-2 (PAR-2) is a member of seven transmembrane domain G protein-coupled receptors activated by proteolytic cleavage whose better known member is the thrombin receptor. The pathophysiological role of PAR-2 remains poorly understood. Because PAR-2 is involved in inflammatory and injury response events, we investigated the role of PAR-2 in experimental myocardial ischemia-reperfusion injury. We show for the first time that PAR-2 activation protects against reperfusion-injury. After PAR-2-activating peptide (2AP) infusion, we found a significant recovery of myocardial function and decrease in oxidation at reflow. Indeed, the glutathione cycle (glutathione and oxidized glutathione) and lipid peroxidation analysis showed a reduced oxidative reperfusion-injury. Moreover, ischemic risk zone and creatine kinase release were decreased after PAR-2AP treatment. These events were coupled to elevation of PAR-2 and tumor necrosis factor α (TNFα) expression in both nuclear extracts and whole heart homogenates. The recovery of coronary flow was not reverted by L-nitroarginine methylester, indicating a NO-independent pathway for this effect. Genistein, a tyrosine kinase inhibitor, did not revert the PAR-2AP effect. During early reperfusion injury in vivo not only oxygen radicals are produced but also numerous proinflammatory mediators promoting neutrophil and monocyte targeting. In this context, we show that TNFα and PAR-2 are involved in signaling in pathophysiological conditions, such as myocardial ischemia-reperfusion. At the same time, because TNFα may exert pro-inflammatory actions and PAR-2 may constitute one of the first protective mechanisms that signals a primary inflammatory response, our data support the concept that this network may regulate body responses to tissue injury.
Although discovered in 1994 by reduced stringency cloning from a murine cDNA library (1), the pathophysiological role in vivo of protease-activated receptor-2 (PAR-2) remains poorly understood. PAR-2 is widely distributed in endothelial (2–5) and smooth muscle cells (5–6) and myocytes of rat jejunum (7), and it is thought to play a role in the regulation of blood pressure and vascular tone (2, 3, 5, 6, 8–10), NO-dependent dilatation of the basilar artery (11), endotoxic shock (9), and inflammation (12, 13). Recently, it has been proposed that NO-mediated vasodilatation to PAR-2 activation is also selectively preserved or augmented in spontaneously hypertensive rats, suggesting a protective role for PAR-2 in the cerebral circulation during chronic hypertension (14).
PAR-2 is a member of seven transmembrane domain G protein-coupled receptors activated by proteolytic cleavage. After its activation, a new amino terminus peptide is exposed that functions as a tethered ligand. Trypsin (1), mast cell tryptase (15, 16), as well as factor Xa (17) may activate PAR-2 in vivo. However, short synthetic peptides [PAR-2-activating peptide (2AP)] corresponding to the new amino terminus exposed after trypsin cleavage (1, 9) are able to activate the receptor in the absence of proteolytic cleavage simulating the effect of trypsin. We have recently shown that inflammatory stimuli, such as endotoxemia induced by bacterial lipopolysaccharide, lead to an increased expression of PAR-2 on endothelial and smooth muscle cells that correlates to an increase in the hypotensive effect of synthetic peptides (9). Because PAR-2 expression is up-regulated in endothelial cells by inflammatory mediators such as interleukin-1 and tumor necrosis factor α (TNFα) (12), this result indicates that inflammatory intracellular signals regulate PAR-2 activity.
Widespread interest in the benefits of myocardial reperfusion has resulted from the positive outcome of several large clinical trials on thrombolysis or percutaneous transluminal angioplasty during acute myocardial infarction (18, 19) or in bypass surgery (18, 19). To investigate the role of PAR-2 in early cardiac inflammation, we analyzed the possible role of PAR-2 receptor in an experimental model of myocardial ischemia-reperfusion injury. This condition produces cardiac necrosis, neutrophil infiltration, and an early broad inflammatory status in the jeopardized tissue (18–21). During myocardial ischemia-reperfusion, there is a generation of oxygen radicals that have been also invoked in the pathophysiology of inflammation (18–21). At the same time, TNFα has been shown to be involved in myocardial ischemia (reviewed in ref. 22). Indeed, TNFα is a potent inflammatory trigger (18–21), but it also exerts cardio-protection (22–25). Pretreatment with TNFα before myocardial ischemia enhances expression of the superoxide radical scavenger Mn-superoxide dismutase (22, 23, 26–29), protecting the heart from oxygen radical-induced damage.
Because our working hypothesis is that PAR-2 is involved in inflammatory and injury response events (6, 9, 13, 30, 31), we attempted to verify whether PAR-2 plays a more general role in cardiac responses to ischemia-reperfusion injury. In the present studies, we provide the first evidence for a role of PAR-2 in cardiac reperfusion injury. Specifically, after treatment with PAR-2AP, we demonstrate a significant recovery of myocardial function. This phenomenon was associated to both PAR-2 and TNFα expression. Intracellular signaling was not mediated by tyrosine kinase, and the increment in coronary flow was not reverted by L-NAME, indicating a NO-independent pathway. Finally, we show that improvement of functional parameters was also associated with a reduction in metabolic indexes of myocardial oxidative injury occurring during the generation of large amounts of oxygen radicals at reperfusion.
Materials and Methods
Isolated Heart Preparation.
The hearts of 54 male rats (6-mo-old, mean body weight of 375 ± 25 g, and mean heart weight 692 ± 45 mg) were excised and perfused in the retrograde Langendorff mode under a constant pressure of 80 mmHg, as previously described in detail (32). In brief, perfusate Tyrode solution contained 120 mM NaCl, 6.0 mM KCl, 2.0 mM CaCl2, 1.0 mM MgSO4, 0.5 mM EDTA, 12.5 mM glucose, and 24 mM NaHCO3, at pH 7.4. The solution was equilibrated at 37°C with a gas mixture of 95% O2 and 5% CO2 and not recirculated. To assess contractile function, a latex balloon was inserted into the left ventricular cavity through the mitral orifice and connected to a pressure transducer (Statham-Gould, Cleveland, OH). A heart electrocardiogram was obtained by an atraumatic epicardial electrode (0.8 mm diameter, silver wire) attached to the free wall of the right ventricle. All subsequent measurements of developed pressure, calculated as the difference between peak systolic and end-diastolic pressure, were made at this same end-diastolic volume. Left ventricular pressure was recorded on a recorder (Statham-Gould). After a 20-min equilibration period, baseline parameters were recorded. Coronary flow rate was measured from timed collection of coronary sinus effluent and related to heart wet weight (ml/min/g). Arrhythmias were scored according to the Lambeth Convention Guidelines as previously described (32). The study was performed in accordance with the Guide for the Care and Use of Laboratory Animals (U.S. Department of Health and Human Services) and the position of the American Heart Association.
Protocol.
After a 20-min equilibration period, baseline parameters were recorded. Twenty minutes of global ischemia was then induced by cross-clamping the perfusion line. Hearts were maintained at 37°C throughout ischemia by immersion in warm perfusate. Hemodynamic parameters were recorded until 60 min of reperfusion. Peptides (see below) were freshly diluted with appropriate amounts of perfusion buffer and administered by a Harvard syringe pump through a side-arm in the perfusion apparatus, at a flow-rate 1/150 that of each heart, to achieve different final concentrations (10–100 μM) in the perfusion line. To verify the effects of PAR-2AP during ischemia-reperfusion, infusion was started at 15 min of stabilization and continued for 5 min into reperfusion. During global ischemia only peptides were infused in treated hearts in small volumes of buffer, controls received equal amounts of buffer alone. Genistein (10 mM) was added together 100 μM PAR-2AP (SLIGRL-NH2). SLIGRL-NH2 and LSIGRL-NH2 (scrambled control peptide) were synthesized by standard solid-phase 9-fluorenylmethoxycarbonyl (FMOC) chemistry with an automated peptide synthesizer (Applied Biosystems, model 432A). Peptides were purified by RP-HPLC, and their identity was confirmed by mass spectroscopy in the Department of Medicinal Chemistry of the Federico II University of Naples, as previously described in detail (9). Cardiac hemodynamics, heart rate, and arrhythmias were monitored throughout the experiment, and samples of coronary sinus effluent were collected every 5 min for biochemical measurements (see below). At the end of the experiment, the hearts were removed from the perfusion apparatus, blotted, and weighed. The cardiac homogenate was used for malondialdehyde (MDA) assay, glutathione reductase, and peroxidase tissue determinations (see below).
Cardiac Tissue Homogenization and Nuclear Extract Preparation.
Both atria and right ventricle were removed, and the left ventricle was homogenized in 9 vol of 1.15% KCl solution (33). To prevent auto-oxidation of the tissutal samples, homogenization was carried out at 4°C in nitrogen-equilibrated solutions, in presence of 10 μM deferoxamine, 0.04% butylated hydroxytoluene, and 2% ethanol (33). The homogenate was centrifuged at 1,000 g for 10 min at 4°C to remove nuclei that were isolated according to the method of Wu (34). In brief, nuclei were resuspended in 1.2 vol of an extraction solution consisting of 10 mM Hepes (pH 7.9), 0.4 M NaCl, 1.5 mM MgCl2, 0.1 mM EGTA, 0.5 mM DTT, 5% glycerol, and the following protease inhibitors: 5 mM leupeptin, 1.5 mM aprotinin, 2 mM phenylethylsulfonylfluoride (PMSF), 3 mM peptastatin A, and 1 mM benzamidine. Protein concentrations were determined by the Lowry method using BSA as a standard (35). In additional hearts, we also performed the classical measurements of risk zone by a 0.5% suspension of zinc-cadmium fluorescent particles in the perfusate to mark the risk zone as nonfluorescent tissue. In brief, the hearts were weighted and frozen overnight before being cut into 2-mm thick slices and stained for 20 min in 1% triphenyl-tetrazolium-chloride. The ischemic risk zone (nonfluorescent under UV light) was traced on acetate by a computer-assisted imaging analyzer (ofoto software, T1–2 version 2.0, Apple, Cupertino, CA).
Biochemical Determinations.
One aliquot of heart tissue supernatant was used for the determination of the peroxidative index MDA by using the thiobarbituric assay (33).
To measure oxidized glutathione (GSSG) release, an additional 0.4 ml aliquots of coronary effluent were simultaneously drawn from a side-arm in the pulmonary artery cannula into a syringe containing 100 μl of 10 mM EDTA and 50 mM N-ethyl-maleimide in 100 mM K-phosphate buffer, pH 7.4, to prevent artifactual oxidation of glutathione (GSH). Concentrations of total glutathione (i.e., GSSG + GSH) were measured by the glutathione reductase/5,5′-dithiobis-(2-nitrobenzoic)acid (DTNB) recirculating assay of Tietze (36), after the oxidation of NADPH at 25°C after addition of GSSG reductase (33). Concentrations of GSSG alone were measured in the presence of N-ethyl-maleimide-EDTA to prevent GSH from reacting. The release of GSH in the coronary effluent was then calculated from the difference between the concentration of total (GSSG + GSH) and oxidized glutathione (GSSG) at each time point (33). Glutathione concentrations were expressed as nanomoles of GSH-equivalents released per min, per gram of wet weight. Total integrated creatine kinase (CK) activity over reperfusion was evaluated as previously described (37). Tissue glutathione reductase and peroxidase and Mn-superoxide dismutase were determined spectrophotometrically as previously described in detail (32). Finally, TNF-α concentrations were determined in the effluent during reperfusion by ELISA according to the manufacturer's instructions (Boehringer Mannheim).
Western Blot Analysis.
Fifty micrograms of protein (homogenate or nuclear extracts) separated by 12.5% SDS-PAGE were transferred to Immobilon-P transfer membranes (Millipore). Western blot analysis was performed according to the standard procedure (38). Membranes were blocked with 5% nonfat milk proteins and incubated with specific antibodies, usually diluted to 1:1,000. Epitopes on proteins recognized specifically by antibodies were visualized by using enhanced chemiluminescence (ECL; Amersham, Milan). We used the specific mAb against PAR-2 receptor raised in rabbit (B5, 1:1,000) (7) and the mAb against TNFα (L-19, catalog no. Sc-1351, goat polyclonal against the epitope corresponding to an amino acid sequence mapping α-amino terminus, Santa Cruz Biotechnology).
To ascertain that blots were loaded with equal amounts of protein lysates, they were also incubated in the presence of the polyclonal antibody against the γ-tubulin protein (Sigma). Densitometric scanning of the Western blots was done by using a Scan LKB (Pharmacia), as previously described in detail (39).
Statistical Analysis.
Data are presented as mean ± SE of the mean. Differences in the time course of the various parameters among the various groups were tested by repeated measure analysis of variance (ANOVA). When the overall trend was significantly different, comparisons at specific time points were made by Bonferroni's corrected t test considering P < 0.05 as significant.
Results
Functional Parameters.
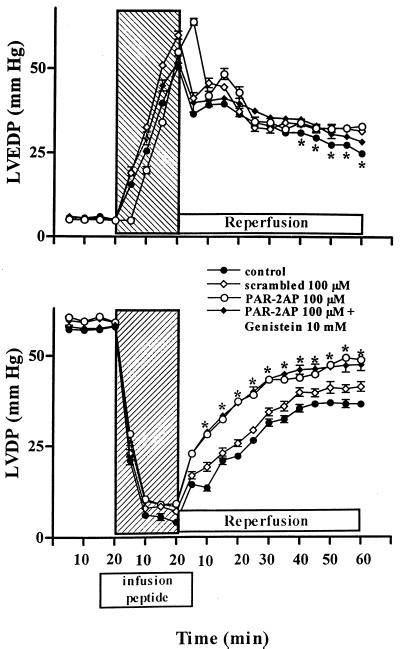
Developed pressure.
Developed pressure before ischemia was similar among groups (Fig. 1, Lower). In untreated hearts reperfused after 20 min of global ischemia, recovery of developed pressure was markedly impaired averaging ≈55% of baseline at the end of 60 min of reflow. Recovery of developed pressure was significantly greater in hearts receiving infusion of PAR-2AP from the 15 min of stabilization to the 5 min of reperfusion (Fig. 1, Lower). In this group, developed pressure was ≈85% of baseline at the end of 60 min of reflow. This phenomenon was not seen when hearts were treated with the scrambled peptide, indicating a selective role of PAR-2. Cotreatment with the inhibitor of tyrosine kinase genistein to PAR-2AP did not revert the recovery of developed pressure (Fig. 1, Lower). In preliminary experiments, genistein alone induced a weak and nonsignificant negative inotropic effect (data not shown).The dose of PAR-2AP (10 μM) caused a modest recovery of developed pressure at the end of the reperfusion period of ≈18% [n = 6; P = not significant (NS)], similar to that achieved with 200 μM trypsin (n = 4; P = NS), whereas the dose of PAR-2AP (30 μM) gave ≈38%.
Figure 1.
Hemodynamic effects of PAR-2AP. Infusion of PAR-2AP in isolated perfused rat heart significantly modulates left ventricular end-diastolic pressure (LVDEP) and left ventricular developed pressure (LVDP) during ischemia-reperfusion injury. Hatched area shows the global ischemia period after 20 min of stabilization period. Scrambled peptide (100 μM) was ineffective whereas genistein did not revert the effects of PAR-2AP alone. Data are expressed as mean ± SD; n = 6. *, P < 0.05 vs. control and scrambled peptide.
End-diastolic pressure.
Ischemia-reperfusion injury also affected recovery of end-diastolic pressure in untreated hearts (Fig. 1, Upper). End-diastolic pressure in control hearts at the end of 60 min of reperfusion was greater than fivefold over baseline values, whereas it was significantly lower in the group treated with PAR-2AP (Fig. 1, Upper). Accordingly to data of developed pressure, no improvement of end-diastolic pressure was seen when hearts were treated with the scrambled peptide. Similarly, cotreatment with genistein to PAR-2AP did not revert the recovery in end-diastolic pressure (Fig. 1, Upper). Also in this case the dose of 10 μM PAR-2AP caused a small but significant decrease in end-diastolic pressure at the end of the reperfusion period of ≈25%, whereas the dose of 30 μM of PAR-2AP gave an improvement of ≈45% (n = 6 for both doses; P < 0.05 from 30 to 60 min of reperfusion period vs. their own controls). Trypsin (200 μM) caused a modest recovery of end-diastolic pressure at the end of the reperfusion period of ≈15% (n = 4; P = NS).
Coronary flow.
Baseline values of coronary flow were similar among groups (Table 1). Hearts treated with PAR-2AP tended to have better recovery of coronary flow than untreated and scrambled-treated hearts after 60 min of reperfusion (Table 1). Pretreatment of hearts with 0.5 mM L-NAME did not revert the effect on coronary flow (n = 3; P = NS) indicating a NO-independent pathway. Also in this case, cotreatment with genistein to PAR-2AP did not revert the recovery of coronary flow (Table 1). At 10 and 30 μM concentrations of PAR-2AP, we observed a tendency of a better recovery of coronary flow that did not reach statistical significance (data not shown). Similarly, trypsin caused a slight recovery of coronary flow of ≈6% (n = 4; P = NS).
Table 1.
Coronary flow and heart rate in different experimental groups
| Coronary flow, ml/min/g tissue wet weight | Control | 100 μM Scrambled peptide | 100 μM PAR-2AP | 100 μM PAR-2AP + Genistein |
|---|---|---|---|---|
| 20 min Baseline | 13.5 ± 1.2 | 13.6 ± 1.1 | 13.2 ± 1.3 | 13.0 ± 1.4 |
| 20 min Isch | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| 5 min Rep | 6.5 ± 1.6 | 6.6 ± 1.8 | 7.2 ± 1.7 | 7.4 ± 1.9 |
| 60 min Rep | 9.1 ± 1.4 | 9.2 ± 1.5 | 10.9 ± 1.6* | 10.8 ± 1.5* |
| Heart Rate, beats/min | ||||
| 20 min Baseline | 247 ± 20 | 248 ± 22 | 247 ± 24 | 249 ± 23 |
| 5 min Rep | 127 ± 29 | 128 ± 28 | 122 ± 28 | 120 ± 25 |
| 60 min Rep | 192 ± 26 | 191 ± 27 | 210 ± 28 | 200 ± 26 |
*, P < 0.05 vs. control and scrambled peptide-treated group. Mean ± SD, n = 6 for each group.
Heart rate.
Baseline values of heart rate were similar among groups (Table 1). PAR-2AP treatment at all concentrations, nor cotreatment with genistein, neither trypsin (data not shown), induced any significant change at each time point of the study protocol (Table 1). In preliminary experiments, genistein alone induced a weak bradycardic effect (data not shown).
Arrhythmia analysis.
The arrhythmic score of controls was 3.8 ± 1.2 similar to that of scrambled peptide-treated group (3.7 ± 1.4) but decreased progressively to 3.5 ± 1.1 and 3.2 ± 1.2 in 10 and 30 μM PAR-2AP treated groups (P = NS vs. controls). The increased score in controls was due mainly to ventricular tachycardia and nonsustained ventricular fibrillation. In hearts treated with 100 μM PAR-2AP, the score was significantly reduced (2.8 ± 1.1; P < 0.05 vs. control and scrambled peptide) and cotreatment with genistein did not have any effect (P = NS, n = 6). Similarly, trypsin (3.8 ± 1.5) did not induce any effect on arrhythmias (P = NS; n = 4).
Biochemical Parameters.
Tissue MDA levels.
Previously we established that MDA content in normally perfused hearts at baseline averaged 0.98 ± 0.1 nmol/mg of protein. This content increased slightly after 20 min of ischemia (1.22 ± 0.15 nmol/mg of protein, P = NS vs. baseline) whereas it significantly increased in 60 min reperfused hearts (1.45 ± 0.2 nmol/mg of protein, P < 0.05 vs. baseline). When detected at the end of reperfusion, this increase in lipid peroxidation was significantly reduced by treatment with 100 μM PAR-2AP (1.11 ± 0.1 nmol/mg of protein, P < 0.05 vs. controls). Scrambled peptide (100 μM), 10–30 μM concentrations of PAR-2AP and trypsin did not reduce significantly the amount of MDA during reperfusion (data not shown).
GSSG and GSH release on the coronary effluent.
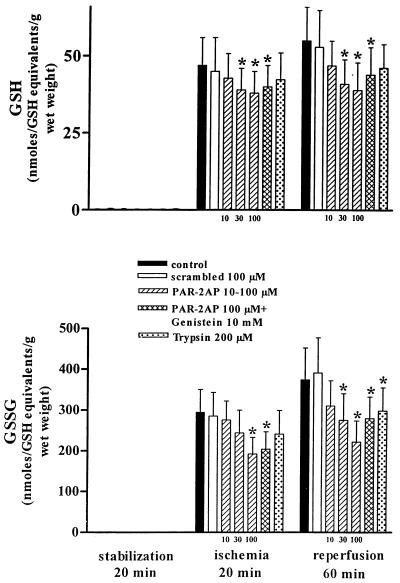
As expected GSSG and GSH release at baseline was negligible in all groups (Fig. 2). Infusion of PAR-2AP significantly reduced the release of both GSSG and GSH after 20 min of ischemia and 60 min of reperfusion in comparison with controls and scrambled-treated hearts (Fig. 2).
Figure 2.
Effect of PAR-2AP administration on GSSG and reduced GSH as indexes of oxidative reperfusion-injury. At 20 min of stabilization, GSSG and GSH were not detected. PAR-2AP dose dependently (10–100 μM) reduced GSSG and GSH levels in effluents from isolated rat heart after 20 min of ischemia and at 60 min of the reperfusion period. Genistein did not revert PAR-2AP effect. Trypsin caused a small but significant inhibition of GSSG only at 60 min of the reperfusion period. Data are expressed as mean ± SD; n = 6; *, P < 0.05 vs. control and scrambled peptide.
Tissue activities of glutathione metabolism enzymes and Mn-superoxide dismutase.
Cardiac tissue activities of gluthatione peroxidase and reductase are showed in Table 2. These levels appeared to be not affected significantly by our protocol of ischemia-reperfusion and were similar among experimental groups. In contrast, Mn-superoxide dismutase was significantly increased after PAR-2AP treatment (Table 2).
Table 2.
Concentrations of cardiac tissutal activities of glutathione peroxidase and reductase and Mn-superoxide dismutase in different experimental groups
| Glutathione peroxidase, mU/mg prot | Glutathione reductase, mU/mg prot | Mn-superoxide dismutase, units/mg prot | |
|---|---|---|---|
| Control nonischemic hearts | 86.7 ± 4.5† | 2.5 ± 0.2 | 2.4 ± 0.4 |
| Control ischemic hearts | 59.8 ± 6.8 | 2.8 ± 0.2 | 2.1 ± 0.2 |
| Scrambled-treated ischemic hearts | 60.8 ± 6.5 | 2.6 ± 0.2 | 2.0 ± 0.2 |
| 100 μM PAR-2AP-treated ischemic hearts | 65.7 ± 7.5 | 2.7 ± 0.2 | 3.2 ± 0.4‡ |
| 30 μM PAR-2AP-treated ischemic hearts | 63.8 ± 8.6 | 2.6 ± 0.2 | 2.8 ± 0.3* |
| 10 μM PAR-2AP-treated ischemic hearts | 63.4 ± 7.9 | 2.5 ± 0.2 | 2.4 ± 0.4 |
| 100 μM PAR-2AP + genistein-treated ischemic hearts | 67.8 ± 8.2 | 2.7 ± 0.2 | 2.8 ± 0.4* |
†, P < 0.05 vs. control ischemic hearts; *, P < 0.05 vs. scrambled and control ischemic hearts; ‡, P < 0.05 vs. scrambled and control ischemic hearts. Mean ± SD, n = 6 for each group.
Creatine kinase activity.
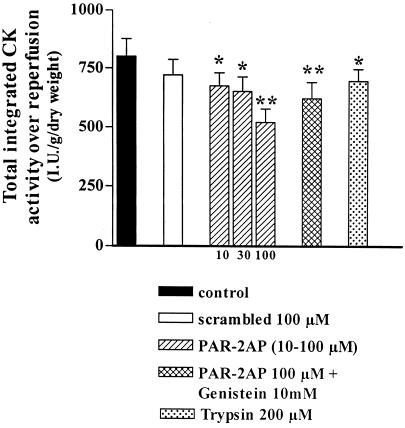
Fig. 3 showed that the total integrated creatine kinase activity over reperfusion was significantly reduced by PAR-2AP treatment (10–100 μM). Also trypsin reduced significantly creatine kinase release (Fig. 3). In contrast, no significant reduction of this release was observed when hearts were treated with the scrambled peptide. Cotreatment with genistein to PAR-2AP did not have any effect with respect to PAR-2AP alone (Fig. 3).
Figure 3.
Effect of PAR-2AP administration on heart damage evaluated as CK release over reperfusion. PAR-2AP dose dependently reduces total integrated CK during reperfusion. Genistein did not revert PAR-2AP effect. Trypsin caused a small but significant reduction of CK release over reperfusion comparable to the lower dose of PAR-2AP used. Data are expressed as mean ± SD, n = 6; *P < 0.05, **P < 0.01 vs. control and scrambled peptide.
Ischemic risk zone.
Mean risk zone was 368 ± 23 mm3 in controls and 360 ± 26 mm3 in scrambled peptide decreasing to 339 ± 19 mm3 in 30 μM PAR-2AP-treated hearts (P = 0.064, NS), and to 289 ± 19 mm3 in 100 μM PAR-2AP-treated hearts (P < 0.02 and P < 0.03 vs. controls and scrambled peptide, respectively). Also, in this case, cotreatment with neither genistein nor trypsin had any effect with respect to PAR-2AP alone and controls, respectively (P = NS for both comparisons).
TNFα release.
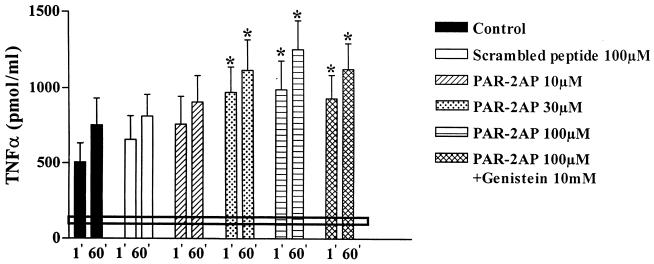
Basal release of TNFα was 92 ± 32 pmol/ml. The increment of TNFα release during reperfusion is shown in Fig. 4. Significant amounts of TNFα were already detected in the coronary effluent during the first minute of reperfusion in control ischemic hearts. TNFα levels correlated with postischemic creatine kinase levels (r = 0.85, P < 0.001) but was inversely correlated with ischemic risk zone (r = −0.47; P < 0.05). It is to note that TNFα levels were significantly increased after infusion of PAR-2AP (Fig. 4). Trypsin or scrambled peptide did not modify TNFα release (P = NS vs. controls).
Figure 4.
Effect of PAR-2AP administration on TNFα release of coronary effluent. TNFα release was measured at 1 min and at 60 min of the reperfusion period. The black continuous line indicates the mean basal TNFα release measured at 20 min of the stabilization period whereas dotted line represents SD. PAR-2AP progressively increases TNFα release whereas genistein did not revert PAR-2AP effect. Data are expressed as mean ± SD, n = 6; *P < 0.05 vs. control and scrambled peptide.
Western Blot Analysis of PAR-2 and TNFα.
Western blot analysis in cardiac homogenate and nuclear extracts revealed a marked increase in the both PAR-2 and TNFα expression after treatment with PAR-2AP. Fig. 5 shows a Western blot analysis in nuclear extracts from hearts treated with 100 μM of scrambled peptide or PAR-2AP. After 20 min of ischemia, there was a marked increase in TNFα expression in both hearts whereas PAR-2 expression increased after PAR-2AP treatment only. After 60 min of reperfusion, we observed a little increase in PAR-2 and TNFα expression in hearts treated with the scrambled peptide. This increase was threefold higher in hearts treated with PAR-2AP. Semi-quantitative scanning analysis of blots showed a positive correlation between PAR-2 and TNFα expression in scrambled-treated hearts (r = 0.672, P < 0.01) and a stronger positive correlation with hearts exposed to 100 μM PAR-2AP (r = 0.892, P < 0.0001). Unspecific expression of atrial natriuretic peptide, used as control peptide, was unchanged between controls and PAR-2AP-treated hearts (data not shown). Similar qualitative results were obtained using cardiac homogenate instead of nuclear extracts and when hearts were infused with 10–30 μM doses of PAR-2AP but not using trypsin (data not shown).
Figure 5.
Western blot analysis in nuclear extracts from hearts treated with 100 μM of scrambled peptide or PAR-2AP at 5 min of baseline, at 20 min of global ischemia, and at 60 min of reperfusion period normalized by γ-tubulin. After 20 min of ischemia, there was a marked increase in TNFα. PAR-2 expression increased after PAR-2AP treatment only. However, at 60 min of reperfusion, we observed a little increase in PAR-2 and TNFα expression in hearts treated with the scrambled peptide whereas hearts treated with PAR-2AP showed a marked increase of PAR-2 and TNFα expression.
Discussion
Studies performed in vivo with PAR-2AP have pointed to a role for PAR-2 in the regulation of cardiovascular functions (5, 6, 8–10). Given the unusual proteinase-mediated mechanism for the activation of PAR-2 and an absence of a conventional circulating hormonal ligand, the in vivo pathophysiological circumstances in which this receptor becomes activated are not well established. PAR-2 may play a role in the setting of an inflammatory response and tissue injury (31). In this context, myocardial ischemia-reperfusion injury may represent an ideal scenario for the action of PAR-2.
We have observed that functional parameters were improved whereas metabolic and oxidative indexes of reperfusion-injury were significantly decreased after stimulation of PAR-2 and these effects were dose-dependent. The specificity of the action of PAR-2AP was further confirmed by the lack of effect on these functional and metabolic parameters by the control scrambled peptide. PAR-2AP significantly improved the functional parameters: left ventricular developed pressure, left ventricular end-diastolic pressure, and coronary flow, and decreased both the ischemic risk zone and the oxidative injury induced by generation of oxygen radicals at reflow. The lack of action of genistein ruled out a possible involvement of tyrosine kinase. Indeed, by using isolated rat arteries, it has been suggested that tyrosine kinase may mediate intracellular signaling of PAR-2 (40). However, in our experimental setting and using genistein, no difference were seen on both functional and biochemical parameters.
Despite the difficulties with making measurements in patients, there is no reason to think that the fundamental biology of ischemia-reperfusion injury is substantially different between humans and experimental models (18, 19). Myocardial ischemia is severe enough to initiate cardiac inflammation during reperfusion but reperfusion injury reduces the amount of benefit that can be achieved by reperfusion. During early reperfusion injury in vivo, not only oxygen radicals and oxidation-related adhesion molecules are produced but also numerous proinflammatory cytokines. In addition, it is activated complement cascade and it is promoted neutrophil and monocyte targeting (41). All these factors induce an early cardiac postischemic inflammation and oxidative injury. In this latter regard, under experimental conditions activation of PAR-2 induced a significant decrease in GSH and GSSG release but did not interfere with tissue activities of glutathione peroxidase and reductase. These results imply that after PAR-2 activation there is a direct effect on the heart because glutathione peroxidase and glutathione reductase, enzymes specifically involved into the metabolism of GSH and GSGG, were not inhibited. The above results also fit well with the dose-dependent reduction in lipid peroxidation measured as MDA content, and cardiac tissue injury measured as total integrated creatine kinase release and ischemic risk zone. In fact, to counteract the effects of toxic oxygen metabolites generated during reperfusion, the cells are endowed with radical scavenging systems. In the glutathione cycle, oxidative compounds are partially metabolized in a glutathione peroxidase-catalyzed reaction with reduced glutathione (GSH), which is present in large amounts inside the cells. The oxidized dimer of glutathione (GSSG) thus formed is subsequently reduced to restore GSH. However, when the cells are exposed to a large amount of oxidants, GSSG formation may exceed the rate of metabolism, resulting in a condition of “oxidative stress” (42, 43). Several studies have established that GSSG is a sensitive and reliable indicator of oxidative stress in the heart (44, 45).
TNFα is a potent inducer of PAR-2 expression and it has been proposed to be cardioprotective (22–29) via increased Mn-superoxide dismutase scavenger activity in the heart and therefore resistance to oxidative reperfusion-injury. Conversely, it has also been shown that rat myocardium synthesizes and releases TNFα in response to reperfusion, which directly correlates with the postischemic cellular necrosis (19–21). The negative inotropic impact of TNFα is frequently ascribed to the stimulation of inducible NO synthase often at higher TNFα concentrations (18). However, whether TNFα is a physiological or a pathological response to injury is still a matter of debate. We have observed that after PAR-2AP administration there was a parallel increase in nuclear TNFα production as well as a coupled expression in the whole heart tissue. These data indicate that the role for TNFα and PAR-2 in ischemia-reperfusion may not be necessarily pathological. It could reflect a more complex situation in which the heart tries to counterbalance the damage caused by ischemia by inducing, a protective mechanical response together with a NO-independent coronary vasodilator effect. PAR-2AP administration also induced an increase in cardiac tissue Mn-superoxide dismutase whose expression is also stimulated by TNFα (26–29), suggesting a final common pathway. In this context, it also has been shown that TNFα induces a dose-dependent protective effect against endotoxin-induced shock and tissue injury in rats (46). Endotoxin pretreatment decreases cardiac ischemia-reperfusion injury and increases myocardial endogenous activity of the hydrogen peroxide scavenger catalase activity in isolated rat heart (47). Endogenous TNFα is protective against the cytotoxicity of exogenous TNFα (48). At the same time, activation of PAR-2, which colocalizes with trypsin in airway epithelium, induces the relaxation of airway preparations causing also a powerful broncho-protection (49). Moreover, bacterial proteinases are able to activate PAR-2 on neutrophils (50), suggesting that this receptor may constitute one of the first alarm protective mechanisms that signal invasion of bacterial pathogens by activating a primary inflammatory response. This consideration might also well fit with the recent hypothesis of the infection-inflammation triggers acute coronary syndromes (51). In conclusion, we have shown that after activation of PAR-2 there is a recovery of functional response associated to an improvement of metabolic parameters of the heart during experimental ischemia-reperfusion injury. These phenomena are coupled to TNFα synthesis implying a common final pathway. Taken together, our data indicate that TNFα and PAR-2 could be involved in both physiological and pathophysiological intracellular signaling.
Trypsin, one of the recognized endogenous activators, did not account for the beneficial effect of PAR-2 activation on ischemia reperfusion. The above finding is consistent with the hypothesis of an in vivo “unknown” activator of the PAR-2 receptor or alternatively raise the possibility that some proteinases, in a similar fashion to PAR-1, “disarm” the receptor (52). This, in turn, could cause more induction of PAR-2 expression coupled to TNFα synthesis and release.
Acknowledgments
This paper is dedicated to the memory of Dr. Russel Ross, Dr. Gaetano Salvatore, and all victims of coronary heart disease. This work was supported by grant ISNIH.99.56980 (to C.N.) and MURST 97/60% (to G.C.). We also acknowledge Dr. Morley D. Hollenberg for the generous gift of the B5 antibody against PAR-2. Finally, we would like to thank Drs. Y. Lee and M. R. Bucci for the excellent technical assistance with Langendorff experiments and very accurate preparations of PAR-2 activating peptide and scrambled peptide.
Abbreviations
- PAR-2
Protease-activated receptor-2
- 2AP
2-activating peptide
- GSGG
oxidized glutathione
- GSH
glutathione
- TNFα
tumor necrosis factor α
- MDA
malondialdehyde
- EDTA
ethylendiaminotetracetate
- CK
creatine kinase
- l-NAME
l-nitroarginine methylester
- NS
not significant
References
- 1.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Proc Natl Acad Sci USA. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwa J J, Ghibaudi L, Williams P, Chintala M, Zhang R, Chatterjee M, Sybertz E. Circ Res. 1996;78:581–588. doi: 10.1161/01.res.78.4.581. [DOI] [PubMed] [Google Scholar]
- 3.Emilsson K, Wahlestedt C, Sun M K, Nystedt S, Owman C, Sundelin J. J Vasc Res. 1997;34:267–272. doi: 10.1159/000159233. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton J R, Nguyen P B, Cocks T M. Circ Res. 1998;82:1306–1311. doi: 10.1161/01.res.82.12.1306. [DOI] [PubMed] [Google Scholar]
- 5.D'Andrea M R, Derian C K, Leturcq D, Baker S M, Brunmark A, Ling P, Darrow A L, Santulli R J, Brass L F, Andrade-Gordon P. J Histochem Cytochem. 1998;46:157–164. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- 6.Molino M, Raghunath P N, Kuo A, Ahuja M, Hoxie J A, Brass L F, Barnathan E S. Arterioscler Thromb Vasc Biol. 1998;18:825–832. doi: 10.1161/01.atv.18.5.825. [DOI] [PubMed] [Google Scholar]
- 7.Kong W, McConalogue K, Khitin L M, Hollenberg M D, Payan D G, Bohm S K, Bunnett N W. Proc Natl Acad Sci USA. 1997;94:8884–8889. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung W M, Andrade-Gordon P, Derian C K, Damiano B P. Can J Physiol Pharmacol. 1998;76:16–25. doi: 10.1139/cjpp-76-1-16. [DOI] [PubMed] [Google Scholar]
- 9.Cicala C, Pinto A, Bucci M, Sorrentino R, Walker B, Harriot P, Cruchley A, Kapas S, Howells G L, Cirino G. Circulation. 1999;99:2590–2597. doi: 10.1161/01.cir.99.19.2590. [DOI] [PubMed] [Google Scholar]
- 10.Damiano B P, Cheung W M, Santulli R J, Fung-Leung W P, Ngo K, Ye R D, Darrow A L, Derian C K, De Garavilla L, Andrade-Gordon P. J Pharmacol Exp Ther. 1999;288:671–678. [PubMed] [Google Scholar]
- 11.Sobey C G, Cocks T M. Stroke. 1998;29:1439–1444. doi: 10.1161/01.str.29.7.1439. [DOI] [PubMed] [Google Scholar]
- 12.Nystedt S, Ramakrishnan V, Sundelin J. J Biol Chem. 1996;271:14910–14915. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]
- 13.Kawabata A, Kuroda R, Minami T, Kataoka K, Taneda M. Br J Pharmacol. 1998;125:419–422. doi: 10.1038/sj.bjp.0702063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sobey C G, Moffatt J D, Cocks T M, Kontos H A. Stroke. 1999;30:1933–1941. doi: 10.1161/01.str.30.9.1933. [DOI] [PubMed] [Google Scholar]
- 15.Molino M, Barnathan E S, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie J A, Schechter N, Woolkalis M, Brass L F. J Biol Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- 16.Corvera C U, Dery O, McConalgue K, Bohm S K, Khitin L M, Caughey G H, Payan D G, Bunnett N W. J Clin Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox M T, Harriott P, Walker B, Stone S R. FEBS Lett. 1997;417:267–269. doi: 10.1016/s0014-5793(97)01298-2. [DOI] [PubMed] [Google Scholar]
- 18.Becker L C, Ambrosio G. Prog Cardiovasc Dis. 1987;30:23–44. doi: 10.1016/0033-0620(87)90009-0. [DOI] [PubMed] [Google Scholar]
- 19.Kukreja R C, Janin Y. J Thromb Thrombol. 1997;4:7–24. doi: 10.1023/a:1017569611074. [DOI] [PubMed] [Google Scholar]
- 20.Zweier J L, Kuppusamy P, Williams R, Rayburn B K, Smith D, Weisfeldt M L, Flaherty J T. J Biol Chem. 1989;264:18890–18895. [PubMed] [Google Scholar]
- 21.McCord J M. Science. 1974;185:529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- 22.Medrum D R. Am J Physiol. 1988;274:R577–R595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 23.Kupatt C, Habazettl H, Goedecke A, Wolf D A, Zahler S, Boekstegers P, Kelly R A, Becker B F. Circ Res. 1999;84:392–400. doi: 10.1161/01.res.84.4.392. [DOI] [PubMed] [Google Scholar]
- 24.Meng X, Banerjee A, Ao L, Meldrum D R, Cain B S, Shames B D, Harken A H. Ann NY Acad Sci. 1999;874:69–82. doi: 10.1111/j.1749-6632.1999.tb09226.x. [DOI] [PubMed] [Google Scholar]
- 25.Li D, Zhao L, Liu M, Du X, Ding W, Zhang J, Mehta J L. Am Heart J. 1999;137:1145–1152. doi: 10.1016/s0002-8703(99)70375-3. [DOI] [PubMed] [Google Scholar]
- 26.Eddy L J, Goeddel D V, Wong G H. Biochem Biophys Res Commun. 1992;184:1056–1059. doi: 10.1016/0006-291x(92)90698-k. [DOI] [PubMed] [Google Scholar]
- 27.Nelson S K, Wong G H, McCord J M. J Mol Cell Cardiol. 1995;27:223–229. doi: 10.1016/s0022-2828(08)80021-1. [DOI] [PubMed] [Google Scholar]
- 28.Nakano M, Knowlton A A, Dibbs Z, Mann D L. Circulation. 1998;97:1392–1400. doi: 10.1161/01.cir.97.14.1392. [DOI] [PubMed] [Google Scholar]
- 29.Wong G H, Goeddel D V. Science. 1988;242:941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- 30.Vergnolle N, Hollenberg M D, Sharkey K A, Wallace J L. Br J Pharmacol. 1999;127:1083–1090. doi: 10.1038/sj.bjp.0702634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollenberg M D. Trends Pharmacol Sci. 1999;20:271–273. doi: 10.1016/s0165-6147(99)01333-4. [DOI] [PubMed] [Google Scholar]
- 32.Abete P, Napoli C, Santoro G, Ferrara N, Tritto I, Chiariello M, Rengo F, Ambrosio G. J Mol Cell Cardiol. 1999;31:227–236. doi: 10.1006/jmcc.1998.0862. [DOI] [PubMed] [Google Scholar]
- 33.Slater T F. Methods Enzymol. 1984;105:283–293. doi: 10.1016/s0076-6879(84)05036-9. [DOI] [PubMed] [Google Scholar]
- 34.Wu C. Nature (London) 1985;317:84–87. doi: 10.1038/317084a0. [DOI] [PubMed] [Google Scholar]
- 35.Lowry O H, Rosebrough H J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 36.Tietze F. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 37.Napoli C, Liguori A, Chiariello M, Di Ieso N, Condorelli M, Ambrosio G. Eur Heart J. 1998;19:411–419. doi: 10.1053/euhj.1997.0748. [DOI] [PubMed] [Google Scholar]
- 38.Harlow E, Lane D. Immunoblotting: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 39.Napoli C, Mancini F P, Corso G, Malorni A, Crescenzi E, Palumbo G. J Biochem. 1997;121:1096–1101. doi: 10.1093/oxfordjournals.jbchem.a021700. [DOI] [PubMed] [Google Scholar]
- 40.Saiffedine M, Bahjat A A, Cheng C H, Wang L, Hollemberg M D. Br J Pharmacol. 1996;118:521–530. doi: 10.1111/j.1476-5381.1996.tb15433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Entman M L, Michael L, Rossen R D, Dreyer W J, Anderson D C, Taylor A A, Smith C W. FASEB J. 1991;5:2529–2537. doi: 10.1096/fasebj.5.11.1868978. [DOI] [PubMed] [Google Scholar]
- 42.Chance B, Sies H, Boveris H. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 43.Harlan J M, Levine J D, Callahan K S, Schwartz B R, Harker L A. J Clin Invest. 1984;73:706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishikawa T, Zimmer M, Sies H. FEBS Lett. 1986;200:128–132. doi: 10.1016/0014-5793(86)80524-5. [DOI] [PubMed] [Google Scholar]
- 45.Ishikawa T, Sies H. J Biol Chem. 1984;259:3838–3843. [PubMed] [Google Scholar]
- 46.Alexander H R, Doherty G M, Block M I, Kragel P J, Jensen J C, Langstein H N, Walker E, Norton J A. Infect Immun. 1991;59:3889–3894. doi: 10.1128/iai.59.11.3889-3894.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown J M, Grosso M A, Terada L S, Whitman G J, Banerjee A, White C W, Harken A H, Repine J. Proc Natl Acad Sci USA. 1989;86:2516–2520. doi: 10.1073/pnas.86.7.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okamoto T, Watanabe N, Yamauchi N, Tsuji Y, Tsuji N, Itoh Y, Neda H, Niitsu Y. Cancer Res. 1992;52:5278–5281. [PubMed] [Google Scholar]
- 49.Cocks T M, Fong B, Chow J M, Anderson G P, Frauman A G, Goldie R G, Henry P J, Carr M J, Hamilton J R, Moffatt J D. Nature (London) 1999;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- 50.Lourbakos A, Chinni C, Thompson P, Potempa J, Travis J, Mackie E J, Pike R N. FEBS Lett. 1998;232:84–89. doi: 10.1016/s0014-5793(98)01036-9. [DOI] [PubMed] [Google Scholar]
- 51.Danesh J, Collins R, Peto R. Lancet. 1997;350:430–436. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 52.Renesto P, Si-Tahar M, Moniatte M, Balloy V, Van Dorsselaer A, Pidard D, Chignard M. Blood. 1997;89:1944–1953. [PubMed] [Google Scholar]