Abstract
Young mated male Caribbean fruit flies [Anastrepha suspensa (Loew)] have greater sexual prowess than their virgin counterparts. After mating for the first time, 6- to 7-day-old males released twice as much sex pheromone and acquired another mate in less than half the time required by virgin males of the same age. Mass spectroscopic analysis of extracts of hemolymph from mated and virgin 7-day-old males resulted in identification of juvenile hormone III bisepoxide and juvenile hormone III in a ratio of 2.5:1. Extracts from mated males contained 3-fold more juvenile hormone than did extracts from virgins. Enhancement of sexual signaling, pheromone release, and mating was induced by topical application of juvenile hormone, methoprene, or fenoxycarb. Newly eclosed adult males treated with juvenoids engaged in sexual signaling, released pheromone, and mated at significantly earlier ages than control males. We conclude that juvenile hormone mediated a positive feedback system that imparted a competitive advantage, guaranteeing that males who mated at an early age would out-compete virgins of the same age for mating opportunities. Additionally, the results support the hypothesis that juvenile hormone is a pivotal hormone coordinating the development of sexual signaling and reproductive maturity in these flies.
Reproductive success among insects requires the use of efficient sexual signaling systems. These systems are, in most cases, correlated with adult reproductive maturity to prevent waste of reproductive effort and energy. Therefore, the endogenous mechanisms that regulate signaling systems must be coordinated tightly with factors responsible for controlling sexual maturity. For example, in the German cockroach, Blatella germanica (1), juvenile hormone (JH) coordinates cycles of gonadotropic activity with cycles of pheromone production. Additionally, in insects that do not have cycles of reproductive activity but that enter reproductive diapause at certain times, for example the moths Pseudaletia unipuncta and Agrotis ipsilon, sexual signaling and reproductive maturity, as indicated by the development of mature eggs, are also closely coordinated (2–6). In both of these moth species, females use JH for initiation of calling behavior and sex pheromone production as well as development of eggs (2–6).
Although no studies have addressed the hormonal coordination of reproductive maturity with sexual signaling in the Caribbean fruit fly [Anastrepha suspensa (Loew)], it is known that sexual signaling by males and response by females are tightly coordinated with reproductive maturity (7–9). Moreover, female reproductive behavior, including response to pheromone, is correlated directly with ovarian maturity (7). Similarly, developmental studies of male salivary and plural glands, both putative sites of pheromone production, showed that these glands reach full size at the same time that ovaries of females become mature and sexual communication begins (8). Thus, neither males nor females engage in sexual behaviors until they have achieved reproductive maturity at 7 or more days after adult emergence (7–9). These facts suggest that adult flies undergo a hormonally regulated period, during which time gametes mature and secondary sexual characters develop. Nonetheless, some males, probably larger individuals who have accumulated sufficient energy reserves, become sexually mature earlier than the majority of the population.
The probability that tropical species of Tephritid fruit flies, like the Caribbean fruit fly, will find mates at oviposition sites is limited because host plants are widely distributed in both time and space (10, 11). Therefore, these flies have evolved non-resource-based mating systems involving formation of male aggregations called “leks” (10–12). It is of distinct advantage for lekking males to possess characters giving them a competitive edge over other males in mating opportunities (13). Consequently, males have evolved complicated mating strategies in which auditory, visual, and chemical signals function to optimize the probability that females will select them from within a lek for mating (7, 12). Nonetheless, female choice, based on male phenotypic characters, results in highly skewed male mating success, and a small number of males account for most of the mating within a lek (13). Indeed, larger, more robust, sexually mature males of the Caribbean fruit fly possess characters that favor their selection as mates in paired male tests (14, 15). However, virgin females avoid recently mated males, even though these males show no overt differences in sexual signaling (16). Therefore, the probability that males will mate more than once in a single day is, at best, very low (17). Consequently, on a given day, young males engaging in sexual signaling have opportunities to mate, despite the presence of established older males, if the established older males have already mated. Interestingly, on subsequent days, previously mated males, regardless of age, are much more likely to attract females and engage in mating than are male virgins (16). We have discovered that male size is not the reason that females prefer young mated males to their virgin counterparts. Herein, we show that young mated male Caribbean fruit flies have greater sexual prowess than similarly aged virgins. We also discovered that the amounts of JH in hemolymph were 3-fold greater in mated 7-day-old males than in virgin males of the same age. Enhancement of sexual signaling and reproduction was induced by JH supplement therapy. From these findings, we conclude that the greater amounts of JH in young mated males enhance sexual behavior and signaling to optimize reproductive effort. Additionally, we discovered that JH supplement therapy induces precocious development of sexual signaling, pheromone release, and mating. Thus, JH is a pivotal hormone responsible for coordination of reproductive maturity and sexual signaling among males of this species. The results have important implications for improvement of efficacy of fruit fly pest control programs that employ release of sterile male insects (the sterile insect technique or SIT), because incorporation of JH supplement therapy into mass sterile fly rearing programs can provide flies that become sexually mature significantly earlier than those currently produced.
Methods
Behavioral and Mating Observations.
Pupae, obtained from laboratory cultures maintained by the Florida Division of Plant Industry, Gainesville, FL, were housed in a greenhouse under natural light (18). On the day of eclosion, adults were segregated by sex, transferred to separate 30 × 30 × 30-cm cages and provided with water and a 3:1 mixture of sugar and hydrolyzed brewers yeast. Experiments were conducted during the period of reproductive activity between 12:00–18:00 h (18, 19). We observed 12 groups of five males for calling behavior (exposure of the lateral abdominal glands and anal glands as well as other behavioral criteria associated with male sexual signaling; ref. 7) throughout the reproductive period on each day until flies were 10 days old. These experiments were also conducted with groups of 10 males that received no treatment, acetone treatment, or hormone supplement therapy (JH III, fenoxycarb, or methoprene) on the day of eclosion.
For initial mating experiments, five virgin males and females were caged together. The flies were selected at random on each day after eclosion from cages containing only virgins. Flies were observed for mating throughout the reproductive period. Mating pairs were removed from cages. Mating studies were also conducted with groups of 10 males that received either no treatment or treatment with just acetone or hormone supplement therapy on the day of eclosion. These males were caged with an equal number of sexually mature (8- to 12-day-old) virgin females on each day subsequent to hormone supplement therapy.
In other experiments, groups of five males, mated on the 5th day after emergence, were caged with five sexually mature, virgin females on the next day. The times it took for these males to mate again were compared with times it took for virgin 6-day-old males to mate. Additionally, groups of five males were combined with females on the 5th day. We removed mating pairs and held them separately. Virgin males were left with females until all had mated. All of these males mated by the end of the second day after pairing. We then caged the males who mated on either the 5th or 6th day with virgin females for a second time on the 7th day. We compared the time it took for these mated males to acquire mates with the time it took for virgin males of the same age to mate. The experiment was repeated with groups of 8-day-old virgins or males mated on either the 6th or 7th day caged with females for a second time on the 8th day.
Collection and Analysis of Pheromone.
Pheromone was collected during the first 4 h of the reproductive period (18, 19) from groups of five virgin males selected at random from holding cages on each day after emergence and from groups of males that received hormone supplement therapy treatments on the day of emergence. We also collected pheromone on the day after mating from groups of five males mated on days 5, 6, and 8 and, for comparison, from groups of virgin males who were 6, 7, and 9 days old at the same time. The total amount of all pheromone components (see ref. 18) released by males was determined by using capillary gas-liquid chromatography (18, 19).
Hormone Supplement Therapy and Identification of JH.
Pure synthetic samples of the diastereomers of JH IIIB were not available. Therefore, we used synthetic JH III and the JH agonists, fenoxycarb and methoprene, which have been used by others for hormone supplement therapy in flies (20–22). We applied the routinely used dose of 5 μg of the juvenoids in a 1-μl drop of acetone (4, 6, 23) or just acetone alone to the thorax of virgin males on the day of adult eclosion. Males were then returned to cages in the greenhouse. We also applied either acetone alone or JH III or methoprene to the thorax of virgin 5-day-old males and collected pheromone on day 6.
We used the technique of Bergot et al. (24) to extract, purify, and identify 397 pg (± 52.4; n = 3) of JH III from 1,000 whole bodies of 10-day-old virgin males. The technique did not allow for identification of JH III bisepoxide (25). We synthesized mixed diastereomers of JH IIIB (25) for use in mass spectral analyses. To identify both JH III and JH III bisepoxide, we collected 15-μl aliquots (≈0.5 μl per fly) of hemolymph separately from 12-day-old males and from mated and virgin 7-day-old males and extracted hemolymph with hexane containing 1 ng of farnesyl acetate as a quantitative internal standard (26). The hexane extracts, without further purification, were subjected to GC-chemical ionization (isobutane) and mass spectral analysis with a Finnigan-MAT (San Jose, CA) ITS 40 ion trap MS interfaced to a Varian Star 3400 GC (26). The GC was equipped with a cool-on-column injector. The 30-m × 0.25-mm (i.d.) analytical column used in the GC, a DB5-MS (J & W Scientific, Folsom, CA), was interfaced to a 10-m × 0.25-mm (i.d.) uncoated, deactivated fused silica retention gap. Conditions of chromatography were as follows: initial injector temperature = 40°C for 30 s; injector temperature increased at 170°C/min to 270°C; initial column temperature = 40°C for 5 min; column temperature increased at 5°C/min to 210°C; He carrier gas linear flow velocity = 24 cm/s; and GC-MS transfer line temperature = 230°C. Under these conditions, farnesyl acetate eluted at 32.3 min; JH III eluted at 33.8 min; and JH IIIB eluted at 34.3 min. Diagnostic ions used for identification and quantification of JH III included m/z = 267 (M+1), 235 (M+1-CH3OH), 217 (M+1-CH3OH-HOH), 189 (M+1-CH3OH-HOH-CO), and 147 (M+1-C2H4O2-C3H8O) (26). Diagnostic ions used for identification and quantification of JH IIIB included m/z = 283 (M+1), 265 (M+1-HOH), 251 (M+1-CH3OH), 233 (M+1-CH3OH-HOH), and 205 (M+1-CH3OH-HOH-CO) (25).
Results and Discussion
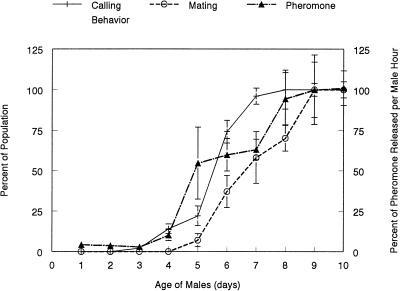
To maximize reproductive potential, it is of distinct advantage for polygamous males to mate early in life and possess characters imparting a competitive edge over virgins in subsequent mating encounters (27–29). Characters imparting male reproductive advantages are important for tropical lekking species of Tephritid flies, because females, who have a prolonged refractory period after mating, select mates from groups of advertising males. In the Caribbean fruit fly, the expression of male and female sexual behavior is coordinated closely with reproductive maturity (7, 9). For example, we found that all males had mated by the 9th day after adult emergence and that, by this time, sex pheromone production by virgin males was maximal (Fig. 1). However, not all males become sexually mature at the same time, and, in our studies, some males engaged in calling behavior, released pheromone, and mated on the 5th day of adulthood. All males who mated on the 5th day mated again in the first 2 h of the mating period on both days 6 and 7. In contrast, only 29 of the 85 6-day-old virgin males mated on day 6. We obtained the same result when we compared directly the time it took for mated and virgin 6-day-old males to mate (Fig. 2a).
Figure 1.
Effect of age on calling behavior, amount of pheromone released, and mating (8) by virgin males of the Caribbean fruit fly. Data for calling behavior (12 groups of five males) and mating (17 replications of five males and females) represent the cumulative percentages of the total number of animals observed over 10 days. Data on pheromone release represent the mean of 10 replicates (five males per replicate) and are expressed as the total amount of all pheromone components released by a single male per hour relative to that released by virgin 10-day-old males.
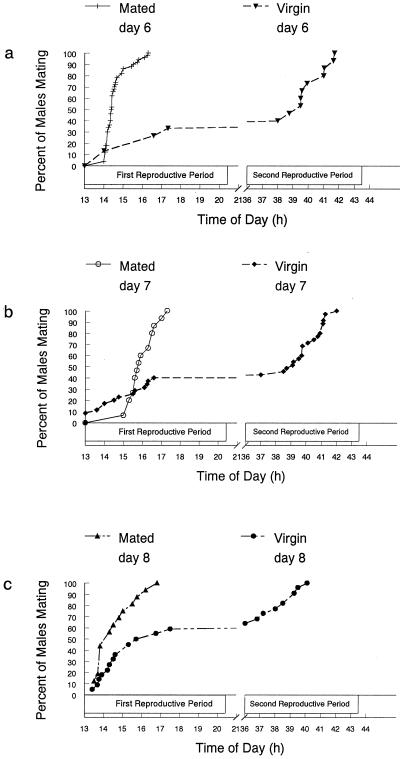
Figure 2.
Comparison of the times required for mating by mated or virgin individuals on the 6th, 7th, or 8th day after eclosion. (a) Comparison of time required for remating on the 6th day by males mated for the first time on day 5 (10 replicates of five males) with the time it took for virgin 6-day-old males to mate (3 replicates of five males). (b) Comparison of time required for remating on the 7th day by males mated for the first time on days 5 or 6 (three replicates of five males) with the time it took for virgin 7-day-old males to mate (four replicates of five males). (c) Comparison of time required for remating on the 8th day by males mated for the first time on days 6 or 7 (four replicates of five males) with the time it took for virgin 8-day-old males to mate (four replicates of five males). Each data point represents the cumulative percentage of the total population of a given treatment mated at that time.
We questioned whether the distinct differences in mating between mated and virgin 6-day-old males could be attributed to artificial selection for two reasons. First, the males that mated on day 5 were clearly sexually mature, whereas the majority of their 6-day-old virgin counterparts may not have been. Secondly, females prefer larger males (14–16), and males that mated on day 5 could have been larger than most of the 6-day-old virgins tested. To remove the selection pressure favoring males who mate early, we paired groups of 5-day-old virgin males, selected at random and regardless of size, with mature females. We did not select males who mated during the first reproductive period. Rather, we collected mating pairs over the next 2 days. Interestingly, all males had mated by the end of the second reproductive period. This result was unexpected, because when we caged 6-day-old virgin males and females together, only 35% mated in a single day (Fig. 1). However, the sexually mature females used could be less choosy and more sensitive to male sexual signals than immature 6-day-old females and thus selected males even though the males were not engaged in optimal sexual signaling. We caged these mated males with females, for a second time, on day 7 and compared the time it took for them to mate with the time it took for virgin 7-day-old males to mate. All mated males coupled with females for a second time within 3 h. Only 40% of the naïve virgin 7-day-old males mated on that day (Fig. 2b).
We repeated the experiment with males that mated, for the first time, on the 6th and 7th days and found they all mated again within 2.5 h on day 8. Only 60% of the 8-day-old virgins mated on that day (Fig. 2c). Results of these studies were strikingly similar and indicated conclusively that prior mating experience influenced positively the males' ability to attract, court, and mate successfully with females on subsequent days.
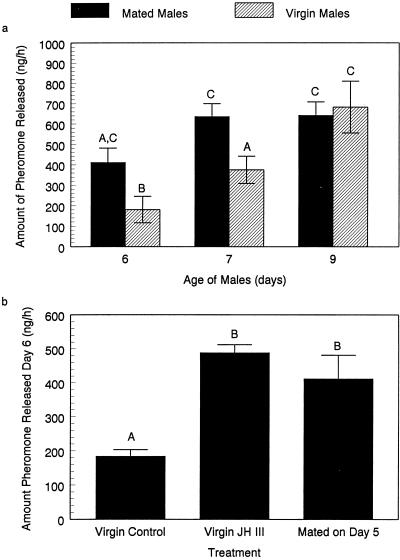
One possible reason that previously mated males required less time to initiate mating was that they became more sexually aggressive. However, we observed no such change in their behavior, nor had Sivinski (16) found evidence of such a change. Male-produced sex pheromones are key elements in sexual communication (7, 9, 18), and we suspected that young mated males might release more pheromone than virgins of the same age. When we determined the amount of pheromone released by virgin and mated males we discovered that 6- and 7-day-old mated males released at least twice as much pheromone as did similarly aged virgins (Fig. 3a). In fact, amounts of pheromone released by mated males were no different from amounts released by 9-day-old males, when all individuals tested were sexually mature. Therefore, we hypothesized endogenous differences existed between young mated and virgin males of the same age.
Figure 3.
Pheromone release by males of the Caribbean fruit fly. (a) Comparison of amount of volatile pheromone released by mated and virgin males 6, 7, and 9 days after emergence. Each data point represents the mean of five replicates and is expressed as the total amount of all pheromone components released by a single male per hour. Means labeled with the same letter are not different in a Fisher's Least Significant Difference test (P = 0.05). (b) Comparison of pheromone released by 6-day-old males treated with either 1 μl of acetone (five replicates) or 1 μl of acetone containing 5 μg of synthetic JH III (five replicates) to that released by 6-day-old males 1 day after mating (five replicates). Means labeled with the different letters were significantly different in a Fisher's Least Significant Difference test (P = 0.05).
JH coordinates development of sexual signaling with gamete maturity in many species of insects (1–6, 30, 31). However, no direct evidence that JH affects mate location, pheromone production, or courtship in flies has been presented (32). Nonetheless, topically applied JH has physiological effects on some adult flies. In Drosophila melanogaster males, JH stimulates replenishment of accessory gland secretions after mating (20), and JH has been shown to have a positive influence on insemination rates in both sexes of Phormia regina (33). We wished to know whether JH affected sex pheromone production by adult male Caribbean fruit flies. Therefore, we conducted hormone supplement therapy experiments and applied methoprene to 5-day-old males. Virgin males treated with JH on the 5th day released three times more pheromone on day 6 than did the control group (Fig. 3b). These data showed that juvenoid supplement therapy induced virgin males to release as much pheromone as did mated males. However, they did not indicate whether JH levels were higher in young mated males than in virgins.
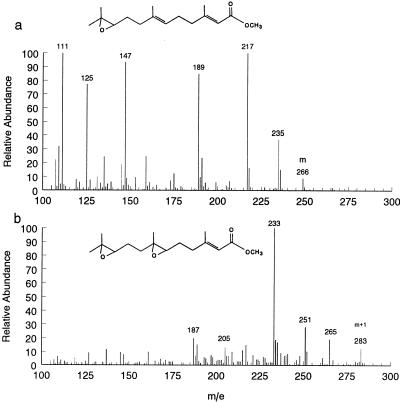
JH has not been identified from any Tephritid fruit fly. Indeed, the most abundant and, in some flies, only homologue of JH, methyl-6,7;10,11-bisepoxy-3,7,11-trimethyl-(2E)-dodecenoate (JH IIIB), synthesized by endocrine glands maintained in tissue culture (32), has never been identified from circulating hemolymph. When we chemically analyzed extracts of hemolymph from 12-day-old virgin males, however, we identified JH IIIB as well as its monoepoxide homology (JH III; Fig. 4) in a 2.35(± 0.33; n = 5):1 ratio. No other JH homologues were detected. However, we could not detect either JH IIIB or JH III from hemolymph extracts of 1-day-old males. More importantly, extracts of hemolymph from 7-day-old mated males contained an average of 2.4 pg/μl (± 0.12; n = 4) of JH IIIB plus JH III. This amount was significantly more than that present in hemolymph from virgins of the same age (0.82 ± 0.10 pg/μl; n = 4; t = 9.331).
Figure 4.
Chemical ionization (isobutane) mass spectra of naturally produced JH III (a) and JH IIIB (b) obtained from analysis of the hemolymph extract obtained from 7-day-old mated males. Diagnostic ions (see Methods) used for quantification and identification are indicated, and the structure of each homologue is shown above the spectrum.
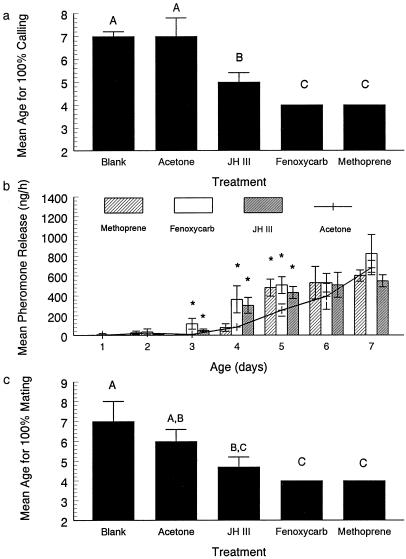
We hypothesized that JH is a pivotal hormone responsible for coordination of development of sexual signaling and reproductive competence, because circulating levels of JH were higher in mated males than in virgins and because application of JH to 5-day-old virgins increased pheromone production significantly. We reasoned that application of juvenoids to newly eclosed males might induce precocious expression of sexual signaling and reproduction. When we applied the juvenoids to males on the day of eclosion and monitored calling behavior and mating, we found that treated males engaged in calling behavior and mated at significantly earlier ages than control treated flies (Fig. 5 a and c). Additionally, males treated with methoprene and fenoxycarb released significantly more pheromone per hour on days 3–5, and JH-treated males released more pheromone than did controls on day 5 (Fig. 5b). Thus, we conclude that JH coordinates all aspects of sexual signaling with reproductive maturity.
Figure 5.
Effects of hormone supplement therapy on calling behavior, pheromone release, and mating by males of the Caribbean Fruit fly. Males were either not treated (calling and mating studies) or treated with JH III, methoprene, fenoxycarb, or acetone alone on the day of eclosion. (a) Mean age (± SEM) at which 100% of treated and untreated males engaged in calling behavior. Means labeled with the same letter are not different in a Fisher's Least Significant Difference test (P = 0.5). Six replicates of 10 males per treatment. (b) Mean amounts of pheromone released per hour by males during the reproductive period on days 1–7 after eclosion (six replicates per treatment). Mean amounts (± SEM) from animals treated with juvenoids labeled with an asterisk (*) were significantly different from amounts released by males treated only with acetone on that day when analyzed with a Fisher's Least Significant Difference test (P = 0.05). All treated and control males released the same amounts of pheromone after the 7th day. (c) Mean age (± SEM) at which 100% of treated and untreated males mated. Means labeled with the same letter are not different in a Fisher's Least Significant Difference test (P = 0.5). Four replicates of 10 males for acetone, JH III, methoprene, and fenoxycarb; 16 replicates for untreated males.
There is an obvious advantage to having an endocrinal coordination of reproductive maturity and sexual signaling. However, it is less obvious why a mechanism would evolve that gives previously mated young males an advantage over virgins for mating opportunities. Indeed, our data indicate that, after mating, young males produce as much pheromone and are as successful in mating as older, well established males. Thus, young mated males in leks can challenge directly older, well established dominant males for their position in the mating hierarchy. Presumably, the long adult prereproductive period in this species is used, at least in part, to acquire resources to be used in lekking behavior later in life (33). If few females are available early in life, it would be more efficient for a male to wait until greater numbers of females arrive before engaging in expensive sexual signaling and territory defense. Alternatively, if large numbers of receptive females are available then a male may use an early sexual encounter as an indication that it is a propitious time to begin full signaling, even if he has not completed sequestering resources. A proximate means of accelerating the development of sexual behavior in young mated males is production of more JH than that produced by similarly aged virgins.
The finding that JH is a pivotal hormone regulating all aspects of reproductive competence has considerable economic significance. Indeed, results have shown that treatment of pharate adults, just before adult eclosion, accelerates reproductive development as well as when males are treated on the day of eclosion (data not shown). Tephritid fruit flies, including the Caribbean, Mexican, and Mediterranean fruit flies, are quarantine pests that pose very serious threats to citrus and vegetable production in subtropical and tropical areas of the world (34). The most environmentally safe method for control of Tephritid fruit flies is the SIT in which males, sterilized by irradiation during rearing, are released into the field and mate with females (35). As females rarely mate more than once, mating with sterile males results in population decline. Consequently, efficacy of SIT requires that sterile males compete equally or preferably better than wild males for females (35, 36). However, significant mortality occurs before reaching sexual maturity if males are released in the field as pupae or newly eclosed adults (37), and if the millions of males used for individual releases are held in rearing until they are sexually mature, holding costs become very expensive. Implementing juvenoid hormone supplement therapy into mass rearing of sterile males could reduce the impact of these problems significantly, because treated males would be sexually mature far sooner than those currently produced. Therefore, efficacy of SIT releases could be enhanced significantly.
Acknowledgments
We thank N. T. Davis (University of Arizona), J. R. Miller (Michigan State University), R. J. Prokopy (University of Massachusetts), A. K. Raina (Agriculture Research Service), W. L. Roelofs (Cornell University), and J. Sivinski, J. H. Tumlinson, and H. Oberlander (all of U.S. Department of Agriculture–Agricultural Research Service) for helpful reviews of the manuscript. Partial support was provided by the International Atomic Energy Agency.
Abbreviations
- JH
juvenile hormone
- SIT
sterile insect technique
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.060034397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.060034397
References
- 1.Schal C, Gu X, Burns E L, Blomquist G J. Arch Insect Biochem Physiol. 1994;25:375–391. doi: 10.1002/arch.940250411. [DOI] [PubMed] [Google Scholar]
- 2.Cusson M, Tobe S S, McNeil J N. Arch Insect Biochem Physiol. 1994;25:329–345. [Google Scholar]
- 3.Gadenne C. J Insect Physiol. 1993;29:25–29. [Google Scholar]
- 4.Cusson M, McNeil J N. Science. 1989;243:210–212. doi: 10.1126/science.243.4888.210. [DOI] [PubMed] [Google Scholar]
- 5.Cusson M, McNeil J N, Tobe S S. J Insect Physiol. 1990;36:139–146. [Google Scholar]
- 6.Picimbon J F, Becard J M, Sreng L, Clement J L, Gadenne C. J Insect Physiol. 1994;41:377–382. [Google Scholar]
- 7.Nation J L. Ann Entomol Soc Am. 1972;65:1364–1367. [Google Scholar]
- 8.Nation J L. Ann Entomol Soc Am. 1974;67:731–734. [Google Scholar]
- 9.Nation J L. J Chem Ecol. 1990;16:553–572. doi: 10.1007/BF01021786. [DOI] [PubMed] [Google Scholar]
- 10.Prokopy R J. In: Proceedings, Symposium on Fruit Fly Problems. Koyama J, editor. Kyoto: XVI International Congress Entomology; 1980. pp. 37–46. [Google Scholar]
- 11.Burke T. Florida Entomol. 1981;64:30–43. [Google Scholar]
- 12.Sivinski J, Burk T. In: World Crop Pests, Fruit Flies, Their Biology, Natural Enemies and Control. Robinson A S, Hooper G, editors. Amsterdam: Elsevier; 1989. pp. 343–351. [Google Scholar]
- 13.Bradbury J W, Gibson R, M. In: Mate Choice. Bateson P, editor. Cambridge, U.K.: Cambridge Univ. Press; 1983. pp. 19–80. [Google Scholar]
- 14.Burk T, Webb J C. Ann Entomol Soc Am. 1983;76:678–682. [Google Scholar]
- 15.Webb J C, Sivinski J, Litzkow C. Environ Entomol. 1984;13:650–656. [Google Scholar]
- 16.Sivinski J. Florida Entomol. 1984;67:126–130. [Google Scholar]
- 17.Hooper G H S. In: World Crop Pests, Fruit Flies, Their Biology, Natural Enemies and Control. Robinson A S, Hooper G, editors. Amsterdam: Elsevier; 1989. pp. 153–164. [Google Scholar]
- 18.Heath R R, Manukian A, Epsky N D, Sivinski J, Calkins C O, Landolt P J. J Chem Ecol. 1993;19:2395–2410. doi: 10.1007/BF00979673. [DOI] [PubMed] [Google Scholar]
- 19.Teal P E A, Gomez-Simuta Y, Meredith J A. Arch Insect Biochem Physiol. 1999;42:225–232. doi: 10.1002/(SICI)1520-6327(199912)42:4<225::AID-ARCH1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Herdon L A, Chapman T, Kalb J M, Lewin S, Partridge L, Wolfner W F. J Insect Physiol. 1997;43:1117–1123. doi: 10.1016/s0022-1910(97)00062-0. [DOI] [PubMed] [Google Scholar]
- 21.Wicker C, Jallon J M. J Insect Physiol. 1995;41:65–70. [Google Scholar]
- 22.Borovsky D, Thomas B R, Carlson D A, Whisenton L R, Fuchs M S. Arch Insect Biochem Physiol. 1995;2:75–90. [Google Scholar]
- 23.Yin C-M, Zou B X, Jaing M G, Li M F, Qin W H, Potter T L, Stoffolano J G. J Insect Physiol. 1995;41:473–479. [Google Scholar]
- 24.Bergot B J, Ratcliff M A, Schooley D A. J Chromatogr. 1981;204:231–244. [Google Scholar]
- 25.Richard D S, Applebaum S W, Sliter T J, Baker F C, Schooley D A, Rueter C C, Henrich V C, Gilbert L I. Proc Natl Acad Sci USA. 1989;86:1421–1425. doi: 10.1073/pnas.86.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teal P E A, Proveaux A T, Heath R R. Anal Biochem. 2000;277:206–213. doi: 10.1006/abio.1999.4377. [DOI] [PubMed] [Google Scholar]
- 27.Lloyd J E. Florida Entomol. 1979;62:17–34. [Google Scholar]
- 28.Parker G A. Annu Rev Entomol. 1978;23:173–196. [Google Scholar]
- 29.Borgia G. In: Sexual Selection and Reproductive Competition in Insects. Blum M S, Blum N A, editors. New York: Academic; 1979. pp. 19–80. [Google Scholar]
- 30.Blomquist G J, Dillwith J W. In: Endocrinology of Insects. Downer R G, Lufer H, editors. New York: Liss; 1983. pp. 527–542. [Google Scholar]
- 31.Vanderwel D, Oehlschlager A C. In: Pheromone Biochemistry. Prestwich G D, Blomquist G J, editors. Orlando, FL: Academic; 1987. pp. 175–215. [Google Scholar]
- 32.Yin C M, Stoffolano J G. Arch Insect Biochem Physiol. 1997;35:513–537. [Google Scholar]
- 33.Yin C M, Qin W H, Stoffolano J G. J Insect Physiol. 1999;45:815–822. doi: 10.1016/s0022-1910(99)00047-5. [DOI] [PubMed] [Google Scholar]
- 34.Hendrichs J. In: Fruit Fly Pests, A World Assessment of Their Biology and Management. McPheron B A, Steck G J, editors. Delray Beach, FL: St. Lucie; 1996. pp. 5–6. [Google Scholar]
- 35.Gilmore J E. In: World Crop Pests, Fruit Flies, Their Biology, Natural Enemies and Control. Robinson A S, Hooper G, editors. Amsterdam: Elsevier; 1989. pp. 353–363. [Google Scholar]
- 36.Knippling E F. The Basic Principals of Insect Population Suppression and Management. Washington, DC: U.S. Dept. of Agric.; 1979. , USDA Agriculture Handbook 512. [Google Scholar]
- 37.Burditt A K, Lopez D, Steiner L F, Von Windeguth D L. Sterility Principles for Insect Control. Vienna: Int. At. Energy Agency; 1974. pp. 93–101. [Google Scholar]