Abstract
During kidney development and in response to inductive signals, the metanephric mesenchyme aggregates, becomes polarized, and generates much of the epithelia of the nephron. As such, the metanephric mesenchyme is a renal progenitor cell population that must be replenished as epithelial derivatives are continuously generated. The molecular mechanisms that maintain the undifferentiated state of the metanephric mesenchymal precursor cells have not yet been identified. In this paper, we report that functional inactivation of the homeobox gene Six2 results in premature and ectopic differentiation of mesenchymal cells into epithelia and depletion of the progenitor cell population within the metanephric mesenchyme. Failure to renew the mesenchymal cells results in severe renal hypoplasia. Gain of Six2 function in cortical metanephric mesenchymal cells was sufficient to prevent their epithelial differentiation in an organ culture assay. We propose that in the developing kidney, Six2 activity is required for maintaining the mesenchymal progenitor population in an undifferentiated state by opposing the inductive signals emanating from the ureteric bud.
Keywords: homeobox, kidney, mouse, nephrogenesis, Six2
Introduction
The development of the mammalian kidney is a paradigm for the reciprocal inductive interactions that control branching morphogenesis and the transition of mesenchyme into epithelia. In mice, the metanephric kidney begins to develop at embryonic day (E) 10.5. Signals from the metanephric blastema, a population of mesenchymal cells in the caudal portion of the intermediate mesoderm, induce the ureteric bud (UB) to evaginate from the Wolffian duct (Gruenwald, 1943; Grobstein, 1955). At around E11.0, the ingrowth of the UB into the metanephric blastema induces the metanephric mesenchyme (MM) at the bud tips to condense around the UB tips. On the ventral side of the UB tips, the condensed cells cluster into pretubular aggregates. Subsequently, these aggregates undergo a mesenchymal–epithelial transition that leads to the formation of epithelial vesicles, which sequentially differentiate into comma-shaped bodies, S-shaped bodies, and eventually functional nephrons (Saxen and Sariola, 1987; Dressler, 2002; Vainio and Lin, 2002; Vize et al, 2003).
As the MM cells condense and differentiate, they also reciprocally induce the UB to continue growing toward the periphery of the kidney and branching repeatedly to form the collecting duct system. The tips of each new UB branch go on to induce additional mesenchymal cells and generate new nephrons. Thus, the pattern of the developing kidney is established along a radial axis, with the oldest nephrons located near the medulla and distributed among interstitial stromal cells, and the youngest nephrons located in the peripheral nephrogenic zone (Saxen, 1987). This reciprocal inductive interaction between mesenchyme and UB epithelia is crucial for kidney development and must be maintained during kidney organogenesis.
Mesenchymal cell progenitors generate the different epithelial cell types of the nephron in response to induction (Herzlinger et al, 1992; Nishinakamura and Osafune, 2006). These mesenchymal stem/progenitor cells must be constantly renewed so that the kidney can continue to grow and induce additional generations of nephrons. Signals from the MM are likely necessary to oppose nephrogenesis and, therefore, maintain an available pool of undifferentiated progenitors within the peripheral nephrogenic zone. However, the identity of the genes and mechanisms required to maintain this mesenchymal stem/progenitor cell population in an undifferentiated condition is not yet known.
Here we investigated the role of the homeobox gene Six2 in kidney nephrogenesis. Our data indicate that Six2 is part of a genetic mechanism that opposes epithelial polarization and regulates renal epithelial precursor cell renewal by maintaining the undifferentiated state of MM progenitors.
Results and discussion
Six2 is expressed in the metanephric mesenchyme
We have previously shown that Six2 is expressed throughout the development of the excretory system, including the nephrogenic cords and metanephroi (Oliver et al, 1995). At around E10.0, Six2 expression was detected in the metanephric blastema before UB invasion (Oliver et al, 1995). Half a day later, Six2 expression was localized in the MM surrounding the UB (Figure 1A). At E11.5, the expression was detected in the induced MM surrounding the UB epithelium (Figure 1B). High levels of Six2 were observed on the dorsal side of the UB, and lower levels were found on the ventral side near the ureteric stalk where the pretubular aggregates will form (arrows in Figure 1B). Later during development (E14.5), Six2 mRNA (Figure 1C and Supplementary Figure S2B) and Six2 protein (arrowhead, Figures 1D and 5O) remained in the Pax2-expressing condensing mesenchyme on the dorsal side of the UB tips (MM progenitor pool; Supplementary Figure S2A) but were downregulated in cells that, following aggregation and subsequent mesenchymal-to-epithelial transition, formed Cadherin-6-, Pax2-, and Wnt4-expressing comma bodies (arrows in Figures 1D and 5O, and Supplementary Figure S2A and C). This expression pattern suggested that, similar to Six1 (Xu et al, 2003), Six2 controls some aspects of early MM development. To directly address this question, we functionally inactivated Six2 in mice by deleting most of the Six2-coding sequence, including the two DNA-binding domains, the homeobox domain and the Six domain (Supplementary Figure S1).
Figure 1.
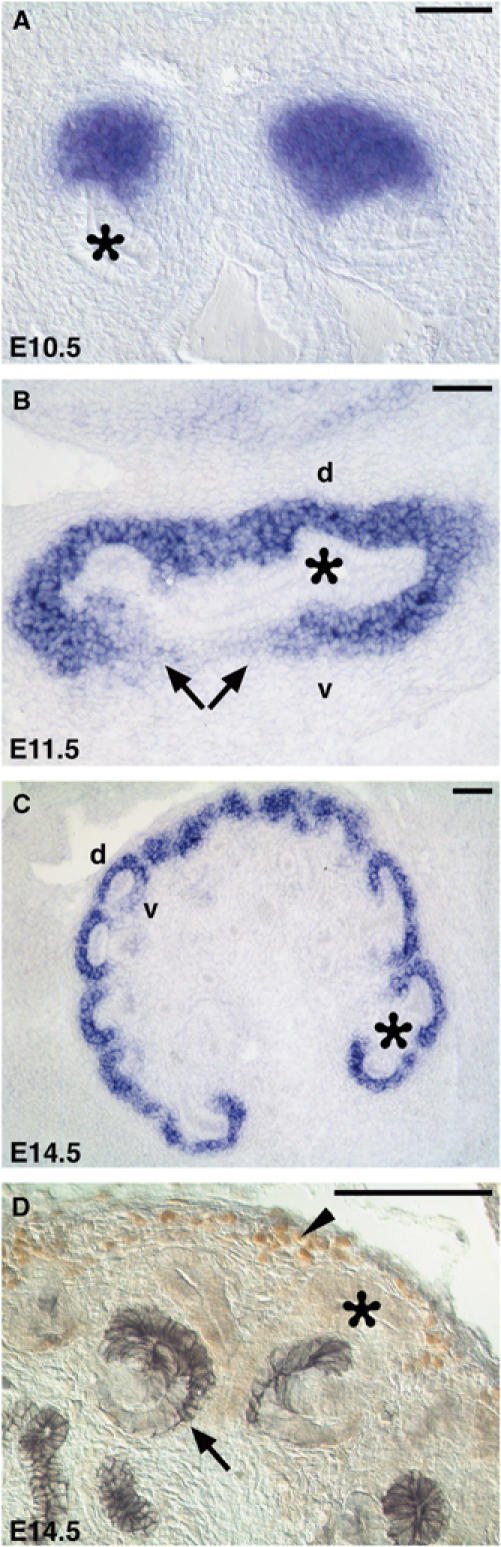
Six2 expression during renal development. (A) At E10.5, Six2 (blue) was expressed in the metanephric blastema, which signals the UB (asterisk) to evaginate from the Wolffian duct. (B) Six2 is expressed at high levels in the dorsal MM (d) at E11.5 and is downregulated where pretubular aggregates will form (arrows) on the ventral side (v) of the UB. (C) At E14.5, Six2 expression persists in the peripheral mesenchyme of the renal cortex. (D) Six2 protein (brown; arrowhead) is localized in the nephrogenic zone but is absent from the epithelial derivatives of the MM, which express Cadherin-6 (gray; arrow). Scale bar, 100 μm.
Six2 activity is required for the normal development of the mammalian kidney
Six2-heterozygous mice did not exhibit any obvious abnormalities. However, Six2-nullizygous mice died soon after birth. An initial morphologic characterization of the mutant mice indicated major defects in the development of the kidney. Isolation of the E14.5 urogenital tract showed that the Six2−/− kidney was approximately 50% smaller than that of the wild-type littermate (Figure 2A); at E16.5, the reduction was even more dramatic at approximately 65% (Figure 2B). These results suggested that Six2 activity is required for the normal development of the mammalian kidney. No obvious alterations were observed in the genital tract or in the mesonephroi.
Figure 2.
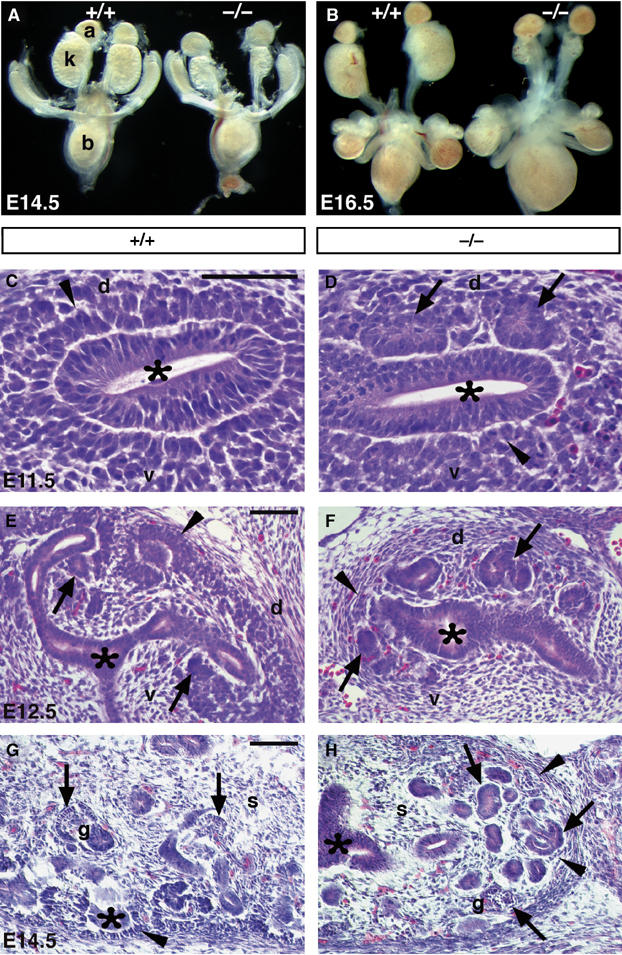
Six2 is crucial for kidney development. Analysis of urogenital tracts dissected at E14.5 (A) and E16.5 (B) revealed that Six2-null kidneys (k) were approximately 50% smaller than those of wild-type (+/+) littermates at E14.5 and 65% smaller at E16.5. The adrenal glands (a) and bladder (b) appeared normal. (C–H) Hematoxylin and eosin staining showed that at E11.5 in the wild-type kidney (C) the UB (asterisk) has induced the MM (arrowhead) to condense, but no pretubular aggregates or epithelia were present at this stage. (D) In Six2-null littermates, the MM has formed ectopic and premature epithelial vesicles (arrows) on the dorsal (d) side of the UB. (E) At E12.5, the wild-type MM on the ventral side of the UB tips has begun to transform into the epithelia of the renal vesicle (arrows). Condensing mesenchyme (arrowhead) was also detected on the dorsal side of the UB tips. (F) In Six2−/− littermates, the MM on the ventral and dorsal sides of the UB transitioned from mesenchyme to epithelia and formed precocious and ectopic renal vesicles (arrows). Note the lack of condensing MM (arrowhead) at the bud tips. (G) At E14.5, the wild-type kidney exhibited its typical uninduced and condensing mesenchyme (arrowhead) and growing branch tips (asterisk) in the cortex, maturing glomeruli (g; arrow) in the medulla, and interstitial stromal cells (s) dispersed throughout. (H) The Six2-null kidney revealed an absence of condensing mesenchyme in the cortex (arrowheads), unorganized epithelial structures (arrows) throughout the kidney including a few glomerular structures (g), and a normal distribution of stromal cells (s). Scale bar, 100 μm.
Histologic analysis of the Six2-null kidneys revealed some intriguing morphologic defects. At E11.5, the wild-type UB is surrounded by condensing mesenchyme (arrowhead, Figure 2C); however, formation of epithelial vesicles is not yet observed at this stage. Instead, the E11.5 Six2−/− kidney displayed ectopic and premature mesenchymal–epithelial transition that lead to the formation of precocious epithelial renal vesicles surrounding the UB (arrows, Figure 2D). At E12.5, the wild-type UB had begun its second round of branching, and the pretubular aggregates on the ventral side of the ureteric branches had undergone mesenchymal–epithelial transition to form renal vesicles (arrows, Figure 2E). In Six2−/− littermates, the UB had not branched beyond the initial ‘T' stage of development (asterisk, Figure 2F), the ectopic renal vesicles surrounding the UB continued to develop further (arrows), and absence of condensing MM was apparent (arrowhead). At E14.5, the wild-type kidney exhibited condensing MM (arrowhead, Figure 2G) and growing UB branches (asterisk) in the cortical nephrogenic zone and interstitial stromal cells dispersed throughout the kidney. In contrast, the Six2-null kidney exhibited abnormal, unorganized masses of nephric epithelia (arrows, Figure 2H) and lacked condensing mesenchyme in the peripheral nephrogenic zone (arrowheads) and UB branches throughout the kidney (asterisk). The interstitial stromal cell population appeared to be normally distributed among the epithelial structures of the Six2-null kidney (Figure 2H).
Six2-null kidneys exhibit premature and ectopic renal vesicles
The presence of precocious ectopic supernumerary renal vesicles in the Six2-null kidney is a rather unique and intriguing phenotype that has not been previously described. In an effort to confirm and better characterize these morphologic alterations, Six2-null kidneys were dissected at E11.5, cultured for 24, 48, and 96 h, and then stained with the following antibodies: anti-pan-cytokeratin to label the UB (Fleming and Symes, 1987), anti-E-cadherin to label the UB and distal tubules (Cho et al, 1998), anti-laminin-A to label all the polarized epithelial structures (Ekblom et al, 1980, 1991), anti-Cadherin-6 to label proximal tubule precursors (Cho et al, 1998), and anti-Wt1 to label podocyte precursors/glomeruli (Buckler et al, 1991; Armstrong et al, 1993; Miner and Li, 2000).
In wild-type explants maintained in culture for 24 h, new epithelial renal vesicles formed on the ventral side of the UB tips and were beginning to express Cadherin-6 in a few cells (arrow, Figure 3A). Instead, Six2-null kidney explants displayed an abundance of Cadherin-6 expression in the precocious renal vesicles that formed on the ventral and dorsal sides of the UB (arrows, Figure 3B). This result confirmed that in the Six2-mutant kidney, developing nephrons form and differentiate prematurely. As expected, in wild-type explants maintained in culture for 48 h, laminin-A-expressing epithelial renal vesicles continued to differentiate into comma- and S-shaped bodies, only on the ventral side of the UB tips (arrows, Figure 3C). In the Six2-null kidney, the UB showed very limited branching (asterisk, Figure 3D) and was completely surrounded on both the ventral and dorsal sides by laminin-A-expressing epithelial vesicles (arrows). The expression of laminin-A and the absence of pan-cytokeratin indicated that the vesicles were polarized epithelia derived from the mesenchyme that formed ectopically on the dorsal side of the UB branch tips.
Figure 3.
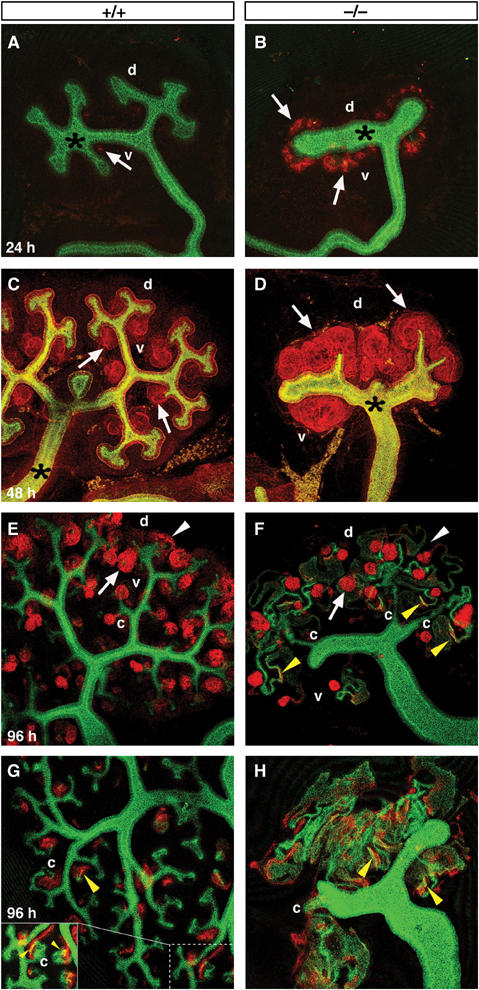
Six2-null kidneys exhibit precocious nephrogenesis. (A, B) Wild-type and Six2-null kidney explants maintained in culture and costained with E-cadherin to label the UB (green; asterisk) and Cadherin-6 to label developing nephrons (red; arrows). After 24 h culture, few cells expressed Cadherin-6 in the wild-type renal vesicles (A); instead, more advanced epithelial structures (arrows) on the dorsal and ventral sides of the UB tips were seen in the Six2−/− explant (B). (C, D) After 48 h, immunohistochemistry was performed using anti-pan-cytokeratin (green) to label the UB (asterisk) and anti-laminin-A (red) to label epithelial structures. (C) Normal developing comma and S-shaped bodies (arrows) were seen on the ventral sides (v) of the bud tips of the wild-type kidney. (D) Numerous ectopic renal epithelial structures (arrows) and decreased branching of the UB were identified in the Six2-null explant. (E, F) Explants cultured for 96 h were labeled with Wt1 (red), Cadherin-6 (red), and E-cadherin (green). (E) A normal reserve of mesenchymal progenitors (arrowhead) at the tips of the UB (green) and normal developing glomeruli (arrow) throughout the kidney were observed in the wild-type explant. (F) Six2−/− explants lacked MM in the periphery (white arrowhead) but formed glomeruli (arrow). (G, H) Explants cultured for 96 h were labeled with only Cadherin-6 (red) and E-cadherin (green) to identify any overlap in their expression at the boundary of the proximal and distal tubules (yellow arrowheads). The Six2-null explant (H) displayed abnormally extensive coexpression of these markers in mispatterned masses of developing tubules and rare connections of the tubules to the UB (c).
To determine whether Six2-null kidneys produce morphologically normal nephrons, we maintained explants in culture for 96 h. As indicated by the nuclear labeling of Wt1, the Six2-null kidney formed glomeruli (arrows, Figure 3F; nephron quantification included in Supplementary Figure S3A), but in contrast to the wild-type kidney, it lacked a reserve of MM in the cortex (white arrowhead, Figure 3F). In the wild-type kidney, Cadherin-6 and E-cadherin were coexpressed at the boundary of the proximal and distal tubules (yellow arrowheads in Figure 3G); in the Six2-null kidney, their expression domains were abnormally expanded and overlapped to a much greater extent (yellow arrowheads, Figure 3F and H). The expression of additional markers of tubule segments including Slc34a1 (Collins and Ghishan, 1994; Murer et al, 2004), Slc12a1 (Gamba et al, 1994), Slc12a3 (Gamba et al, 1994; Hebert et al, 2004), and Calbindin-3 (Shamley et al, 1992) was also detected in the E15.5 Six2-mutant kidney (Supplementary Figure S3).
Wild-type nephrons normally contain a single glomerular structure connected to the UB via the distal tubule (‘c' in Figure 3E and G). In contrast, despite the presence of numerous glomeruli, only a few (1–3) connections between the nephric structures and the UB were identified in the Six2-null explants (‘c' in Figure 3F and H). Together, these results indicate that the Six2-null kidney forms glomeruli and expresses markers for distinct tubule segments; however, the lack of Six2 activity leads to defects in patterning and regionalization of nephric tubules and defects in the connections of nephrons to the UB. These defects are likely secondary to the lack of UB branching.
The metanephric kidney forms through reciprocal interactions between the MM and the UB epithelium, and these interactions lead to the formation of functional nephrons (Grobstein, 1955). During this process, a number of well-characterized genes, including Wt1, Eya1, Bmp7, Pax2, Six1, Lim1, Sall1, Wnt4, Sfrp2, Wnt11, Gdnf, Ret, and Foxd1, are essential for normal kidney morphogenesis (Vainio and Lin, 2002; Yu et al, 2004). To identify the cause of the phenotypic alterations observed in Six2-null kidneys, we analyzed the expression of these genes at different developmental stages.
The Wilms' tumor suppressor Wt1 encodes a zinc-finger transcription factor necessary for UB outgrowth and survival of the metanephric blastema (Kreidberg et al, 1993; Donovan et al, 1999; Moore et al, 1999). Wt1 is expressed weakly in the metanephric blastema at E10.5 (arrowhead, Figure 4A) but as development progresses, its expression increases in MM (arrowhead, Figure 4G), aggregates, comma bodies, and S-shaped bodies and persists in podoctye precursors and epithelia of the Bowman's capsule (arrows, Figure 4M). The eyes absent 1 (Eya1) gene encodes a protein tyrosine phosphatase that acts as a transcriptional coactivator (Li et al, 2003), is expressed in the MM (Figure 4C, I, and O) (Kalatzis et al, 1998), and is required for UB invasion of the MM (Xu et al, 1999). In Eya1-null embryos, the UB fails to invade the kidney mesenchyme, and the kidneys do not develop (Xu et al, 1999). Bone morphogenetic protein-7 (Bmp7), a member of the TGF-β family of secreted growth factors, is expressed initially in the UB, the MM, and in the early tubules derived from the mesenchyme (Figure 4E, K, and Q) (Dudley et al, 1995; Lyons et al, 1995; Dudley and Robertson, 1997). In Bmp7-null embryos, the condensed MM cells are gradually lost after E12.5, which leads to hypoplastic kidneys with few glomeruli at birth. This result suggests that Bmp7 acts as a survival factor for the nephrogenic progenitor pool during kidney development (Dudley et al, 1995; Luo et al, 1995).
Figure 4.
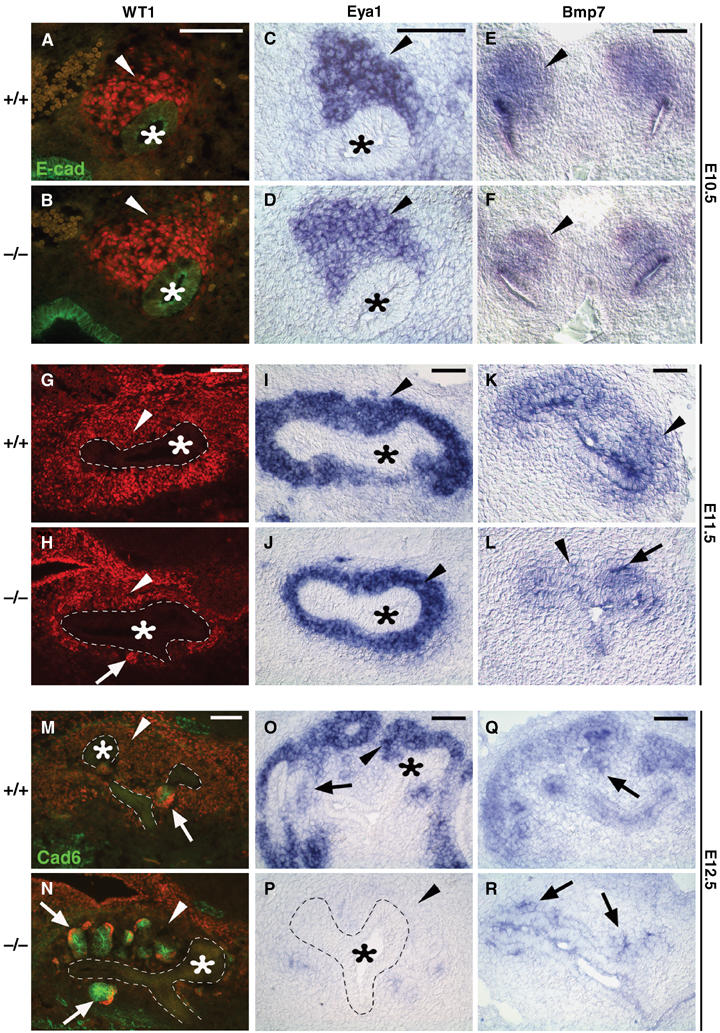
Molecular characterization of the Six2-null kidney. At E10.5, no obvious differences in the levels of expression of Wt1 (A, B; red), Eya1 (C, D), or Bmp7 (E, F) were detected in the MM (arrowheads) localized at the tip of the UB (asterisk) in Six2-null kidneys. No changes in their expression levels were also detected at E11.5 (G–L), although the size of the MM (arrowheads) surrounding the UB (asterisk and outlined) was reduced and premature and ectopic renal vesicles were already present (arrows) in Six2−/−. At E12.5, epithelial vesicles (arrows) with MM progenitors residing in the cortex (arrowheads) are seen in control kidneys (M, O, Q). Six2-null kidney (N) displayed Wt1 (red)- and Cadherin-6 (green)-expressing ectopic renal vesicles (arrows) on the dorsal and ventral sides of the UB and an absence of MM in the cortex (arrowhead). (P) In agreement with the depletion of the MM surrounding the UB (asterisk; outlined with dashes), expression of Eya1 was lost in E12.5 Six2-null kidneys. (R) Bmp7 was expressed at normal levels in the ectopic renal vesicles and UB at this stage. Scale bar, 100 μm.
Expression of all the above-mentioned molecular markers was normal in the E10.5 Six2-null blastema (Figure 4A–F). In the wild-type kidney, epithelial vesicles were first detected exclusively on the ventral side of the branched UBs at around E12.5 (Figure 4M). In agreement with the earliest morphological indications of developmental defects in Six2-null kidney, premature and ectopic Wt1-expressing (arrow, Figure 4H) and Bmp7-expressing (Figure 4L) epithelial vesicles were detected at around E11.5 in the mutant kidney. In addition, we observed a reduction in the size of the mesenchymal population that expresses Wt1, Eya1, and Bmp7 (arrowheads, Figure 4H, J, and L). At E12.5, the presence of ectopic Wt1-, Cadherin-6-, and Bmp7-expressing epithelial vesicles surrounding the whole UB of the Six2-null kidney (arrows, Figure 4N and R) most likely contributed to the abnormal depletion of the Eya1-expressing MM (Figure 4P) that is normally present in the wild-type kidney at this stage (Figure 4M, O, and Q).
Pax2, a paired-domain protein expressed in the UB, MM, and in epithelial derivatives of the MM (Figure 5A, G, and M), is a key player in kidney morphogenesis (Dressler et al, 1990; Dressler and Douglass, 1992). In Pax2-deficient embryos, the kidney and genital tract never develop (Torres et al, 1995; Favor et al, 1996). Sall1, the murine homologue of the Drosophila homeotic spalt gene, which is necessary for UB invasion of the MM (Nishinakamura et al, 2001; Nishinakamura and Takasato, 2005), is expressed in the mesenchymal population of the E10.5 blastema (Figure 5A) and in the MM, comma bodies, and faintly in a population of cells excluded from the expression domain of Pax2 at later stages (green arrowhead, Figure 5M). At around E10.5, before (data not shown) and after UB invasion (Figure 5B), expression of Pax2 and Sall1 was normal in the uninduced MM of Six2-null embryos. At E11.5, the expression level of Pax2 was also normal in the MM and UB of Six2−/− kidneys; however, the number of Pax2-positive MM cells surrounding the ingrown UB was reduced (Figure 5H). As indicated by the expression of Sall1 and Pax2, ectopic comma bodies were detected in the mutant kidney at E12.5 (arrow, Figure 5N). As already demonstrated by the markers used in Figure 4, depletion of MM precursors in the periphery of the Six2-null kidney was further supported by the absence of Pax2 expression; however, cells faintly expressing Sall1 remained in the cortical stromal population (Figure 5N).
Figure 5.
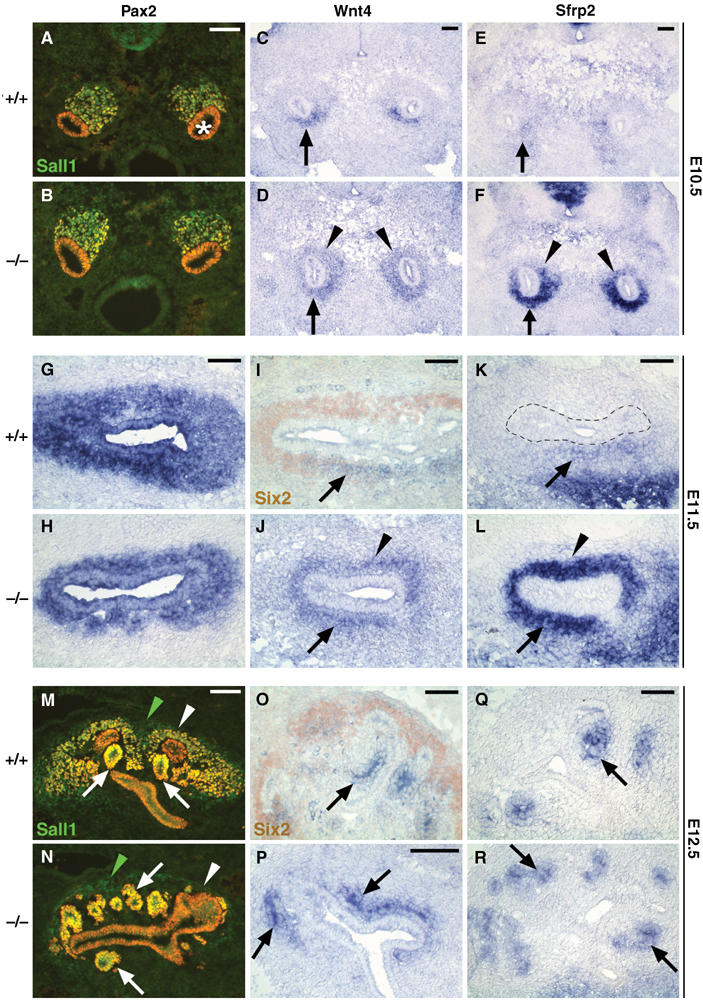
Expression of members of the Wnt pathway is ectopically and prematurely detected in Six2-null kidneys. (A, B) Pax2 (red) and Sall1 (green) were used to identify the E10.5 MM. At this stage, expression of both these markers was normal in Six2−/− (B). (C) Wnt4 expression is localized in the ventral-most mesenchyme (arrow) of an E10.5 wild-type kidney; instead, it was ectopically expanded into the dorsal-most mesenchyme (arrowheads) of the Six2-null littermate (D). (E) At this stage, normal low levels of Sfrp2 were present in the wild-type mesenchyme surrounding the Wolffian duct (arrow). Sfrp2 expression was also ectopically expanded into the dorsal side of the UB (arrowheads) and its expression was highly upregulated in the mutant littermate (F). (G, H) At E11.5, Pax2 expression in the UB and MM of the Six2-null kidney was similar to that of the wild type, although the size of the MM was reduced. (I) At this stage, the expression of Six2 (brown) and that of Wnt4 (blue) in the MM were complementary, that is, Six2 was expressed predominantly on the dorsal side of the UB and Wnt4 (arrow) only on the ventral side. (J) In the Six2−/− kidney, Wnt4 expression ectopically expanded to the dorsal MM (arrowhead). (K) At E11.5, Sfrp2 expression in the wild-type MM was similar to that of Wnt4; dotted line indicates UB epithelium. (L) In the Six2−/− kidney, the level of Sfrp2 was upregulated and ectopically expanded to the dorsal side (arrowhead). (M) At E12.5, Pax2 and Sall1 expression remained in the MM (white arrowhead) and was also detected in renal vesicles (arrows). Sall1 was also expressed by the stromal population (green arrowhead). (N) At E12.5, Pax2 expression in the Six2−/− kidney highlighted the presence of ectopic supernumerary renal vesicles (arrows) surrounding the UB. Pax2 expression was normal in the UB and renal vesicles, but mesenchymal cells (white arrowhead) expressing Pax2 were absent. However, Sall1-expressing stromal cells (green arrowhead) were still present in the Six2-null kidney at this stage. (P, R) Wnt4 and Sfrp2 were expressed in the ectopic renal vesicles (arrows) of the E12.5 Six2−/− kidney at levels comparable to those of wild-type (O, Q) kidney. Scale bar, 100 μm.
Wnt4 and Sfrp2 are ectopically expressed in the Six2-null metanephric mesenchyme
The Wnt signaling pathways regulate various key morphogenetic steps during embryogenesis, including the conversion of renal mesenchyme into epithelia. Wnt4 encodes an essential mesenchyme-derived signal required for the transition of pretubular aggregates into epithelial renal vesicles, and its activity is necessary for nephron formation (Stark et al, 1994; Vainio and Uusitalo, 2000). In Wnt4-null kidneys, the mesenchyme initially condenses around the UB outgrowth, but aggregates of cells that would normally form nephrons fail to epithelialize, and few renal vesicles form (Stark et al, 1994). In addition, Wnt4 is sufficient to trigger tubulogenesis in isolated MM (Kispert et al, 1998). However, Wnt4 is unlikely to be the primary UB-derived inductive signal, as it is expressed first in the mesenchymal aggregates on the ventral side of the UB tips (Stark et al, 1994).
It has been previously shown that Wnt activity is modulated by the secreted frizzled-related proteins (Sfrp). The stroma expresses Sfrp1, a factor that blocks epithelialization of the mesenchyme presumably by competing with the frizzled receptor for Wnt4 binding (Yoshino et al, 2001). The inhibitory activity of Sfrp1 is suppressed by Sfrp2, another member of this family whose expression pattern is similar to that of Wnt4 (Leimeister et al, 1998; Lescher et al, 1998). Thus, the ability of Sfrp2 to antagonize the suppressive function of Sfrp1 could promote epithelial polarization (Yoshino et al, 2001).
The precocious nephrogenesis observed in the Six2−/− kidney suggests that Wnt signaling may be altered in the mutant MM. At E10.5, Wnt4 expression was detected only in the ventral-most MM (that closest to the Wolffian duct) of the wild-type kidney (arrow, Figure 5C), whereas in Six2-null littermates, Wnt4 expression ectopically extended into the MM dorsal to the UB (arrowhead, Figure 5D). As shown in Figure 5E, Sfrp2 expression was detected at low levels in the wild-type MM surrounding the Wolffian duct. Similar to Wnt4, Sfrp2 expression was also ectopically expanded and strongly upregulated in the Six2−/− MM (Figure 5F). These results determined that as early as E10.5, the lack of Six2 activity promoted ectopic expansion of the mesenchymal territory permissive to inductive signals.
Interestingly, at around E11.5, the expression pattern of Six2 was mostly complementary to those of Wnt4 and Sfrp2 in the wild-type MM, with minimal overlap in their expression domains; Six2 expression was mostly localized on the dorsal side of the UB (brown, Figure 5I), whereas Wnt4 and Sfrp2 expression remained restricted to pretubular aggregates on the ventral side of the UB (arrows, Figure 5I and K). In the Six2−/− kidney, Wnt4 expression remained ectopically expanded into the dorsal MM (arrowhead, Figure 5J), colocalizing with strong ectopic Sfrp2 expression (Figure 5L). The presence of ectopic premature nephrogenesis and the depletion of the MM in the Six2−/− kidney were confirmed by analysis of the expression of these same signaling molecules at E12.5. At this stage in a normal kidney, Wnt4 and Sfrp2 were expressed in pretubular aggregates, comma bodies, and S-shaped bodies (arrows, Figure 5O and Q), all of which were derived from the Six2-expressing mesenchymal population. As shown previously in the E12.5 Six2-null kidney, the MM population has already been depleted owing to the premature formation of supernumerary ectopic renal vesicles dorsal and ventral to the UB tips (Figure 4N, P, and R), which continue to express these molecular markers (Figure 5P and R).
Recently, Wnt9b has also been reported to be expressed in the Wolffian duct and UB branches during kidney development (Qian et al, 2003). Furthermore, this gene acts upstream of Wnt4 in the induction of nephrogenesis and is essential for induction of MM and subsequent formation of epithelial renal tubules (Carroll et al, 2005). At E11.5 and E12.5, Wnt9b expression was normal in the Six2-null Wolffian duct and its derivative, the UB (Supplementary Figure S4). The finding that the expression of Wnt4 is ectopically expanded and that of Wnt9b remains normal in the Six2-mutant kidney suggests that Six2 is a MM regulator of the Wnt-promoted nephrogenesis cascade (downstream of Wnt9b but upstream of Wnt4). In this scenario, Six2 activity will be normally required to repress this inductive signal in the dorsal mesenchyme and thus suppress nephrogenesis in the mesenchymal progenitor pool.
Reciprocal inductive interactions are defective in Six2-null kidneys
Next, we analyzed whether in addition to the identified changes in the expression of some genes regulating MM differentiation, expression of other genes whose activities are necessary for UB branching was also affected in the Six2-null kidney. UB branching requires cooperative interactions between Wnt11, Gdnf, and Ret (Majumdar et al, 2003). Expression of Wnt11 is normally detected in the branching UB tips (Figure 6A, G, and M; Kispert et al, 1996). Functional inactivation of Wnt11 results in mild renal hypoplasia caused by subtle defects in branching morphogenesis of the UB (Majumdar et al, 2003). The glial-derived neurotrophic factor (Gdnf), which is expressed in the mesenchyme (Figure 6E, K, and Q), and its receptor tyrosine kinase Ret, which is expressed by the UB (Figure 6C, I, and O), are essential to promote UB outgrowth from the nephric duct (Pachnis et al, 1993; Durbec et al, 1996; Hellmich et al, 1996; Moore et al, 1996; Pichel et al, 1996; Sanchez et al, 1996; Trupp et al, 1996; Vega et al, 1996; Schuchardt et al, 1996; Sariola and Saarma, 1999). As the UB invaded the metanephric blastema at E10.5, Wnt11 expression in the UB tips of the Six2−/− kidney appeared normal (Figure 6B), confirming that initial UB induction was not affected. By E11.5, the invading UB has branched into a T-shaped structure with two expanding ampullae at both tips that express Wnt11 (Figure 6G). At this stage, Wnt11 was reduced in the UB tips of the Six2−/− kidney (Figure 6H). This reduced expression of Wnt11 was the earliest indication that the reciprocal interactions between the MM and UB were affected in the Six2-null kidney. At E12.5, Wnt11 expression was extinguished in the Six2-null UB (Figure 6N), a result suggesting that the inductive mechanisms for UB branching morphogenesis had been prematurely disrupted in the mutant kidney. The levels of expression of Gdnf and Ret were normal in Six2-null kidneys at E10.5, E11.5, and E12.5 (Figure 6D, F, J, L, P, and R); therefore, this downregulation in the expression of Wnt11 might be due to defects in some other alternative mechanism such as changes in the proteoglycan environment (Kispert et al, 1996).
Figure 6.
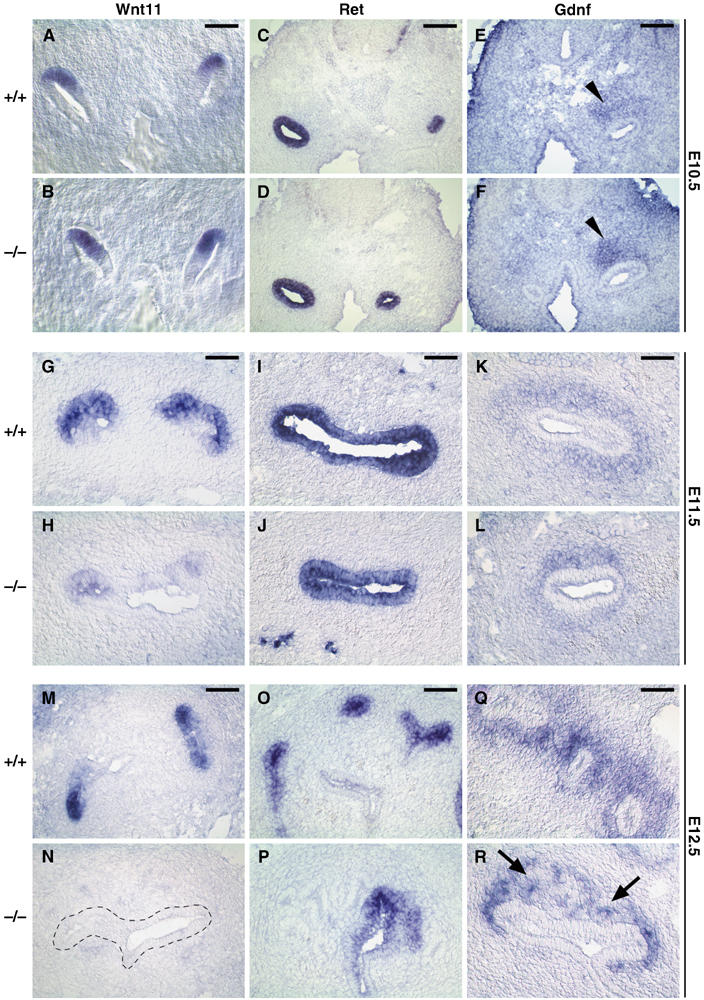
Reciprocal inductive interactions are lost in Six2-null kidney. Wnt11 (A, B), Ret (C, D), and Gdnf (E, F) expression was normal in Six2−/− kidney at E10.5, indicating that the initial inductive mechanism of the UB was unaffected. (G, H) At E11.5, Wnt11 expression was downregulated in the UB tips of the Six2−/− kidney as compared to wild-type littermates but that of Ret (I, J) and Gdnf (K, L) was normal. (M, N) At E12.5, reciprocal inductive interactions were lost in the Six2−/− kidney as indicated by the lack of Wnt11 expression in the UB (dashed outline). (O, P) Ret expression remained at normal levels in the Six2-null UB at E12.5. (Q, R) Gdnf expression confirmed the abnormal reduction in the size of the MM population and the presence of ectopic developing nephrons (arrows). Scale bar, 100 μm.
In addition to the genes analyzed in Figures 4, 5 and 6, we also characterized the expression of other well-known regulators of kidney development such as Six1 (Oliver et al, 1995; Xu et al, 2003), Pax8 (Plachov et al, 1990), Fgf8 (Crossley and Martin, 1995), and Lim1 (Fujii et al, 1994). Changes in the expression pattern of Six1, Pax8, Fgf8 (data not shown), and Lim1 (Supplementary Figure S4) were in agreement with those we previously observed when using other markers (e.g., Pax2, Eya1, Wnt4) that recognize similar cell populations. Those results, together with the kidney phenotypes resulting from the functional inactivation of these genes (Shawlot and Behringer, 1995; Mansouri et al, 1998; Tsang et al, 2000; Bouchard et al, 2002; Laclef et al, 2003; Li et al, 2003; Xu et al, 2003; Grieshammer et al, 2005; Perantoni et al, 2005), indicated that most likely they did not contribute to the Six2-null phenotype. Also, no obvious changes were identified in the stromal cell population that expresses Foxd1 (Hatini et al, 1996), although this cell population was reduced owing to the smaller size of the Six2-null kidney (Supplementary Figure S4). Together, these results indicated that the primary reciprocal induction between the UB and the E10.5 metanephric blastema was largely unaffected in Six2-null kidney and suggested that UB invasion and MM induction do not require Six2 activity. However, the precocious and ectopic nephrogenesis promoted by removal of Six2 activity caused the depletion of the mesenchymal progenitor/stem cell population and loss of reciprocal inductive interactions required for continued kidney growth. Later during development (E14.5), most of the essential mesenchymal and ureteric genes (e.g., Eya1, Pax2, Ret) were downregulated or absent in the mutant kidney, whereas expression of Wnt4 remained in the developing nephrons and that of stromal markers (e.g., Foxd1, Sfrp1, Raldh2) remained unchanged (Supplementary Figure S5, and data not shown).
Apoptosis contributes to the loss of the mesenchymal progenitor pool in Six2-null kidneys
The detection of precocious ectopic epithelial vesicles suggested that MM lacking Six2 undergoes premature nephrogenesis, resulting in a rapid reduction in the size of the uninduced mesenchymal cell population. A reduction in the size of the E11.5 MM population before the appearance of renal vesicles was also revealed by the analysis of the MM markers Eya1, Pax2, Bmp7, and Gdnf (Figures 4, 5 and 6). To confirm the reduction of the E11.5 MM population and to analyze proliferation in the Six2-null kidney, we used antibodies against phosphohistone H3 and Pax2 on adjacent sections. Quantification of the number of PH3-positive cells in the Pax2-positive population revealed that the rate of proliferation and size of the MM were unaltered in E10.5 Six2-null kidney (Figure 7A and B). However, although the rate of proliferation was similar in the E11.5 wild-type and mutant kidneys, the size of the MM population was reduced by approximately 40% in the Six2-null kidney (Figure 7C and D). In addition, the amount of cell death detected by TUNEL assay increased significantly in the MM and stroma of the E11.5 Six2-null kidney (Figure 7H); this increase of apoptosis in the progenitor pool is also likely to contribute to the observed reduction in the size of both populations throughout kidney development. No significant changes in apoptosis were detected at E10.5 or E12.5 (Figure 7E, F and I, J); however, cell death increased from E14.5 to E17.5 (Figure 7K and L, and data not shown). The result of these phenotypic alterations is a severely hypoplastic and nonfunctional kidney at birth.
Figure 7.
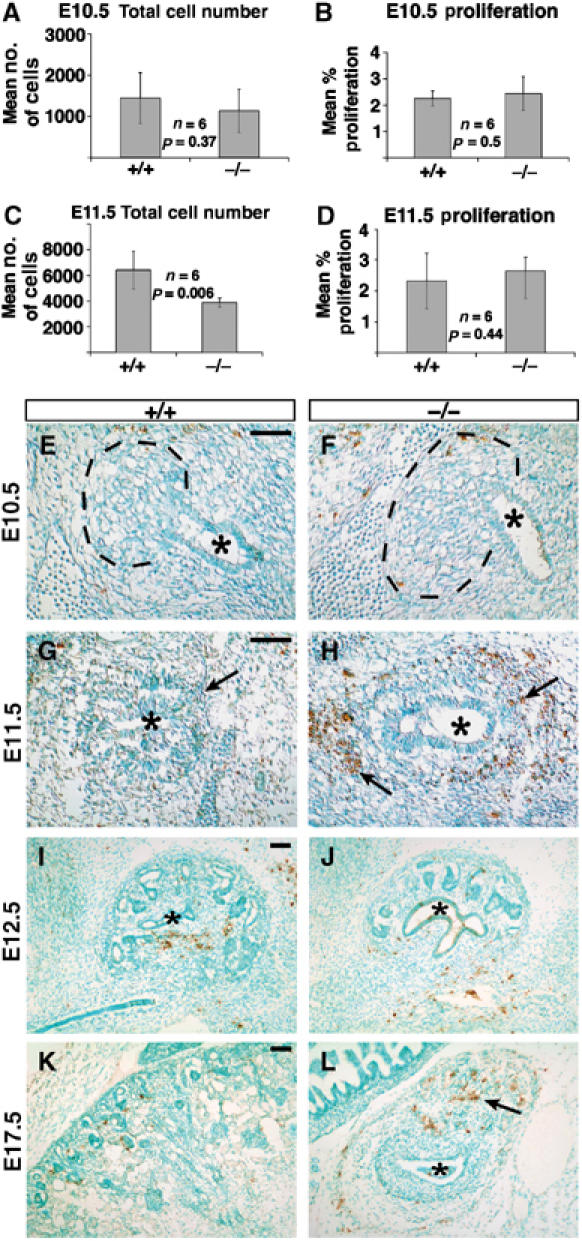
Six2−/− kidneys exhibit increased apoptosis at E11.5. (A, B) No differences in the mean total number of cells (A) or in the mean percentage of proliferating cells (B) were measured in the E10.5 Six2-null MM. (C, D) At E11.5, Six2−/− kidneys were significantly smaller than wild type (C; P=0.006); however, the number of proliferating cells was comparable. (E, F) Similar to its wild-type littermate, no apoptotic cells were detected at E10.5 in Six2-mutant kidneys. The UB (asterisk) and the area of the metanephric blastema (dashed line) are indicated. (G, H) An abnormal increase in the number of apoptotic cells was identified in the Six2-null MM (arrows) surrounding the UB at this stage. (I, J) At E12.5, there was no significance difference in the number of apoptotic cells between wild-type and Six2−/− kidneys. (K, L) An increase in the number of apoptotic cells was again detected at E17.5 in the mutant kidney (arrow) as compared to wild-type controls. Scale bar, 100 μm.
Together, these results indicated that in the Six2-null kidney, the MM aggregated rapidly and the renal vesicles formed ectopically and prematurely. These events, along with the presence of abnormal apoptosis, depleted the mesenchymal progenitor/stem cell population, which was not replenished. Thus, Six2 activity is required to repress epithelial polarization, at least in a portion of MM, thus allowing for the renewal of undifferentiated mesenchymal progenitors as the organ grows. Six2 opposes tubulogenesis promoted by the UB-derived inductive signal and thus reserve a subset of mesenchymal progenitor/stem cells in an undifferentiated state for future rounds of nephrogenesis.
Six2 ectopic expression repressed the differentiation of mesenchymal cells into epithelia in an organ culture system
To further test this working model, we expressed Six2 ectopically under the control of the chicken β-actin promoter in mouse kidney organ cultures. Plasmids expressing either EGFP or FLAG-tagged Six2 were introduced into wild-type E12.5 kidneys grown on Transwell filters using a modified version of a previously published electroporation protocol (Gao et al, 2005). Expression of EGFP was robust after 24 h in culture and could be maintained for several days. After 48 h in culture, the electroporated kidneys were fixed and sectioned to identify the distribution of EGFP and FLAG-Six2 expression. In the control cultures (n=8), EGFP-labeled cells were found in both the mesenchymal and epithelial components of the developing kidney at near equal proportions (Figure 8A, B and E, F, and Table I). In agreement with previous results indicating that MM cells can be incorporated into the UB epithelia (Qiao et al, 1995), we observed EGFP-positive cells in branching epithelial tubules and developing nephrons; both these epithelial structures were Pax2-positive (Figure 8A and B) and were surrounded by a laminin-containing basement membrane (Figure 8E and F). In contrast, electroporation of the Six2 expression vector (n=8) resulted in high levels of FLAG-Six2 labeling almost exclusively in the mesenchymal population (Figure 8C, D and G, H, and Table I). The FLAG-Six2-positive cells were localized along the peripheral mesenchyme and in the interstitial cells, as shown by Foxd1 expression (Figure 8I and J). FLAG-Six2 was rarely detected in epithelial structures derived from MM or UB epithelia. Thus, ectopic expression of Six2 repressed the differentiation of mesenchymal cells into epithelia in the organ culture system. These results support the hypothesis that Six2 opposes epithelial polarization and helps maintain an undifferentiated population of renal blastemal cells.
Figure 8.
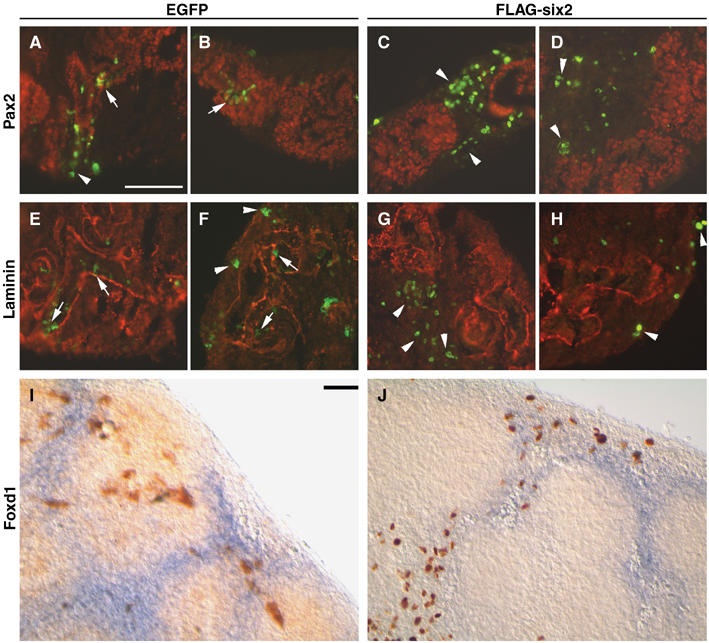
Overexpression of Six2 in wild-type kidney organ cultures. Forty-eight hours after microinjection and electroporation of EGFP or FLAG-Six2 expression plasmids, sections of E12.5 kidney organ cultures were labeled with antibodies specific for Pax2 (red; A–D), laminin (red; E–H), EGFP (green), or FLAG-Six2 (green). (A, B) EGFP and Pax2 were coexpressed in epithelial structures (arrows), and EGFP was also expressed in peripheral mesenchyme (arrowhead). (C, D) Cells expressing FLAG-Six2 were almost exclusively found in peripheral and interstitial mesenchyme (arrowheads), separated from Pax2-positive cells. (E, F) EGFP-positive cells were located within the developing tubules (arrows), as demarcated by laminin-containing basement membranes, and in the peripheral mesenchyme (arrowheads). (G, H) FLAG-Six2-expressing cells (arrowheads) were not surrounded by laminin-containing basement membranes and exhibited a mesenchymal phenotype. (I, J) In situ hybridization for Foxd1 followed by immunohistochemistry using anti-GFP (I) or anti-FLAG (J) antibodies indicated that the cells expressing FLAG-Six2 resided mainly in the interstitial stroma, whereas cells expressing the EGFP control vector resided in all cell populations. Scale bar, 100 μm.
Table 1.
Electroporation of E12.5 kidney organ cultures
| Vector | Laminin (+) | Laminin (−) | Pax2 (+) | Pax2 (−) | Cells/section |
|---|---|---|---|---|---|
| EGFP | 7.6±4.0 | 13.8±7.9 | 9.7±5.2 | 11.3±6.4 | 21.2±7.6 (n=24)a |
| Fl-Six2 | 0.44±0.89 | 18.8±12.4 | 1.2±1.4 | 20.4±4.5 | 20.6±12.6 (n=32)a |
| aNumber of sections from eight electroporated cultures. | |||||
Six2 activity maintains kidney blastemal cells in an undifferentiated state
Before induction, the MM is a small aggregate of a few thousand cells. In response to inductive signals, these cells coordinate a precise program of proliferation and differentiation to generate most of the epithelial cells in the nephrons. Because of the sequential nature of kidney patterning, new nephrons are induced in the periphery as the kidney grows. Thus, some of the MM cells aggregate and become polarized early, whereas others proliferate and remain mesenchymal to generate nephrons at subsequent stages. Our results identify an essential role of Six2 in maintaining and renewing this undifferentiated population of MM progenitor cells. Although mutations in genes such as Pax2, Wt1, Eya1, Six1, and Sall1 (Kreidberg et al, 1993; Torres et al, 1995; Xu et al, 1999, 2003; Nishinakamura et al, 2001) affect the response to inductive signaling resulting in complete developmental arrest and kidney agenesis, the Six2-mutant phenotype is unique in that it exhibits premature and ectopic epithelial differentiation. Most likely, these events and the ectopic apoptosis detected during early stages of MM development deplete the peripheral mesenchymal progenitor population so that no new nephrons are generated. In addition, the defective maintenance of reciprocal inductive interactions we identified in the mutant kidneys most likely leads to the arrest in UB branching and mispatterning of nephrons observed at later stages. Furthermore, gain-of-function experiments in kidney organ culture demonstrated that persistent Six2 expression inhibits conversion of mesenchyme to epithelia. Therefore, Six2 must be downregulated in mesenchyme cells for epithelialization of mesenchymal pretubular aggregates to proceed. As previously mentioned, it has been suggested that Bmp7 acts as a survival factor for the nephrogenic progenitor pool during kidney development (Dudley et al, 1995; Luo et al, 1995); therefore, the Bmp7 signaling pathway may mediate Six2 function. However, no obvious phenotypic alterations were observed in the Bmp7-null kidneys before E12.5 (Dudley et al, 1995; Luo et al, 1995; Dudley and Robertson, 1997), and we did not detect obvious changes in the expression level of Bmp7 in the E11.5 Six2−/− MM (Figure 4L). Together, these results indicated that most likely Bmp7 does not play a significant role in the Six2−/− phenotype and, therefore, is not a key player in the maintenance of the mesenchymal progenitor pool at the earliest stages of kidney development (i.e., E10.5–E11.5).
It is important to stress that although premature tubulogenesis resulted from a lack of Six2 activity, none of the genetic markers for the stromal population were significantly changed. This finding indicates that Six2 differentially controls the fate of progenitor cells that are committed toward the epithelial lineage. This is consistent with the hypothesis that stromal and nephric lineages are specified separately at an early stage during kidney development (Hatini et al, 1996).
As suggested by our expression data, Six2 opposes tubulogenesis by directly or indirectly repressing the expression of Wnt4 and Sfrp2 within the MM. Importantly, although Six2 and its closely related family member Six1 are expressed in the same population of the embryonic MM, their loss-of-function phenotypes suggest that they are functionally nonredundant and control different aspects of kidney development. On the basis of these results, we propose that Six2 controls the fate of the renal epithelial progenitor/stem cell population by suppressing the inductive signals that promote epithelial differentiation and maintaining available pools of blastemal cells in an undifferentiated state.
Materials and methods
Functional inactivation of Six2
Six2−/− mice were generated by replacing the NcoI–EcoRI fragment of the Six2 gene containing exon 1, the transcriptional initiation site, the Six domain, and the homeodomain with a 1.6-kb fragment containing the pGK-Neo cassette (Supplementary Figure S1). The vector pKO Scrambler NTKV-1901 (Stratagene) was used as a backbone. The HindIII site upstream of exon 2 was deleted during the cloning of the 3′ arm for genotyping purposes. Electroporation and selection of embryonic stem cells was performed using standard methods. Positive clones were identified and injected into blastocysts to generate chimeras. The mutated Six2 allele was identified by Southern blot analysis and amplified by PCR.
In situ hybridization
Embryos were fixed in 4% paraformaldehyde and either processed for whole-mount in situ hybridization (Wilkinson, 1995) or cryopreserved for cryosectioning. Whole-mount tissue was sectioned on a vibratome. Nonradioactive in situ hybridization was performed on sections as described previously (Schaeren-Wiemers and Gerfin-Moser, 1993).
Microinjection, electroporation, and culture of metanephric explants
E11.5 kidneys were dissected in L-15 medium (Gibco) and maintained in culture on Costar Transwell filters (0.4-μm pore size). The culture medium consisted of DMEM/F12 (1:1 mix), 10% fetal calf serum, and penicillin and streptomycin (Cellgro). Explants were maintained in culture for 24, 48, and 96 h at 37°C with 5% CO2 for immunohistochemical analysis.
For transfection experiments, E12.5 kidneys were microdissected at room temperature in Dulbecco's PBS and placed on Transwell plates (24-mm diameter, 8-μm pore size, polycarbonate membrane; Corning) with 1 ml of DMEM. Using a glass capillary microelectrode controlled by an Eppendorf FemtoJet microinjection system, we injected 0.02–0.03 μl of PBS containing purified plasmid DNA (1.5 μg/μl) into different regions of the kidney. Immediately after injection, we delivered five square electrical pulses of 40 V for 50 ms each at 100 ms intervals through platinum electrodes (7-mm diameter, 1-cm distance) by using a BTX ECM 830 (San Diego) electroporator. The kidneys were then maintained in culture for 48 h at 37°C with 5% CO2. The kidneys were fixed in 4% paraformaldehyde at 4°C for 20 min, rinsed in PBS, cryoprotected in 0.5 M sucrose for 4 h, embedded in OCT medium, and stored at −80°C. Sections of kidney (14-μm thick) were cut on a cryostat and immunolabeled with anti-GFP (1:50, Invitrogen), anti-Flag (1:200, Sigma), anti-Pax2 (1:200), and anti-laminin (1:200, Sigma) antibodies. Whole-mount in situ hybridization was performed as described previously (Wilkinson, 1995) to detect Foxd1 mRNA expression followed by whole-mount immunohistochemistry for either anti-GFP or anti-Flag antibodies using diaminobenzidine as a substrate.
Immunohistochemistry, TUNEL, and proliferation studies
See Supplementary data.
Supplementary Material
Supplementary Information
Supplementary Figure Legends
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Acknowledgments
We thank J Kreidberg for teaching the electroporation procedure to GRD. We also thank G Murti and colleagues of the St Jude Scientific Imaging Department for their help with imaging and A McMahon, T Carroll, G Martin, S Vainio, S Potter, R Maas, C Krull, and K Kawakami for plasmids. We would like to thank T Valerious, T Carroll, and A McMahon for discussions and A McArthur for excellent scientific editing of the manuscript. This work was supported in part by the National Institutes of Health grant R21DK068560, Cancer Center Support grant CA-21765, and the American Lebanese Syrian Associated Charities (ALSAC) to GO, DK054740 and DK062914 to GRD, and a PKD Foundation fellowship to YC.
References
- Armstrong JF, Pritchard-Jones K, Bickmore WA, Hastie ND, Bard JB (1993) The expression of the Wilms' tumour gene, WT1, in the developing mammalian embryo. Mech Dev 40: 85–97 [DOI] [PubMed] [Google Scholar]
- Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M (2002) Nephric lineage specification by Pax2 and Pax8. Genes Dev 16: 2958–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler AJ, Pelletier J, Haber DA, Glaser T, Housman DE (1991) Isolation, characterization, and expression of the murine Wilms' tumor gene (WT1) during kidney development. Mol Cell Biol 11: 1707–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP (2005) Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9: 283–292 [DOI] [PubMed] [Google Scholar]
- Cho EA, Patterson LT, Brookhiser WT, Mah S, Kintner C, Dressler GR (1998) Differential expression and function of cadherin-6 during renal epithelium development. Development 125: 803–812 [DOI] [PubMed] [Google Scholar]
- Collins JF, Ghishan FK (1994) Molecular cloning, functional expression, tissue distribution, and in situ hybridization of the renal sodium phosphate (Na+/P(i)) transporter in the control and hypophosphatemic mouse. FASEB J 8: 862–868 [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martin GR (1995) The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development 121: 439–451 [DOI] [PubMed] [Google Scholar]
- Donovan MJ, Natoli TA, Sainio K, Amstutz A, Jaenisch R, Sariola H, Kreidberg JA (1999) Initial differentiation of the metanephric mesenchyme is independent of WT1 and the ureteric bud. Dev Genet 24: 252–262 [DOI] [PubMed] [Google Scholar]
- Dressler GR (2002) Development of the excretory system. In Mouse Development: Patterning, Morphogenesis, and Organogenesis, Rossant J, Tam PPL (eds) pp 395–420. San Diego, CA: Academic Press [Google Scholar]
- Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P (1990) Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development 109: 787–795 [DOI] [PubMed] [Google Scholar]
- Dressler GR, Douglass EC (1992) Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proc Natl Acad Sci USA 89: 1179–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ (1995) A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev 9: 2795–2807 [DOI] [PubMed] [Google Scholar]
- Dudley AT, Robertson EJ (1997) Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn 208: 349–362 [DOI] [PubMed] [Google Scholar]
- Durbec P, Marcos-Gutierrez CV, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, Smith D, Ponder B, Costantini F, Saarma M, Sariola H, Pachnis V (1996) GDNF signalling through the Ret receptor tyrosine kinase. Nature 381: 789–793 [DOI] [PubMed] [Google Scholar]
- Ekblom P, Alitalo K, Vaheri A, Timpl R, Saxen L (1980) Induction of a basement membrane glycoprotein in embryonic kidney: possible role of laminin in morphogenesis. Proc Natl Acad Sci USA 77: 485–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P, Klein G, Ekblom M, Sorokin L (1991) Laminin isoforms and their receptors in the developing kidney. Am J Kidney Dis 17: 603–605 [DOI] [PubMed] [Google Scholar]
- Favor J, Sandulache R, Neuhauser-Klaus A, Pretsch W, Chatterjee B, Senft E, Wurst W, Blanquet V, Grimes P, Sporle R, Schughart K (1996) The mouse Pax2(1Neu) mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of the brain, ear, eye, and kidney. Proc Natl Acad Sci USA 93: 13870–13875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming S, Symes CE (1987) The distribution of cytokeratin antigens in the kidney and in renal tumours. Histopathology 11: 157–170 [DOI] [PubMed] [Google Scholar]
- Fujii T, Pichel JG, Taira M, Toyama R, Dawid IB, Westphal H (1994) Expression patterns of the murine LIM class homeobox gene lim1 in the developing brain and excretory system. Dev Dyn 199: 73–83 [DOI] [PubMed] [Google Scholar]
- Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC (1994) Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium–(potassium)–chloride cotransporter family expressed in kidney. J Biol Chem 269: 17713–17722 [PubMed] [Google Scholar]
- Gao X, Chen X, Taglienti M, Rumballe B, Little MH, Kreidberg JA (2005) Angioblast-mesenchyme induction of early kidney development is mediated by Wt1 and Vegfa. Development 132: 5437–5449 [DOI] [PubMed] [Google Scholar]
- Grieshammer U, Cebrian C, Ilagan R, Meyers E, Herzlinger D, Martin GR (2005) FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development 132: 3847–3857 [DOI] [PubMed] [Google Scholar]
- Grobstein C (1955) Inductive interactions in the development of the mouse metanephros. J Exp Zool 130: 319–340 [DOI] [PubMed] [Google Scholar]
- Gruenwald P (1943) Stimulations of nephrogenic tissues by normal and abnormal inductors. Anat Rec 86: 321–335 [Google Scholar]
- Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E (1996) Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev 10: 1467–1478 [DOI] [PubMed] [Google Scholar]
- Hebert SC, Mount DB, Gamba G (2004) Molecular physiology of cation-coupled Cl− cotransport: the SLC12 family. Pflugers Arch 447: 580–593 [DOI] [PubMed] [Google Scholar]
- Hellmich HL, Kos L, Cho ES, Mahon KA, Zimmer A (1996) Embryonic expression of glial cell-line derived neurotrophic factor (GDNF) suggests multiple developmental roles in neural differentiation and epithelial–mesenchymal interactions. Mech Dev 54: 95–105 [DOI] [PubMed] [Google Scholar]
- Herzlinger D, Koseki C, Mikawa T, al-Awqati Q (1992) Metanephric mesenchyme contains multipotent stem cells whose fate is restricted after induction. Development 114: 565–572 [DOI] [PubMed] [Google Scholar]
- Kalatzis V, Sahly I, El-Amraoui A, Petit C (1998) Eya1 expression in the developing ear and kidney: towards the understanding of the pathogenesis of Branchio-Oto-Renal (BOR) syndrome. Dev Dyn 213: 486–499 [DOI] [PubMed] [Google Scholar]
- Kispert A, Vainio S, McMahon AP (1998) Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 125: 4225–4234 [DOI] [PubMed] [Google Scholar]
- Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP (1996) Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development 122: 3627–3637 [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R (1993) WT-1 is required for early kidney development. Cell 74: 679–691 [DOI] [PubMed] [Google Scholar]
- Laclef C, Souil E, Demignon J, Maire P (2003) Thymus, kidney and craniofacial abnormalities in Six 1 deficient mice. Mech Dev 120: 669–679 [DOI] [PubMed] [Google Scholar]
- Leimeister C, Bach A, Gessler M (1998) Developmental expression patterns of mouse sFRP genes encoding members of the secreted frizzled related protein family. Mech Dev 75: 29–42 [DOI] [PubMed] [Google Scholar]
- Lescher B, Haenig B, Kispert A (1998) sFRP-2 is a target of the Wnt-4 signaling pathway in the developing metanephric kidney. Dev Dyn 213: 440–451 [DOI] [PubMed] [Google Scholar]
- Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, Rosenfeld MG (2003) Eya protein phosphatase activity regulates Six1–Dach–Eya transcriptional effects in mammalian organogenesis. Nature 426: 247–254 [DOI] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G (1995) BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 9: 2808–2820 [DOI] [PubMed] [Google Scholar]
- Lyons KM, Hogan BL, Robertson EJ (1995) Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech Dev 50: 71–83 [DOI] [PubMed] [Google Scholar]
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP (2003) Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130: 3175–3185 [DOI] [PubMed] [Google Scholar]
- Mansouri A, Chowdhury K, Gruss P (1998) Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet 19: 87–90 [DOI] [PubMed] [Google Scholar]
- Miner JH, Li C (2000) Defective glomerulogenesis in the absence of laminin alpha5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev Biol 217: 278–289 [DOI] [PubMed] [Google Scholar]
- Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A (1999) YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development 126: 1845–1857 [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A (1996) Renal and neuronal abnormalities in mice lacking GDNF. Nature 382: 76–79 [DOI] [PubMed] [Google Scholar]
- Murer H, Forster I, Biber J (2004) The sodium phosphate cotransporter family SLC34. Pflugers Arch 447: 763–767 [DOI] [PubMed] [Google Scholar]
- Nishinakamura R, Matsumoto Y, Nakao K, Nakamura K, Sato A, Copeland NG, Gilbert DJ, Jenkins NA, Scully S, Lacey DL, Katsuki M, Asashima M, Yokota T (2001) Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development 128: 3105–3115 [DOI] [PubMed] [Google Scholar]
- Nishinakamura R, Osafune K (2006) Essential roles of sall family genes in kidney development. J Physiol Sci 56: 131–136 [DOI] [PubMed] [Google Scholar]
- Nishinakamura R, Takasato M (2005) Essential roles of Sall1 in kidney development. Kidney Int 68: 1948–1950 [DOI] [PubMed] [Google Scholar]
- Oliver G, Wehr R, Jenkins NA, Copeland NG, Cheyette BN, Hartenstein V, Zipursky SL, Gruss P (1995) Homeobox genes and connective tissue patterning. Development 121: 693–705 [DOI] [PubMed] [Google Scholar]
- Pachnis V, Mankoo B, Costantini F (1993) Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 119: 1005–1017 [DOI] [PubMed] [Google Scholar]
- Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M (2005) Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 132: 3859–3871 [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H (1996) Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 382: 73–76 [DOI] [PubMed] [Google Scholar]
- Plachov D, Chowdhury K, Walther C, Simon D, Guenet JL, Gruss P (1990) Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development 110: 643–651 [DOI] [PubMed] [Google Scholar]
- Qian J, Jiang Z, Li M, Heaphy P, Liu YH, Shackleford GM (2003) Mouse Wnt9b transforming activity, tissue-specific expression, and evolution. Genomics 81: 34–46 [DOI] [PubMed] [Google Scholar]
- Qiao J, Cohen D, Herzlinger D (1995) The metanephric blastema differentiates into collecting system and nephron epithelia in vitro. Development 121: 3207–3214 [DOI] [PubMed] [Google Scholar]
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M (1996) Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382: 70–73 [DOI] [PubMed] [Google Scholar]
- Sariola H, Saarma M (1999) GDNF and its receptors in the regulation of the ureteric branching. Int J Dev Biol 43: 413–418 [PubMed] [Google Scholar]
- Saxen L (1987) Organogenesis of the kidney. In Developmental and Cell Biology Series 19, Bard JBL, Barlow PW, Kirk DL (eds) Cambridge: Cambridge University Press [Google Scholar]
- Saxen L, Sariola H (1987) Early organogenesis of the kidney. Pediatr Nephrol 1: 385–392 [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A (1993) A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry 100: 431–440 [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D'Agati V, Pachnis V, Costantini F (1996) Renal agenesis and hypodysplasia in ret-k- mutant mice result from defects in ureteric bud development. Development 122: 1919–1929 [DOI] [PubMed] [Google Scholar]
- Shamley DR, Opperman LA, Buffenstein R, Ross FP (1992) Ontogeny of calbindin-D28K and calbindin-D9K in the mouse kidney, duodenum, cerebellum and placenta. Development 116: 491–496 [DOI] [PubMed] [Google Scholar]
- Shawlot W, Behringer RR (1995) Requirement for Lim1 in head-organizer function. Nature 374: 425–430 [DOI] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP (1994) Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372: 679–683 [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Dressler GR, Gruss P (1995) Pax-2 controls multiple steps of urogenital development. Development 121: 4057–4065 [DOI] [PubMed] [Google Scholar]
- Trupp M, Arenas E, Fainzilber M, Nilsson AS, Sieber BA, Grigoriou M, Kilkenny C, Salazar-Grueso E, Pachnis V, Arumae U (1996) Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature 381: 785–789 [DOI] [PubMed] [Google Scholar]
- Tsang TE, Shawlot W, Kinder SJ, Kobayashi A, Kwan KM, Schughart K, Kania A, Jessell TM, Behringer RR, Tam PP (2000) Lim1 activity is required for intermediate mesoderm differentiation in the mouse embryo. Dev Biol 223: 77–90 [DOI] [PubMed] [Google Scholar]
- Vainio S, Lin Y (2002) Coordinating early kidney development: lessons from gene targeting. Nat Rev Genet 3: 533–543 [DOI] [PubMed] [Google Scholar]
- Vainio SJ, Uusitalo MS (2000) A road to kidney tubules via the Wnt pathway. Pediatr Nephrol 15: 151–156 [DOI] [PubMed] [Google Scholar]
- Vega QC, Worby CA, Lechner MS, Dixon JE, Dressler GR (1996) Glial cell line-derived neurotrophic factor activates the receptor tyrosine kinase RET and promotes kidney morphogenesis. Proc Natl Acad Sci USA 93: 10657–10661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vize PD, Woolf AS, Bard JBL (2003) The Kidney, from Normal Development to Congenital Disease. London: Academic Press [Google Scholar]
- Wilkinson DG (1995) RNA detection using non-radioactive in situ hybridization. Curr Opin Biotechnol 6: 20–23 [DOI] [PubMed] [Google Scholar]
- Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R (1999) Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet 23: 113–117 [DOI] [PubMed] [Google Scholar]
- Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D (2003) Six1 is required for the early organogenesis of mammalian kidney. Development 130: 3085–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino K, Rubin JS, Higinbotham KG, Uren A, Anest V, Plisov SY, Perantoni AO (2001) Secreted Frizzled-related proteins can regulate metanephric development. Mech Dev 102: 45–55 [DOI] [PubMed] [Google Scholar]
- Yu J, McMahon AP, Valerius MT (2004) Recent genetic studies of mouse kidney development. Curr Opin Genet Dev 14: 550–557 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Figure Legends
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5


