Abstract
A combination of two cationic lipid derivatives having the same headgroup but tails of different chain lengths has been shown to have considerably different transfection activity than do the separate molecules. Such findings point to the importance of investigating the hydrophobic portions of cationic amphiphiles. Hence, we have synthesized a variety of cationic phosphatidylcholines with unusual hydrophobic moieties and have evaluated their transfection activity and that of their mixtures with the original molecule of this class, dioleoyl-O-ethylphosphatidylcholine (EDOPC). Four distinct relationships between transfection activity and composition of the mixture (plotted as percent of the new compound added to EDOPC) were found, namely: with a maximum or minimum; with a proportional change; or with essentially no change. Relevant physical properties of the lipoplexes were also examined; specifically, membrane fusion (by fluorescence resonance energy transfer between cationic and anionic lipids) and DNA unbinding (measured as accessibility of DNA to ethidium bromide by electrophoresis and by fluorescence resonance energy transfer between DNA and cationic lipid), both after the addition of negatively charged membrane lipids. Fusibility increased with increasing content of second cationic lipid, regardless of the transfection pattern. However, the extent of DNA unbinding after addition of negatively charged membrane lipids did correlate with extent of transfection. The phase behavior of cationic lipids per se as well as that of their mixtures with membrane lipids revealed structural differences that may account for and support the hypothesis that a membrane lipid-triggered, lamellar→nonlamellar phase transition that facilitates DNA release is critical to efficient transfection by cationic lipids.
INTRODUCTION
Cationic lipids are useful reagents for the transfection of mammalian cells both in vitro and in vivo; however, their transfection efficiency is still rather low relative to that of viral vectors. (The term “lipoid” is more appropriate than “lipid” because the latter refers to natural products, whereas “lipoid” describes molecules that are similar to lipids in structure and in properties but are not natural products). An additional drawback is that the mechanism of transfection remains poorly understood, and there is no consensus on the correlation between physicochemical properties and transfection efficiency (1–7). Hence, more efficient reagents would be desirable.
Previously we demonstrated that a combination of two cationic lipid derivatives having the same headgroup but tails of different chain lengths and saturation behave considerably differently than do the separate molecules and that the transfection efficiency can be tuned by adjusting the ratio of two components (8). For example, the combination of the dilauroyl (12-carbon chain) and the dioleoyl (18-carbon chain) homologs of O-ethylphosphatidylcholine transfected DNA into primary human umbilical artery endothelial cells (HUAECs) more than 30-fold-times more efficiently than either compound separately.
Since the introduction of the first practical liposomal transfection agent (9), a large variety of such compounds has been synthesized and tested by many different laboratories. Enormous attention has been given to altering the headgroup of these compounds and, collectively, this research revealed that a large number of different polar structures can be used for the headgroup. Much less attention has been given to hydrophobic portions of cationic lipids, which seems to have been an unfortunate development, for our investigations suggest that the nonpolar portions may be critical for the effectiveness of these transfection agents. Thus, we have extended our previous study of mixtures to include assessing how transfection activity changes with different tails and with different kinds of combinations of those tails. These changes have been made on cationic phosphatidylcholines by modifying and combining their hydrophobic moieties. Furthermore, to shed some light to the mechanism by which the transfection efficiency changes, we examined some relevant physical properties of the lipoplexes; specifically, membrane fusion (by fluorescence resonance energy transfer) and DNA unbinding (measured both as accessibility of DNA to ethidium bromide by electrophoresis and by fluorescence resonance energy transfer between DNA and cationic lipid), in both cases after addition of negatively charged liposomes to the lipoplexes. No correlation between membrane fusion and transfection activity was found. Membrane fusion consistently increased with addition of a second cationic lipid, regardless of its effect on transfection. In contrast, the DNA unbinding assay revealed a correlation between extent of transfection and the extent of DNA release induced by addition of negatively charged membrane lipids, suggesting that, while both processes may be necessary, fusion itself is generally not rate-limiting for transfection. Small-angle x-ray diffraction experiments illustrated that facilitated DNA release from the most effective lipoplex formulations could be accounted for by an intracellular lamellar→nonlamellar phase transition in the cationic lipid/anionic membrane lipid mixtures.
MATERIALS AND METHODS
Materials
1,2-Dioleoyl-sn-glycero-3-ethylphosphocholine (EDOPC), 1,2-diphytanoyl-sn-glycero-3-ethylphosphocholine (EDΦPC), 1,2-dioleoyl-sn-glycero-3-stearylphosphocholine (SDOPC), 1,2-didecanoyl-sn-glycero-3-myristylphosphocholine (C14DC10PC), 1,2-didecanoyl-sn-glycero-3-octylphosphocholine (C8DC10PC), 1,2-diphytanoyl-sn-glycero-3-palmitylphosphocholine (PDΦPC), 1-oleyol-2-decanoyl-sn-glycero-3-ethylphosphocholine (EC18C10PC), and 1,2-ditridecanoyl-sn-glycero-3-ethylphosphocholine (EDC13PC) were synthesized in our laboratory according to Rosenzweig et al. (10).
1,2-Dilauroyl-sn-glycero-3-ethylphosphocholine (EDLPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-ethylphosphocholine (EPOPC), 1,2-dioleoyl-sn-glycero-3-L-serine (DOPS) and lissamine rhodamine B-labeled as 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (Rh-DOPE) were purchased from Avanti Polar Lipids (Alabaster, AL).
3,3′-Dioctadecyloxacarbocyanine perchlorate (DiO), 1,1′-dioctadecyl-3,3,3′,3′- tetramethylindocarbocyanine perchlorate (DiI), and YOYO-1 iodide were obtained from Molecular Probes (Eugene, OR).
Fluorescein di-β-D-galactopyranoside (FDG), X-gal, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and L-α-phosphatidylcholine extracted from egg yolk (egg PC) were purchased from Sigma (St. Louis, MO).
A β-galactosidase plasmid was purchased from Clontech Laboratories (Palo Alto, CA) and propagated and purified by Bayou Biolabs (Harahan, LA).
Methods
Cell culture
Human umbilical artery endothelial cells (HUAECs) from Cambrex (Walkersville, MD) were used for all experiments. The cells were maintained in EGM-2 MV containing 5% fetal bovine serum (Cambrex, Walkersville, MD) at 37°C under 5% CO2. At confluence, the cells were passaged using 0.25 mg/ml Trypsin/EDTA (Cambrex, Walkersville, MD) and were used at passages 5–10 for these experiments.
Lipoplex preparation
For preparation of lipoplexes used for transfection, chloroform solutions of cationic lipids were mixed in glass vials at the desired ratios, after which the chloroform was removed under an Argon or N2 stream. Vials were placed under high vacuum for several hours to remove the last traces of the solvent. The lipid mixtures were hydrated in HEPES buffered saline solution (Cambrex, Walkersville, MD) at 1 mg/ml to form liposomes. Liposomes and plasmid DNA were each diluted in OptiMEM (Gibco, Gaithersburg, MD) to 160 μg/ml for lipid and to 40 μg/ml for DNA, and liposomes were pipetted into an equal volume of plasmid DNA solution at a 4:1 weight ratio and mixed gently. DNA/lipid mixtures were allowed to stand at room temperature for 15 min before transfection.
For x-ray diffraction sample preparation, 50 mM phosphate buffer and 100 mM NaCl, pH 7.2 (PBS) was added to cationic lipids after removal of chloroform with a N2 stream and, subsequently, high vacuum. The dispersions were hydrated overnight at room temperature and vortex-mixed for several minutes; then they were subjected to several cycles of freezing and thawing. Herring sperm DNA (Invitrogen, Carlsbad, CA) was used for preparation of lipoplexes. DNA/lipid (4:1 w/w) dispersions were prepared by adding an aqueous DNA solution to the dry lipid film and immediately vortexing, as previously described (11). Samples were equilibrated for 1–3 days at room temperature before measurement.
Transfection and cell viability
The cells were seeded in 96-well plates at 24 h before transfection at densities to give ∼80% confluence at the time of transfection. Before transfection, the cells were washed with HEPES buffered saline solution and then covered by either medium lacking serum or medium containing 5% fetal bovine serum. The lipoplexes were added to cells and allowed to incubate for 2 h. After removal of medium (and unattached lipoplexes), the cells were incubated for 24 h with fresh medium containing serum and then evaluated for β-galactosidase activity with a microplate fluorometric assay. Briefly, after aspiration of the medium from each well, the cells were washed once with PBS and then lysed by addition of 100 μl lysis buffer (0.03% Triton X-100 in 100 mM HEPES, 8 mM MgSO4, and pH 7.8). Ten microliters of 100 μM fluorescein di-β-D-galactopyranoside (FDG) was added into each well and fluorescence was measured at 20 h with a microplate fluorimeter (model No. 7620, Cambridge Technology, Cambridge, MA). Viability of cells after transfection was assessed with the MTT method (12).
Membrane fusion
For this procedure (13), cationic liposomes were labeled with 1% DiO and DiI and the lipoplexes were prepared according to the procedure described above. Labeled lipoplexes were placed in 6 × 50 mm borosilicate glass tubes. Then, an equal amount (by wt) of unlabeled negatively charged liposomes (20% DOPS in egg PC) was added and the fluorescence of DiO was recorded with an Alphascan fluorometer (Photon Technology International, Princeton, NJ) with excitation and emission monochromators set at 489 nm and 506 nm, respectively. For calibration of the fluorescence scale, the initial residual fluorescence intensity before the addition of anionic lipid was set to zero and the intensity at infinite probe dilution was set to 100%. The latter value was obtained by lysis of the lipoplexes with Triton X-100.
Anionic lipid-induced unbinding of DNA from lipoplexes
DNA release measured by electrophoresis.
Lipoplexes were prepared as described above, treated with a threefold molar excess of liposomes consisting of 20% DOPS in egg PC, and then incubated for 1 h at 37°C. After addition of 6× loading buffer (0.25% bromophenol blue, 0.25% xylene cyanol FF, and 40% (w/v) sucrose in water), samples were electrophoresed on 0.7% agarose gels containing 10 μg/ml ethidium bromide (EtBr) in Tris-acetate EDTA, pH 8.0 at 75 V for 1 h. The EtBr-stained DNA bands were visualized and photographed on an ultraviolet transilluminator (Fotodyne, New Berlin, WI).
DNA unbinding measured by fluorescence resonance energy transfer (FRET) between DNA and cationic lipid.
Cationic liposomes were labeled with 3% Rh-DOPE and DNA was labeled with YOYO-1 at a ratio of 2 nmol dye per 10 μg DNA, and then the lipoplexes were prepared according to the procedure described above. Labeled lipoplexes were placed in 6 × 50 mm borosilicate glass tubes. Then, a portion of unlabeled negatively charged liposomes (20% DOPS in egg PC) was added in an amount to give a threefold molar excess of negative over positive charge, and the fluorescence of YOYO-1 was recorded with an Alphascan fluorometer with excitation and emission monochromators set at 491 nm and 509 nm, respectively. The intensity of free DNA without any lipids was set to 100%.
Synchrotron small-angle x-ray diffraction (SAXD)
Measurements were performed at Argonne National Laboratory (Argonne, IL), Advanced Photon Source, DND-CAT (beamline 5-IDD) and BioCAT (beamline 18-ID), using 12 keV x-rays, as previously described (14). The lipid concentration of the dispersions was 20 wt %. Samples were filled into glass capillaries (Charles Supper, Natick, MA) and flame-sealed. A Linkam thermal stage (Linkam Sci Instruments, Surrey, England) provided temperature control. Linear heating and cooling scans were performed at rates of 0.8–5°C/min. Exposure times were typically ∼0.5 s. Data were collected using a MAR-CCD detector. Sample-to-detector distance was 1.8–2 m. Diffraction intensity versus Q plots were obtained by radial integration of the two-dimensional patterns using the interactive data-evaluating program FIT2D (15). SAXD measurements were also performed using Ni-filtered CuKα radiation from a rotating anode generator ELLIOTT-GX6 (Elliott Automation Radar Systems, Marconi Avionics, Herts, England), as previously described (16).
Dynamic light scattering (DLS)
Measurements were performed with a Brookhaven Instruments BI-200SM goniometer and BI-9000 digital correlator (Brookhaven, NY). Cationic lipid dispersions in PBS were prepared at 50 μg/ml. DNA (1 mg/ml in PBS) was added to generate lipoplex samples at a 4:1 lipid/DNA weight ratio. Measurements were carried out at 37°C. Borosilicate glass, 250 μl, 3 × 30 mm, flat bottom tubes were used. Delay times of 10 μs–1 s were examined. The correlation data were fitted with quadratic cumulants, using the algorithm provided with the instrument.
RESULTS
Transfection in mixtures with EDOPC of compounds with different tails
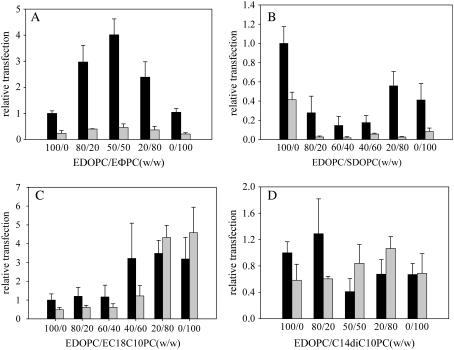
In our previous study (8), we showed that the addition of EDLPC dramatically enhanced the transfection by EDOPC of HUAECs and that transfection reached a maximum at EDLPC/EDOPC = 60:40 (w/w). To extend that research, we asked whether other cationic phosphatidylcholines have a similar synergistic effect in mixtures. We also sought to determine whether the transfection curve is always convex-shaped, that is, with a maximum at intermediate compositions. To address these questions, we synthesized a variety of cationic phosphatidylcholines with different hydrophobic moieties (see Fig. 1 for the structures), combined each of them with the original molecule of this class (EDOPC), and observed how transfection changed. All mixtures tested fell into one of four kinds of relationship between transfection activity and composition of the mixture (plotted as percent of the new compound in EDOPC). The first (Fig. 2 A) is a convex curve, similar to that of the mixture of EDOPC/EDLPC, in which there is a maximum in transfection for intermediate compositions. The second (Fig. 2 B) is concave and has the opposite curvature from that of EDOPC/EDLPC mixture, with the transfection pattern exhibiting a minimum at intermediate compositions. The third case (Fig. 2 C) has a skewed shape, with the transfection changing monotonically with the second cationic lipid. The transfection pattern in the fourth case (Fig. 2 D) does not exhibit obvious trend and is thus described “no clear change”; there is no statistically significant difference (p > 0.01) among transfection activities of the different compositions, signifying the absence of a mixture effect. Although the transfection pattern of one representative combination in each category is given in Fig. 2, several other combinations that gave these kinds of patterns were also examined. EDOPC/EDΦPC illustrates the convex relationship, and the EDOPC/C8DC10PC mixture also conforms to this type (as does, of course, the previously reported EDOPC/EDLPC mixture). While the effect is pronounced both with and without serum, transfection without serum was generally more efficient. A concave relationship, like that shown for EDOPC/SDOPC, was also given by EDOPC/PDΦPC. In addition to the skewed relationship shown for EDOPC/EC18C10PC (Fig. 2 C), EDOPC/EDC13PC also exhibited this relationship, and did so in both the presence and absence of serum. The other case of a “no clear change” curve besides the EDOPC/C14DC10PC mixture (Fig. 2 D) was EDOPC/EPOPC. Although the magnitude of the increase (in the convex and “skewed” cases) or the decrease (in concave case) of transfection activity relative to that of pure EDOPC varies with second cationic lipid, the overall trend in transfection efficiency as a function of composition is quite invariant, and what is significant is that transfection efficiency alters significantly with a minor modification in the ratio of two components. The weight ratio of lipid to DNA was kept constant for convenience; and although that meant that the ±charge ratio varied somewhat with cationic lipid composition, separate measurements of charge ratio changes showed that these had minor effects relative to those of lipid composition. For example, in the case of EDOPC/EDLPC, when the weight ratio was kept at 4:1, the ±ratio changes from 1.3:1 (100% EDOPC) to 1.9:1 (100% EDLPC); the transfection activity, however, decreased only 50%. So we can exclude the possibility that the four kinds of relationship between transfection activity and composition of the mixture are qualitatively altered by the change of ±charge ratio.
FIGURE 1.
Structures of synthetic cationic phosphatidylcholines with different hydrophobic moieties.
FIGURE 2.
Extent of transfection of HUAEC varies with the composition of the cationic lipid mixture in the presence and absence of serum. Lipoplexes were made as described in Materials and Methods. Fifty-microliters per well (1 μg DNA/well) of the resultant lipoplexes were added to the cells that were either in medium lacking serum (solid bars) or medium containing 5% serum (shaded bars). Lipoplexes were removed at 2 h and the cells were assayed for β-galactosidase activity at 24 h after transfection. Data represent the mean ±SD of a representative experiment performed in quadruplicate. The experiments were repeated three times with consistent results. The β-Gal expression of HUAECs with EDOPC lipoplexes in the absence of serum was ∼0.1 mU per well.
Membrane fusion
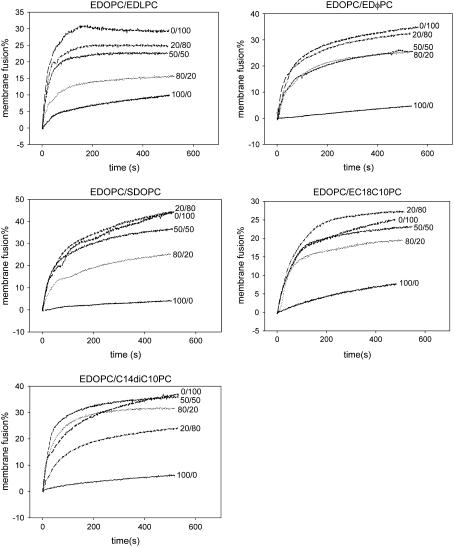
To be expressed, the plasmid DNA must dissociate from the cationic lipids and move into the nucleus. Several investigators have demonstrated that anionic membranes promote cationic lipid/DNA complex dissociation and postulated that, after endocytosis of the cationic lipid/DNA complex, anionic lipids can migrate from the endosomal membrane into the complex where they displace DNA from the cationic lipid, releasing it into cytoplasm (17–19). Indeed, our previous research (8) showed that EDLPC facilitates the membrane fusion of lipoplex and negatively charged liposomes; the extent of fusion of EDOPC/EDLPC lipoplexes is significantly higher than that of pure EDOPC. It would hence be useful to know if increased membrane fusion is the reason why the mixtures of EDOPC and other cationic phosphatidylcholines with different hydrophobic moieties function so differently; that is, whether the highest transfection activity is due to the most extensive membrane fusion. To address this issue, membrane fusion was measured using a fluorescence resonance energy transfer (FRET) assay (13,20), which is dependent on the dilution of fluorescent lipid probes in the participating membranes. Similar to the transfection experiments, one mixture from each category was chosen as a representative to characterize lipid mixing between lipoplex lipid with anionic lipid. The results, shown in Fig. 3, indicate that increases in membrane fusion occur upon addition of other cationic lipids to EDOPC, regardless of the extent of transfection by the mixture. These data hence suggest that some perturbation or modification of the bilayer of EDOPC is induced by the presence of a second cationic lipid, with the consequence that membrane fusion is increased. The lack of correlation between membrane fusion and transfection activity, however, may be attributed to the fact that lipid mixing of lipoplexes with anionic membranes is not the limiting step in transfection; in particular, it suggests that the kind of membrane mixing registered by the energy transfer fusion assay does not necessarily correlate with the extent of release of DNA from the lipoplex (21,22). We therefore measured the extent of DNA release from different formulations induced by anionic lipid.
FIGURE 3.
Membrane fusion of cationic lipoplexes with anionic liposomes by fluorescence resonance energy transfer. Two hundred microliters of lipoplex labeled with 1% each of DiO and DiI were treated with an equal amount of unlabeled egg PC liposomes containing 20% DOPS. Ex = 489 nm; Em = 506 nm. The percentage of membrane fusion = (Fn-F0)/(F100-F0) × 100%, where Fn is the fluorescence after the addition of anionic lipid, F0 is the initial fluorescence of lipoplexes, and F100 is the fluorescence after the addition of Triton X-100 at the end of the experiments.
DNA unbinding induced by negatively charged membrane lipid
DNA release measured by electrophoresis.
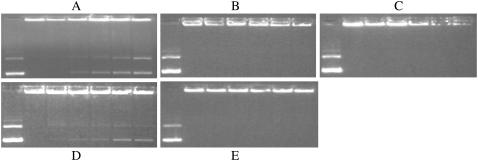
The extent of DNA release from different lipoplex formulations after interaction with negatively charged liposomes was examined by electrophoresis on agarose gels. From Fig. 4, one can see that the amount of free DNA was significantly greater for formulations with higher transfection activity (such as EDOPC/EDLPC (40:60), EDOPC/EDLPC (20:80), EDOPC/EDLPC (0:100), EDOPC/EC18C10PC (20:80), and EDOPC/EC18C10PC (0:100)), whereas the formulations with low transfection activity (such as EDOPC/SDOPC mixtures and EDOPC/C14DC10PC mixtures) showed no free DNA at all. Noteworthy is that a new band can be seen just below the wells after the addition of negatively charged liposomes. We interpret the new band as DNA that is released from the lipid surface so that it is available to EtBr, but still confined by cationic-anionic lipid aggregates, so it cannot migrate very far from the wells.
FIGURE 4.
DNA release from cationic lipoplexes upon interaction with 20% DOPS in egg PC liposomes measured by electrophoresis. Lane 1, free DNA; lanes 2–7, EDOPC/the other cationic lipid (100:0, 80:20, 60:40, 40:60, 20:80, 100:0) lipoplexes. (A) EDOPC/EDLPC; (B) EDOPC/EDΦPC, (C) EDOPC/SDOPC; (D) EDOPC/EC18C10PC; and (E) EDOPC/C14DC10PC.
DNA unbinding measured by FRET between DNA and cationic lipid.
Given the electrophoresis results, it was desirable to directly measure the release of DNA from the lipid surface, so we measured DNA unbinding using a procedure involving FRET between DNA and cationic lipid. We regard this assay as a DNA unbinding assay rather than a DNA release assay, because “DNA release” usually refers to DNA that dissociates from the lipid surface and is subsequently liberated from the confines of the lipid aggregate. However, FRET between DNA and cationic lipid can change either when DNA is free in solution or when DNA is only released from lipid surface but remains trapped within the confines of the lipid array. Given these considerations, “DNA unbinding” is the more accurate term. Comparison of Table 1 with Fig. 2 reveals that the percentage of DNA release induced by negatively charged membrane lipid is correlated to transfection activity. Formulations with higher transfection activity (such as EDOPC/EDΦPC (40:60) and EC18C10PC) gave higher percentage of DNA unbinding (31.2 and 68.1%, respectively, compared with that of pure EDOPC, 11.5%). It is noteworthy that the formulations with low transfection activity, EDOPC/SDOPC (50:50) and SDOPC, gave a negative value for DNA unbinding, and that the unbinding DNA in these two formulations before addition of anionic lipid was significantly higher than that of other formulations and the negative change means that some of the initially unbound DNA became associated with the lipid aggregates after anionic lipid was added. Indeed, we did observe under the microscope, that, especially for SDOPC, there were initially few large lipoplexes particles, but more such large particles formed after the addition of anionic lipids. In addition, examination of the intracellular distribution of lipoplexes revealed more DNA from SDOPC and EDOPC/SDOPC lipoplexes was trapped in lysosomes than that from EDOPC lipoplexes (data not shown). As measured by electrophoresis, pure EDLPC lipoplexes released DNA very well after treatment with anionic lipid, but the transfection activity of EDLPC was low. This apparent exception to the correlation between ease of DNA release and transfection activity is considered in Discussion, below.
TABLE 1.
DNA unbinding induced by the addition of negatively charged membrane lipid
| Lipoplex composition | Unbound DNA in lipoplexes before the addition of anionic lipid (%) | Unbound DNA in lipid mixture after the addition of anionic lipid (%) | DNA unbinding induced by anionic lipid (%) |
|---|---|---|---|
| EDOPC | 23.7 ± 0.8 | 35.2 ± 0.9 | 11.5 |
| EDOPC/EDLPC (50:50) | 16.1 ± 0.6 | 39.3 ± 1.1 | 23.3 |
| EDLPC | 15.9 ± 0.6 | 66.7 ± 0.9 | 50.9 |
| EDOPC/EDΦPC (40:60) | 2.2 ± 0.2 | 53.2 ± 0.8 | 31.2 |
| EDΦPC | 22.8 ± 0.7 | 31.8 ± 0.8 | 8.9 |
| EDOPC/EC18C10PC (40:60) | 2.0 ± 0.2 | 49.0 ± 1.1 | 47.0 |
| EC18C10PC | 2.8 ± 0.2 | 70.9 ± 1.4 | 68.1 |
| EDOPC/SDOPC (50:50) | 48.7 ± 1.5 | 38.8 ± 1.0 | −10.0 |
| SDOPC | 78.3 ± 2.1 | 64.7 ± 1.7 | −13.6 |
Structural organization of lipoplexes before and after treatment with membrane lipids
In search of the reason why the DNA in lipoplex formulations with high transfection activity is more extensively liberated upon treatment of the lipoplexes with negatively charged liposomes, we did structural experiments using small-angle x-ray diffraction (SAXD).
Mixtures with a minimum in the transfection efficiency versus composition curve: EDOPC/SDOPC, EDOPC/PDΦPC.
According to our previous SAXD data, both the lipid dispersions and the lipid/DNA complexes of EDOPC are arranged into lamellar arrays, with repeat periods of 4.9 nm and 6.6 nm, respectively (16). In addition to sharp lamellar reflections, an additional low-intensity diffuse peak was also present in lipoplex diffraction patterns (see Fig. 8 in (16)). Such peaks have been interpreted as coming from the in-plane packing of the DNA strands intercalated between the lipid lamellae (23,24). Its spacing was 3.2 nm in the samples of EDOPC/DNA 4:1 w/w ratio.
FIGURE 8.
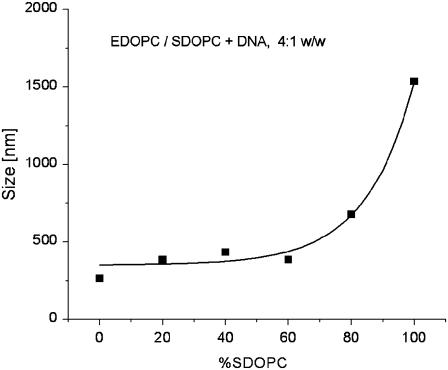
Sizes of the EDOPC/SDOPC lipoplexes, estimated by DLS.
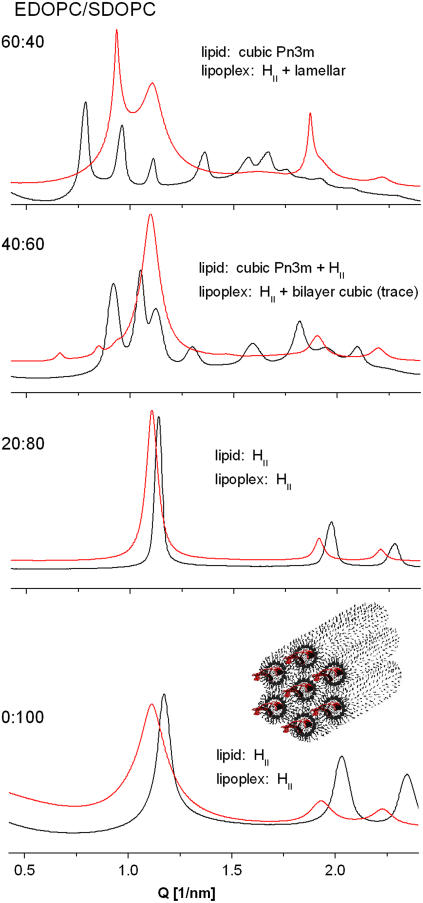
The structural organization of SDOPC dispersions was significantly different from that of EDOPC dispersions. At room temperature, this lipid was arranged as an inverted hexagonal HII phase, with a lattice parameter a = 2d/√3 = 6.2 nm (Fig. 5, bottom). This structure was retained upon heating to 90°C. On cooling below room temperature, it transformed into a lamellar phase at 1°C (not illustrated). The transition was reversible, with a large temperature hysteresis, as is typical for this type of phase transition: the Lα→HII transition was observed at 12°C on heating. Addition of DNA at a lipid/DNA 4:1 w/w ratio did not change the phase structure of SDOPC; the x-ray diffraction pattern was characteristic of that for a hexagonal phase arrangement at room temperature (a = 6.5 nm) and this arrangement was retained on heating to 80°C, as well as upon cooling. The lower intensity of the diffraction peaks from the 11 and 20 planes in the presence of DNA relative to the patterns without DNA (Fig. 5, bottom) is a likely result of the higher electron density of DNA relative to water (see, e.g., (25)). It is thus an indication of the presence of DNA in the core of the hexagonal phase cylinders, i.e., for the formation of columnar inverted hexagonal phase lipoplexes (as shown in the cartoon on Fig. 5, bottom).
FIGURE 5.
SAXD patterns of EDOPC/SDOPC cationic lipid mixtures (black) and their lipoplexes (red) at 37°C; lipid/DNA 4:1 w/w.
In the aqueous dispersions of EDOPC/SDOPC mixtures at room temperature, as the SDOPC fraction was increased, the structure changed from lamellar for the pure EDOPC, to bilayer cubic Pn3m (a = 11.3 nm) at EDOPC/SDOPC 60:40 w/w, to coexisting cubic (Pn3m) + hexagonal HII at 40:60 w/w, and then to pure hexagonal HII (a = 6.4 nm) at 20:80 w/w and at 100% SDOPC (Fig. 5). In the lipoplexes of the EDOPC/SDOPC 60:40 and 40:60 samples, for which the transfection activity was minimal, lamellar, and hexagonal phases coexisted (Fig. 5), virtually epitaxially matched (ahex ≈ dlam) (Fig. 6), while the 20:80 EDOPC/SDOPC lipoplexes formed only the hexagonal phase, as did the pure SDOPC.
FIGURE 6.
Coexisting lamellar and inverted hexagonal phases in lipoplexes of EDOPC/PDΦPC 60:40 (A), and EDOPC/SDOPC 60:40 (B) at 37°C as revealed by their SAXD patterns. (C) Cartoon representation of the suggested aggregate morphology (see Discussion).
The structural organization and the phase behavior of the EDOPC/PDΦPC dispersions and lipid/DNA complexes wererather similar to those of EDOPC/SDOPC. Thus, pure PDΦPC formed an inverted hexagonal phase, as did its lipoplexes. The EDOPC/PDΦPC 60:40 mixture formed a bilayer cubic Pn3m phase, while in the lipoplexes at that lipid composition (exhibiting minimum transfection potency), lamellar and inverted hexagonal phases coexisted, as they did in the EDOPC/SDOPC lipoplexes of minimum transfection activity; the close similarity of their diffraction patterns is apparent in Fig. 6, A and B.
Thus, the minimum in the transfection activity for these two mixtures corresponded to extensive lamellar + hexagonal phase coexistence in the lipoplexes. Noteworthy is that the hexagonal-phase lipoplexes formed by the pure SDOPC and PDΦPC exhibited lower transfection efficiency than did the lamellar lipoplexes formed by EDOPC, contrary to what has been reported before for other cationic lipids (26,27).
Mixtures with maxima in the transfection efficiency versus composition curve: EDOPC/EDΦPC, EDOPC/C8DC10PC.
The phase behavior of the two mixtures exhibiting maxima in their transfection versus composition curves, EDOPC/EDΦPC and EDOPC/C8DC10PC, differed from one another. EDΦPC and its mixtures with EDOPC formed a lamellar phase, similar to pure EDOPC; the lamellar repeat distances of the mixtures increased monotonically from 4.9 nm for EDOPC to 5.35 nm for EDΦPC. In contrast, C8DC10PC formed a cubic phase, identified by eight diffraction peaks, indexing as the Pn3m cubic symmetry (a = 8.6 nm). A cubic phase of the same Pn3m symmetry, but with a significantly larger lattice parameter, a = 14.5 nm, was formed in the EDOPC/C8DC10PC 50:50 w/w mixture. The lipoplexes of all EDOPC/EDΦPC and EDOPC/C8DC10PC samples (lipid/DNA 4:1 w/w) were arranged in lamellar arrays. Their structural characteristics (lamellar repeat distance, in-plane spacing between DNA strands) changed monotonically with the lipid composition and did not exhibit an extremum. Thus, the structural organization of the EDOPC/EDΦPC and EDOPC/C8DC10PC lipid aggregates and their lipoplexes did not provide insight into the basis of the maximum in the transfection activity versus composition curve.
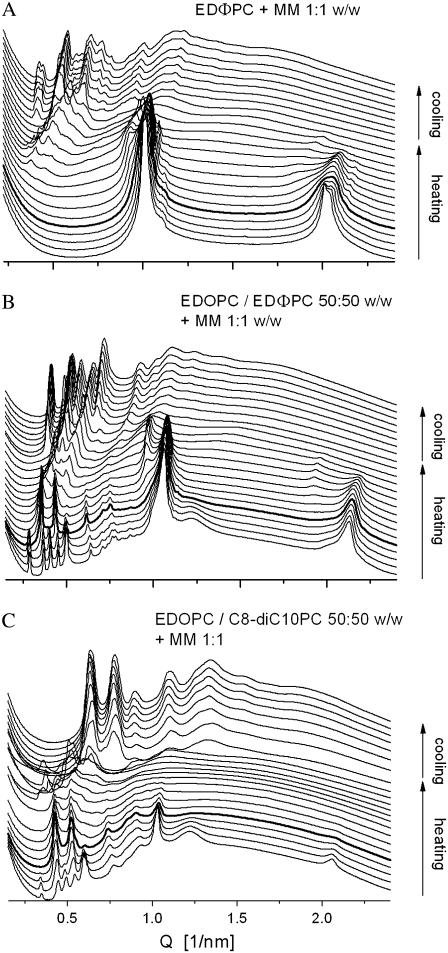
EDOPC/EDLPC is another cationic lipid mixture that exhibited maximal transfection activity at intermediate compositions (8). It is noteworthy that, according to our earlier work, it also formed lamellar lipoplexes only, with gradually changing structural characteristics as a function of composition. A clue to the origin of that maximal efficiency was suggested by an examination of the structural changes that took place as a result of the interaction of the lipoplexes with negatively charged membrane lipids (28). Thus, we studied the structures that formed when EDOPC/EDΦPC and EDOPC/C8DC10PC formulations were mixed with a lipid mixture that mimics natural membranes, MM = DOPC/DOPE/DOPS/Chol, 45:20:20:15 (w/w) (29). This membrane-mimicking formulation was chosen to better simulate the conditions that exist when lipoplexes interact with cellular lipids.
An EDΦPC/MM 1:1 mixture formed a lamellar phase (d = 6.15 nm) at room temperature. Upon heating, it converted into the cubic Pn3m phase at ∼70°C. The transition was irreversible, with the cubic phase persisting on cooling and on subsequent incubation at room temperature (Fig. 7 A). The phase behavior of the EDOPC/MM 1:1 mixture was rather similar to that of EDΦPC/MM, in that it was dominated by a lamellar phase at room temperature that irreversibly converted into cubic Pn3m phase at 80°C upon heating. The picture was significantly different, however, when the maximum-activity EDOPC/EDΦPC 50:50 formulation was mixed with the membrane-mimicking lipid blend MM. At room temperature, extensive phase coexistence was observed; 18 diffraction maxima, indexing as a mixture of one lamellar and two cubic phases, of Pn3m and Im3m symmetry (Fig. 7 B), were present. Upon heating, the Im3m cubic phase disappeared at physiological temperature, 37°C. The lamellar phase also gradually disappeared at higher temperature, and only the cubic Pn3m phase was observed at 80°C and on subsequent cooling. Rather similar was the phase behavior of the maximum-efficiency EDOPC/C8DC10PC 50:50 formulation, when it was also in a mixture with the membrane-mimicking preparation. In this case too, there was extensive phase coexistence of two cubic phases and a lamellar phase at room and physiological temperatures, along with a phase transition (disappearance of the Im3m cubic phase) at physiological temperature, after which only the Pn3m cubic phase remained at high temperatures and on subsequent cooling (Fig. 7 C). Thus, the maximal transfection potency in these formulations correlated with development of nonlamellar phases and phase transformation at physiological temperature in mixtures containing membrane lipids. Lipid vehicles are known to exhibit maximum leakiness and contents release in the vicinity of phase transitions, especially those involving formation of nonlamellar phases.
FIGURE 7.
SAXD patterns of mixtures of the membrane-mimicking lipid formulation MM combined with EDΦPC (A), with EDOPC/EDΦPC 50:50 (B), and with EDOPC/C8DC10PC 50:50 (C), all recorded during 20°–90°–20°C heating-cooling scans; the pattern in bold was taken at 37°C on heating.
Mixtures with gradually changing transfection activity (skewed patterns): EDOPC/EC18C10PC.
Aqueous dispersions of the EDOPC/EC18C10PC mixture formed a lamellar phase at all compositions, as did their lipoplexes (Table 2). The structural characteristics (lamellar repeat distance, DNA interstrand distance) changed insignificantly and did not provide an explanation for the increasing transfection activity. Thus, we again sought the origin of the improved transfection activity of this lipid composition in its interaction with the negatively charged membrane lipid formulation MM. As described above, the EDOPC/MM mixture is characterized by an irreversible lamellar→cubic (Pn3m) transition at ∼80°C. When the EDOPC/EC18C10PC 40:60 mixture interacted with the membrane-mimicking composition MM, it also arranged into a lamellar phase at room temperature. Upon heating above 40–50°C, it became progressively more disordered, and no diffraction maxima were observed at above those temperatures. A cubic Pn3m phase developed at ∼80°C. The EC18C10PC sample exhibited an irreversible lamellar→cubic Pn3m phase transition at 37°C on heating. Thus, the increasing transfection efficiency as a function of the lipoplex composition correlates with a decreasing lamellar-nonlamellar phase transition temperature in the cationic/membrane lipid arrays. It appears that increased instability and diminished retention of DNA in the vicinity of that phase transformation can account for the enhanced transfection activity.
TABLE 2.
Structural organization and parameters of EDOPC / EC18C10PC aggregates
| Cationic lipid | Lipoplex | Cationic lipid + MM 1:1 |
|---|---|---|
| EDOPC | ||
| lamellar | lam d = 6.6 nm | lam → cubic Pn3m ∼80°C; cubic on cooling |
| d = 4.9 nm | dDNA = 3.2 nm | |
| EDOPC/EC18C10PC 40:60 | ||
| lamellar | lam d = 6.4 nm | lam → disordered ∼45–50°C → cubic Pn3m ∼80°C; cubic on cooling |
| d = 4.8 nm | dDNA = 3.8 nm | |
| EC18C10PC | ||
| lamellar | lam d = 6.2 nm | lam → cubic Pn3m ∼37°C; cubic on cooling |
| d = 4.6 nm | dDNA = 3.4 nm |
Mixtures with a no-clear-change transfection activity profile: EDOPC/EPOPC.
The aqueous dispersions of these two cationic lipids exhibited rather similar physico-chemical properties that remained unchanged in their mixtures. The same was true for their lipoplexes, with the structural parameters of the EDOPC/EPOPC lipoplexes at all compositions remaining virtually identical to those of the pure EDOPC. The structures developing upon interaction of the EDOPC/EPOPC mixtures with the membrane-mimicking formulation MM was also very similar to those observed with EDOPC. We regard the absence of changes in phase structure of EDOPC/EPOPC, both alone and in mixtures with MM, as responsible for their virtually unchanged transfection activity.
Lipoplex size—dynamic light scattering (DLS)
Particle size is important for transfection. The structure of the EDOPC/SDOPC lipoplexes changed with increasing proportion of SDOPC, first from lamellar to coexisting lamellar and hexagonal, and then to hexagonal (see above). Since hexagonal phase lipoplexes are only rarely observed and the relationship between their physical properties and their transfection activity is not understood, we used DLS to estimate the sizes of the EDOPC/SDOPC lipoplexes.
The mixed EDOPC/SDOPC lipoplexes containing 20–60% SDOPC are in the size range 380–430 nm, by ∼30–50% larger than the pure EDOPC lipoplexes (Fig. 8). At higher SDOPC content, however, the particle size increased dramatically, up to 1500 nm for the pure SDOPC lipoplexes.
Cell viability
Cell viability after transfection with different formulations is shown in Fig. 9 for transfection in the absence of serum. The viability of HUAECs treated with EDOPC/EDLPC lipoplexes dropped considerably with increased EDLPC content such that viability was ∼45% for pure EDLPC. The other formulations were considerably less toxic and cell viabilities for other formulations remained ∼70–80% (except in the case of 100% SDOPC). As will be discussed below, the toxicity of pure EDLPC seems to contribute to its low transfection activity.
FIGURE 9.
Viability of HUAEC as a function of the composition of cationic lipid mixtures. The cells were treated in the same way as for Fig. 2 and their viability was assayed by the MTT method 24 h after transfection. The viability of cells that had not been treated with lipoplexes was set at 100%. Data represent the mean ±SD of a representative experiment performed in quadruplicate. The experiments were repeated twice with consistent results.
X-gal staining
To distinguish whether higher transfection was due to more transfected cells or to increased copy number, X-gal assays were done, and, as can be seen from Table 3, there was a large influence from the higher number of transfected cells. EDOPC/EC18C10PC (20/80) had comparable transfection efficiency to EDOPC/EDLPC, which was previously reported as 15%. It should be noted that although many established cell lines can be transfected at high efficiencies, the values we obtained for HUAECs are very high for transfection of primary cells.
TABLE 3.
Transfection efficiency measured by X-gal staining
| Formulation | Serum | X-gal staining % |
|---|---|---|
| EDOPC | − | ∼5 |
| EDOPC | + | ∼4 |
| EDOPC/EC18C10PC (20/80) | − | ∼14 |
| EDOPC/EC18C10PC (20/80) | + | ∼14 |
| EDOPC/EDΦPC (50/50) | − | ∼10 |
| EDOPC/SDOPC (60/40) | − | ∼2 |
DISCUSSION
To probe the effect of the hydrophobic portion of cationic amphiphiles on their efficacy in transfecting nucleic acids into cells, we synthesized a variety of cationic phosphatidylcholines having unusual hydrophobic moieties. The first set of molecules (EDLPC, EDC13PC, and EPOPC) had shorter acyl chains and the alkyl group was ethyl. The second set of molecules was characterized by shorter acyl groups but an extended alkyl group, as in C8DC10PC and C14DC10PC. The third set comprised compounds with three long chains, two acyl and one alkyl (SDOPC and PDΦPC), which may present a different intrinsic curvature and interfere with packing as a normal bilayer. The fourth set of molecules (EDΦPC and PDΦPC) involved replacement of a linear with a branched chain acyl group, such that hydrophobicity was increased without changing chain length. Finally, EC18C10PC, a compound in which the two acyl groups are of significantly different length, was tested. The transfection activity of these compounds, both alone and in mixtures with the original molecule of this cationic phospholipid class, EDOPC, was evaluated. Four relationships between transfection activity and composition of the mixture (plotted as percent of the new compound in EDOPC) were found (Fig. 2), namely:
Convex—exhibiting maximum transfection activity at intermediate compositions.
Concave—exhibiting minimum transfection activity at intermediate compositions.
Skewed—with monotonically changing transfection activity upon composition change.
No clear change—little dependence or no clear trend of transfection activity on composition.
Several tendencies were identified in these activity-composition relationships:
Increasing the hydrophilic/hydrophobic balance by introducing a shorter acyl group was beneficial to transfection as in the case of EDLPC, EDC13PC, C8DC10PC, and EC18C10PC.
Inclusion of compounds with three long chains (SDOPC and PDΦPC), so as to increase the negative intrinsic curvature as well as total hydrophobicity, decreased transfection activity.
In the mixtures characterized by a no-clear-change transfection-composition relationship, neither carbon number nor cross-sectional area were much different from those of EDOPC.
Our initial hypothesis was that better transfection agents fused more rapidly with cellular membranes, a presumptive first step in DNA delivery. Support for this explanation was provided by the correlation of increased transfection activity of EDOPC/EDLPC mixtures with increased fusion with negatively charged liposomes (8). As shown here, however, membrane fusion with negatively charged liposomes increased with increasing proportion of the addition of second cationic lipid (Fig. 3), regardless of the effect of the latter on transfection. Although evidently not critically related to transfection activity in these systems, the effect is nonetheless striking and potentially important for fusion of other membranes (including cell membranes). A possible explanation of the effect is that after addition of the second cationic lipid, particularly one with significantly different structural properties than the first, the system is no longer in a single, fully miscible phase. That is, the lipids may be partially phase-separated with regions (perhaps only transient) that pack poorly enough that they could constitute a defect. Packing defects should facilitate the fusion of bilayers, because bilayer fusion, at some stage in the process, must involve merging of the hydrophobic cores of the two participating membranes. One might argue that if this were the case, the compounds giving a no-clear-change relationship would be those that do not show any increase in fusion when constituted into mixtures. Our results, however, also contradicted this hypothesis. Nevertheless, it should be recognized that although those cationic lipids with no-clear-change transfection curves were not much different from EDOPC, the addition of a second lipid does increase the degrees of freedom and thus also leads to some perturbation of the lipid packing. In any case, the fact that all mixture formulations exhibited increased propensity for membrane fusion indicates that some step after membrane fusion is rate-limiting for transfection.
The lack of correlation between transfection activity and the extent of lipid mixing of cationic lipoplexes with anionic cellular membranes has also been observed by other investigators (30–34) who attributed their findings to the fact that lipid mixing of lipoplexes with anionic membranes does not necessarily cause concomitant release of DNA from the lipoplex. Support of such suggestions was provided by Zabner et al. (22), who observed highly ordered structures that were located in intracellular vesicles after transfection with cationic lipid/DNA complexes. Much of the delivered DNA was in these structures. Also, a thickening of intracellular membranes has been observed after the uptake of cationic lipids, which could reflect an interaction, perhaps the mixing, between cationic lipids and intracellular membranes (21,22). In any case, these results suggest that lipid mixing does not necessarily lead to DNA escape from the confines of the lipid array. Hence, we next sought to determine the relationship between transfection activity and unbinding of the DNA from the lipid surfaces when the lipoplexes were treated with negatively charged membrane lipid.
DNA release data revealed a correlation between the extent of transfection by a given lipoplex formulation and the extent of DNA release that was induced by treating that lipoplex formulation with negatively charged membrane lipid (Fig. 4 and Table 1). Large amounts of unbound DNA were strongly correlated with formulations exhibiting convex and skewed curves and high transfection activity such as EDOPC/EDLPC (40:60), EDOPC/EDLPC (20:80), EDOPC/EC18C10PC (20:80), and EDOPC/EC18C10PC (0:100).
The case of the EDOPC/EDLPC (0:100) mixture appears to be an exception to the correlation between activity and anionic lipid-induced DNA unbinding, in that the transfection activity-composition relationship described convex curve, with 100% EDLPC lipoplexes having little activity. Nevertheless, EDLPC lipoplexes let go of their DNA when treated with the negatively charged membrane lipids, behavior that otherwise correlated with good transfection. The reason for the deviant behavior of this particular composition is evidently its toxicity; the viability of EDLPC-treated cells was only 45%, and such cells are likely too compromised to express of high levels of β-galactosidase. Thus, if low transfection activity of high proportions of EDLPC is due to cell toxicity, then EDOPC/EDLPC should be assigned to the skewed curve category.
The fact that transfection was improved when either EDLPC or EDC13PC was combined with EDOPC suggests that there is an optimal hydrophobicity for cationic phosphatidylcholine transfection agents. Also, comparing EDOPC/C8DC10PC with EDOPC/C14DC10PC shows that the presence of C8DC10PC led to enhanced transfection, whereas inclusion of the more hydrophobic C14DC10PC had no effect on transfection. Moreover, increased hydrophobicity (substituting PDΦPC for EDΦPC) changed the convex-shaped EDOPC/EDΦPC to the concave-shaped EDOPC/PDΦPC. It is hence clear that there are large effects associated with changing the hydrophobicity of transfection agents.
The physical property most closely related to the hydrophilic/hydrophobic balance in amphiphiles is their mesomorphic phase organization. We thus examined the phase structures of the cationic lipid mixtures and their lipoplexes; the results are discussed in the paragraphs that follow.
SDOPC and PDΦPC, which are effectively triple-chained, form the inverted hexagonal phase, HII, in both the absence and presence of DNA and represent an uncommon case of hexagonal-phase columnar lipoplexes. These compounds were less effective transfection agents than EDOPC (and, in mixtures with EDOPC, led to reduced activity; Fig. 2). Correlations between the mesomorphic phase state of the lipoplexes (lamellar versus inverted hexagonal) and their transfection activity have been sought (26,27,35–38), but it remains questionable whether hexagonal phase lipoplexes have advantages for transfection. Moreover, in most reports, the lipid phase state was controlled by adding the neutral helper lipid DOPE, and DOPE may well have effects beyond its influence on phase state. This cannot be true of the newly synthesized cationic lipids SDOPC and PDΦPC, which form hexagonal-phase lipoplexes per se, without helper lipid. Furthermore, since the headgroup of these molecules is the same as that of the biologically more active EDOPC, the observed different activity seems a likely result of their different phase organization. SDOPC and PDΦPC thus represent clear examples of the hexagonal-phase lipoplexes that are disadvantageous for transfection of HUAECs.
EDOPC/SDOPC and EDOPC/PDΦPC were the two cationic lipid mixtures that exhibited a minimum in the transfection versus composition curve. It is therefore potentially significant that the phase behavior of these two mixtures is remarkably similar to each other and quite different from that of other lipids and mixtures. As mentioned in the previous paragraph, the lipoplexes of both SDOPC and PDΦPC were arranged in the HII phase, but their mixtures with EDOPC—especially those mixtures with minimum transfection activity—exhibited extensive Lα-HII phase coexistence, characterized by especially similar diffraction patterns (Fig. 6).
Although the diffraction patterns of these mixtures clearly show the presence of two phases, structural ambiguities remain since we cannot specify the topological modes of phase coexistence. For example, Lα and HII phases might coexist either in separate aggregates or in the same aggregate, and the distinction might be critical for DNA unbinding and release. Additional information on the lipoplex organization was hence sought through size measurement by DLS; HII phases are normally highly aggregated and hence their dispersions tend to have large particles. Indeed, the pure SDOPC lipoplexes, which are HII structures, were significantly larger than those of the lamellar phase EDOPC lipoplexes. In contrast, the EDOPC/SDOPC lipoplexes with intermediate lipid compositions were similar in size to those of EDOPC (Fig. 8). These observations, as well as the fact that the coexisting Lα and HII phases are nearly epitaxially matched (Fig. 6), were interpreted as suggesting that the mixed-phase lipoplexes may have a topology like that depicted in Fig. 6 C. Such an arrangement would be consistent with reduced transfection activity, because the release of DNA would be obstructed by the outer lamellae. In addition to the topological relationship between the two phases, another question that arises with respect to samples having two coexisting phases is whether both phases contain DNA. The expanded d-spacing of the lamellar phase clearly indicates the presence of DNA, but what about the hexagonal phase? The structural characteristics of the hexagonal arrays in Fig. 6 suggest that they also contain DNA; the lattice parameter of the hexagonal-phase arrays that coexist with the lamellar ones in the EDOPC/SDOPC 60:40 lipoplexes is a = 2d/√3 = 6.5 nm, identical to that of the SDOPC lipoplexes, which, as indicated by the relative intensities of the x-ray diffraction peaks, contain DNA (see analysis in Results, above).
The EDOPC/EDΦPC and EDOPC/C8DC10PC mixtures both exhibited a maximum in their activity versus composition curve, but the phases of these lipoplexes could not be correlated to their transfection efficiency because their lipoplexes were lamellar at all compositions. Nevertheless, there is considerable evidence that the origin of the high transfection activity of the intermediate compositions of these lipids does ultimately depend on lipid phase behavior. This conclusion is derived from recent research in this laboratory, and is further supported by data in the present report. Specifically, we found that DNA release from lipoplexes induced by negatively charged membrane lipids unambiguously correlates with the mesomorphic phases developed in the cationic/anionic lipid mixtures, with most extensive release correlating with highest negative curvature of the phases generated when lipoplex lipids mix with cellular lipids (39). Not surprisingly, the curvature of the phase of the cationic/anionic lipid mixture depends both on the composition of negatively charged membranes and on the cationic transfection lipids (28).
The phase behavior of EDOPC/EDΦPC and EDOPC/C8DC10PC—the mixtures that exhibited a maximum in the relationship between transfection activity and composition—was consistent with the hypothesis outlined in the previous paragraph in that the compositions of maximal activity (50:50) generated nonlamellar phases (in these cases, two cubic phases, Pn3m and Im3m) when mixed with the membrane-mimicking lipid blend, MM. Moreover, the Im3m phase converts to the Pn3m phase at physiological temperature. This could be highly significant with respect to DNA release, for not only do lipid vehicles exhibit maximal leakiness and contents release in the vicinity of phase transitions (40–43), but increased leakiness is also seen for transitions involving nonlamellar phase formation (44–47). (Such increases in leakiness are presumed to be due to the accumulation of defects and increased disorder along the phase boundaries within the transition region.) Thus, the maximum efficiency of the EDOPC/EDΦPC 50:50 and the EDOPC/C8DC10PC 50:50 samples appears to be due to the coincidence that they not only tend to form nonlamellar arrays when mixed with membrane lipids, but also that they are within a phase transition region at physiological temperature, phenomena which both strongly facilitate DNA release. As we predict from the lower transfection activity of EDΦPC and EDOPC, their combination with MM lipids exhibited lower tendency for nonlamellar phase formation (such phases only formed at temperatures that were considerably above physiological temperature; see Fig. 7 A).
In the EDOPC/EC18C10PC formulations, the increasing transfection activity with increasing proportion of EC18C10PC is also explicable on the basis of superior transfection activity being associated with the process: lipoplex + MM lipids→nonlamellar phase. The lamellar→nonlamellar phase transition in the mixtures of those formulations with MM shifts to lower temperature and approaches physiological values when EC18C10PC becomes dominant. For the pure EC18C10PC, a lamellar-cubic phase transition precisely at 37°C in its mixture with MM (Table 2) seems to be the reason for its enhanced DNA release and excellent transfection outcome.
The effects of phase changes on DNA retention by transfection lipids after their treatment with MM lipids are visible by electrophoresis. Three bands are visible in a number of the lanes of Fig. 4. The two bands most distant from the wells are free DNA that has been released from the lipid surface and subsequently escaped from the confines of the cationic-anionic lipid aggregate. The other new band is just below the wells and represents DNA that barely entered the agarose; its considerable retardation indicates a large reduction in negative charge and/or formation of a supramolecular complex. The latter, in the form of a DNA-cationic-anionic lipid aggregate in which DNA has been released from the lipid surface but is still retained inside the lipid array, is most likely. Such electrophoretic patterns suggest caution in interpreting “free” DNA as that measured by a diminished fluorescence resonance energy transfer value between DNA and cationic lipid, for it is evident that such a method could overestimate DNA release. The fluorescence change actually includes both that of the completely released DNA and that from DNA no longer electrostatically associated with a cationic lipid surface, but still entrapped in the lipid aggregate. The gel electrophoresis assay can distinguish these two circumstances and determine how much DNA becomes completely free from lipid, whereas the fluorescence resonance energy transfer assay shows how much DNA is has unbound from the lipoplex surface and is potentially able to subsequently escape from the lipid aggregate.
It is clear from the electrophoresis data that even in the case of the formulation with the best transfection, only a small part of DNA was completely freed, and most remained entrapped within the aggregate. An obviously important question is whether this also holds true in cells and accounts for the low extent of transfection by cationic lipidic agents even under optimal conditions. Lipoplexes are highly-ordered multilamellar structures; in cells, they may be presumed to interact with a number of cellular membranes, during which DNA may be released gradually only after the lipoplex has acquired enough anionic lipids to neutralize the cationic charge and to rearrange into a structure from which the DNA can escape. Indeed, other investigators have observed that some DNA remained within exogenous intracellular structures after transfection with cationic lipid/DNA complexes. If poor DNA release from lipoplexes proves to be a critical attribute of poor transfection by cationic lipids, then judicious manipulation of critical molecular design parameters such as headgroup size and lipophilic segment dimensions to generate the proper interaction between cationic lipids and DNA and between cationic lipids and cellular anionic lipids should be helpful in optimizing transfection agents.
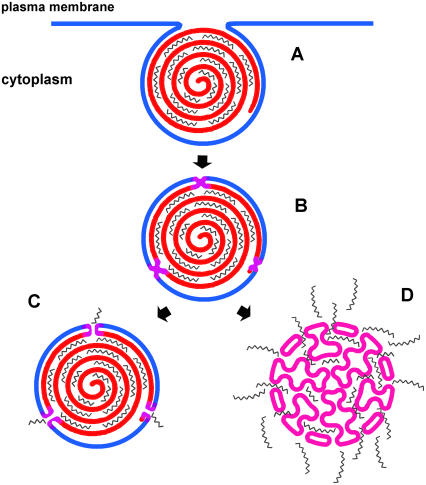
In summary, in this study we established that the hydrophobic core of cationic phospholipid mixtures can strongly modulate their DNA delivery efficacy. This efficacy correlates well with the phase behavior of the mixtures of cationic lipids with negatively charged membrane lipids, as well as with the extent of DNA unbinding from lipoplexes induced by negatively charged membrane lipids; however, transfection activity did not show a consistent relationship with cationic-anionic membrane fusion. Together, these findings indicate that some step after membrane fusion is rate-limiting for transfection. They also emphasize the topological difference between the multilamellar lipid/DNA complexes and the single-bilayer cell membranes; efficient DNA delivery evidently involves substantial lipid mixing between cell membranes and the lipoplex and such processes would require persistent or multiple interactions. It appears that, in some proportion of the lipoplexes, a cell membrane lipid-triggered, lamellar→nonlamellar phase transition generates regions of high interfacial curvature, which facilitate the extensive unbinding of DNA that is required for efficient transfection by cationic lipids (Fig. 10).
FIGURE 10.
Cartoon representation of the cellular uptake of lipoplexes and DNA release. (A) Lipoplexes are internalized into the cytoplasm by endocytosis. (B) Lipoplexes fuse with the negatively charged cellular membrane; mixed lipoplexes are more fusogenic than those of single lipids, a phenomenon that is presumably related to nonideal mixing, domain formation, and/or higher probability for packing defects in the cationic lipid mixtures relative to single component bilayers. (C) In the case of those cationic lipid compositions for which the lipoplex retains its lamellar organization after fusion, DNA release is constrained to its outmost layer of the lipoplex and is thus inefficient. (D) In the case of cationic lipid compositions for which the nonlamellar phase develops after fusion, DNA release is facilitated and much more extensive, with the consequence that transfection is more efficient.
Acknowledgments
We thank Yaeko Hiyama (Northwestern University) for synthesis of some cationic lipids. We are grateful to Ruby MacDonald and Lucy Gutierrez (Northwestern University) for help in electrophoresis. We thank Theodore Jardetzky (Northwestern University) for use of his microplate reader.
Supported by National Institutes of Health grants No. GM52329, GM74429, and GM57305. BioCAT is a National Institutes of Health-supported Research Center, through grant No. RR08630. DND-CAT is supported by the E.I. DuPont de Nemours & Co., The Dow Chemical Company, the U.S. National Science Foundation through grant No. DMR-9304725, and the State of Illinois through the Department of Commerce and the Board of Higher Education grant No. IBHE HECA NWU 96. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Energy Research under contract No. W-31-102-Eng-38. We also acknowledge the use of the small-angle x-ray diffraction instrument at the Central Facilities supported by the Materials Research Science and Engineering Center program of the National Science Foundation (grant No. DMR-0076097) at the Material Research Center of Northwestern University, and the dynamic light scattering instrument in the Keck Biophysics Facility at Northwestern University.
References
- 1.Lobo, B. A., J. A. Vetro, D. M. Suich, R. N. Zuckermann, and C. R. Middaugh. 2003. Structure/function analysis of peptoid/lipitoid: DNA complexes. J. Pharm. Sci. 92:1905–1918. [DOI] [PubMed] [Google Scholar]
- 2.Gaucheron, J., T. Wong, E. F. Wong, N. Maurer, and P. R. Cullis. 2002. Synthesis and properties of novel tetraalkyl cationic lipids. Bioconjug. Chem. 13:671–675. [DOI] [PubMed] [Google Scholar]
- 3.Niculescu-Duvaz, D., J. Heyes, and C. J. Springer. 2003. Structure-activity relationship in cationic lipid mediated gene transfection. Curr. Med. Chem. 10:1233–1261. [DOI] [PubMed] [Google Scholar]
- 4.Subramanian, M., J. M. Holopainen, T. Paukku, O. Eriksson, I. Huhtaniemi, and P. K. J. Kinnunen. 2000. Characterisation of three novel cationic lipids as liposomal complexes with DNA. Biochim. Biophys. Acta Biomembr. 1466:289–305. [DOI] [PubMed] [Google Scholar]
- 5.Jaaskelainen, I., B. Sternberg, J. Monkkonen, and A. Urtti. 1998. Physicochemical and morphological properties of complexes made of cationic liposomes and oligonucleotides. Int. J. Pharm. 167:191–203. [Google Scholar]
- 6.Song, Y. K., F. Liu, S. Y. Chu, and D. X. Liu. 1997. Characterization of cationic liposome-mediated gene transfer in vivo by intravenous administration. Hum. Gene Ther. 8:1585–1594. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh, Y. K., S. S. Visweswariah, and S. Bhattacharya. 2002. Advantage of the ether linkage between the positive charge and the cholesteryl skeleton in cholesterol-based amphiphiles as vectors for gene delivery. Bioconjug. Chem. 13:378–384. [DOI] [PubMed] [Google Scholar]
- 8.Wang, L., and R. C. MacDonald. 2004. New strategy for transfection: mixtures of medium-chain and long-chain cationic lipids synergistically enhance transfection. Gene Ther. 11:1358–1362. [DOI] [PubMed] [Google Scholar]
- 9.Felgner, P. L., T. R. Gadek, M. Holm, R. Roman, H. W. Chan, M. Wenz, J. P. Northrop, G. M. Ringold, and M. Danielsen. 1987. Lipofection—a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA. 84:7413–7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenzweig, H. S., V. A. Rakhmanova, T. J. McIntosh, and R. C. MacDonald. 2000. O-alkyl dioleoylphosphatidylcholinium compounds: the effect of varying alkyl chain length on their physical properties and in vitro DNA transfection activity. Bioconjug. Chem. 11:306–313. [DOI] [PubMed] [Google Scholar]
- 11.Koynova, R., and R. C. MacDonald. 2004. Columnar DNA superlattices in lamellar o-ethylphosphatidylcholine lipoplexes: mechanism of the gel-liquid crystalline lipid phase transition. Nano Lett. 4:1475–1479. [Google Scholar]
- 12.Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival—application to proliferation and cyto-toxicity assays. J. Immunol. Methods. 65:55–63. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald, R. C., G. W. Ashley, M. M. Shida, V. A. Rakhmanova, Y. S. Tarahovsky, D. P. Pantazatos, M. T. Kennedy, E. V. Pozharski, K. A. Baker, R. D. Jones, H. S. Rosenzweig, K. L. Choi, R. Z. Qiu, and T. J. McIntosh. 1999. Physical and biological properties of cationic triesters of phosphatidylcholine. Biophys. J. 77:2612–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koynova, R., and R. C. MacDonald. 2003. Mixtures of cationic lipid O-ethylphosphatidylcholine with membrane lipids and DNA: phase diagrams. Biophys. J. 85:2449–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammersley, A. P., S. O. Svensson, M. Hanfland, A. N. Fitch, and D. Hausermann. 1996. Two-dimensional detector software: from real detector to idealised image or two-θ scan. High Press. Res. 14:235–248. [Google Scholar]
- 16.Koynova, R., and R. C. MacDonald. 2003. Cationic O-ethylphosphatidylcholines and their lipoplexes: phase behavior aspects, structural organization and morphology. Biochim. Biophys. Acta Biomembr. 1613:39–48. [DOI] [PubMed] [Google Scholar]
- 17.Xu, Y. H., and F. C. Szoka. 1996. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 35:5616–5623. [DOI] [PubMed] [Google Scholar]
- 18.Zelphati, O., and F. C. Szoka. 1996. Mechanism of oligonucleotide release from cationic liposomes. Proc. Natl. Acad. Sci. USA. 93:11493–11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharya, S., and S. S. Mandal. 1998. Evidence of interlipidic ion-pairing in anion-induced DNA release from cationic amphiphile-DNA complexes. Mechanistic implications in transfection. Biochemistry. 37:7764–7777. [DOI] [PubMed] [Google Scholar]
- 20.Struck, D. K., D. Hoekstra, and R. E. Pagano. 1981. Use of resonance energy-transfer to monitor membrane-fusion. Biochemistry. 20:4093–4099. [DOI] [PubMed] [Google Scholar]
- 21.Friend, D. S., D. Papahadjopoulos, and R. J. Debs. 1996. Endocytosis and intracellular processing accompanying transfection mediated by cationic liposomes. Biochim. Biophys. Acta Biomembr. 1278:41–50. [DOI] [PubMed] [Google Scholar]
- 22.Zabner, J., A. J. Fasbender, T. Moninger, K. A. Poellinger, and M. J. Welsh. 1995. Cellular and molecular barriers to gene-transfer by a cationic lipid. J. Biol. Chem. 270:18997–19007. [DOI] [PubMed] [Google Scholar]
- 23.Radler, J. O., I. Koltover, T. Salditt, and C. R. Safinya. 1997. Structure of DNA-cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science. 275:810–814. [DOI] [PubMed] [Google Scholar]
- 24.Lasic, D. D., H. Strey, M. C. A. Stuart, R. Podgornik, and P. M. Frederik. 1997. The structure of DNA-liposome complexes. J. Am. Chem. Soc. 119:832–833. [Google Scholar]
- 25.Francescangeli, O., M. Pisani, V. Stanic, P. Bruni, and T. M. Weiss. 2004. Evidence of an inverted hexagonal phase in self-assembled phospholipid-DNA-metal complexes. Europhys. Lett. 67:669–675. [Google Scholar]
- 26.Koltover, I., T. Salditt, J. O. Radler, and C. R. Safinya. 1998. An inverted hexagonal phase of cationic liposome-DNA complexes related to DNA release and delivery. Science. 281:78–81. [DOI] [PubMed] [Google Scholar]
- 27.Smisterova, J., A. Wagenaar, M. C. A. Stuart, E. Polushkin, G. ten Brinke, R. Hulst, J. B. F. N. Engberts, and D. Hoekstra. 2001. Molecular shape of the cationic lipid controls the structure of cationic lipid/dioleylphosphatidylethanolamine-DNA complexes and the efficiency of gene delivery. J. Biol. Chem. 276:47615–47622. [DOI] [PubMed] [Google Scholar]
- 28.Koynova, R., L. Wang, Y. Tarahovsky, and R. C. MacDonald. 2005. Lipid phase control of DNA delivery. Bioconjug. Chem. 16:1335–1339. [DOI] [PubMed] [Google Scholar]
- 29.Gennis, R. B. 1989. Biomembranes: Molecular Structure and Function. Springer-Verlag, New York.
- 30.Stegmann, T., and J. Y. Legendre. 1997. Gene transfer mediated by cationic lipids: lack of a correlation between lipid mixing and transfection. Biochim. Biophys. Acta Biomembr. 1325:71–79. [DOI] [PubMed] [Google Scholar]
- 31.Mui, B., Q. F. Ahkong, L. Chow, and M. J. Hope. 2000. Membrane perturbation and the mechanism of lipid-mediated transfer of DNA into cells. Biochim. Biophys. Acta Biomembr. 1467:281–292. [DOI] [PubMed] [Google Scholar]
- 32.Pires, P., S. Simoes, S. Nir, R. Gaspar, N. Duzgunes, and M. C. P. de Lima. 1999. Interaction of cationic liposomes and their DNA complexes with monocytic leukemia cells. Biochim. Biophys. Acta Biomembr. 1418:71–84. [DOI] [PubMed] [Google Scholar]
- 33.Leventis, R., and J. R. Silvius. 1990. Interactions of mammalian-cells with lipid dispersions containing novel metabolizable cationic amphiphiles. Biochim. Biophys. Acta. 1023:124–132. [DOI] [PubMed] [Google Scholar]
- 34.Harvie, P., F. M. P. Wong, and M. B. Bally. 1998. Characterization of lipid DNA interactions. I. Destabilization of bound lipids and DNA dissociation. Biophys. J. 75:1040–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simberg, D., D. Danino, Y. Talmon, A. Minsky, M. E. Ferrari, C. J. Wheeler, and Y. Barenholz. 2003. Phase behavior, DNA ordering and size instability of cationic lipoplexes: relevance to optimal transfection activity. J. Liposome Res. 13:86–87. [DOI] [PubMed] [Google Scholar]
- 36.Congiu, A., D. Pozzi, C. Esposito, C. Castellano, and G. Mossa. 2004. Correlation between structure and transfection efficiency: a study of DC-Chol-DOPE/DNA complexes. Colloids Surf. B Biointerfaces. 36:43–48. [DOI] [PubMed] [Google Scholar]
- 37.Ross, P. C., M. L. Hensen, R. Supabphol, and S. W. Hui. 1998. Multilamellar cationic liposomes are efficient vectors for in vitro gene transfer in serum. J. Liposome Res. 8:499–520. [Google Scholar]
- 38.Rakhmanova, V. A., T. J. McIntosh, and R. C. MacDonald. 2000. Effects of dioleoylphosphatidylethanolamine on the activity and structure of O-alkyl phosphatidylcholine-DNA transfection complexes. Cell. Mol. Biol. Lett. 5:51–65. [Google Scholar]
- 39.Tarahovsky, Y. S., R. Koynova, and R. C. MacDonald. 2004. DNA release from lipoplexes by anionic lipids: correlation with lipid mesomorphism, interfacial curvature, and membrane fusion. Biophys. J. 87:1054–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mouritsen, O. G., K. Jorgensen, and T. Honger. 1995. Permeability of lipid bilayers near the phase transition. In Permeability and Stability of Lipid Bilayers. E. A. Disalvo and S. A. Simon, editors. CRC Press, Boca Raton, FL.
- 41.Papahadjopoulos, D., K. Jacobson, S. Nir, and T. Isac. 1973. Phase-transitions in phospholipid vesicles—fluorescence polarization and permeability measurements concerning effect of temperature and cholesterol. Biochim. Biophys. Acta. 311:330–348. [DOI] [PubMed] [Google Scholar]
- 42.Blok, M. C., L. L. M. Vandeenen, and J. Degier. 1976. Effect of gel to liquid-crystalline phase-transition on osmotic behavior of phosphatidylcholine liposomes. Biochim. Biophys. Acta. 433:1–12. [DOI] [PubMed] [Google Scholar]
- 43.Marsh, D., A. Watts, and P. F. Knowles. 1976. Evidence for phase boundary lipid—permeability of tempo-choline into dimyristoylphosphatidylcholine vesicles at phase transition. Biochemistry. 15:3570–3578. [DOI] [PubMed] [Google Scholar]
- 44.Langner, M., and S. W. Hui. 1993. Dithionite penetration through phospholipid-bilayers as a measure of defects in lipid molecular packing. Chem. Phys. Lipids. 65:23–30. [DOI] [PubMed] [Google Scholar]
- 45.Lamson, M. J., L. G. Herbette, K. R. Peters, J. H. Carson, F. Morgan, D. C. Chester, and P. A. Kramer. 1994. Effects of hexagonal phase induction by dolichol on phospholipid membrane-permeability and morphology. Int. J. Pharm. 105:259–272. [Google Scholar]
- 46.Kronke, M. 1999. Biophysics of ceramide signaling: interaction with proteins and phase transition of membranes. Chem. Phys. Lipids. 101:109–121. [DOI] [PubMed] [Google Scholar]
- 47.Alonso, A., F. M. Goni, and J. T. Buckley. 2000. Lipids favoring inverted phase enhance the ability of aerolysin to permeabilize liposome bilayers. Biochemistry. 39:14019–14024. [DOI] [PubMed] [Google Scholar]