Abstract
α-Hemolysin (HlyA) is an extracellular protein toxin (117 kDa) secreted by Escherichia coli that targets the plasma membranes of eukaryotic cells. We studied the interaction of this toxin with membranes using planar phospholipid bilayers. For all lipid mixtures tested, addition of nanomolar concentrations of toxin resulted in an increase of membrane conductance and a decrease in membrane stability. HlyA decreased membrane lifetime up to three orders of magnitude in a voltage-dependent manner. Using a theory for lipidic pore formation, we analyzed these data to quantify how HlyA diminished the line tension of the membrane (i.e., the energy required to form the edge of a new pore). However, in contrast to the expectation that adding the positive curvature agent lysophosphatidylcholine would synergistically lower line tension, its addition significantly stabilized HlyA-treated membranes. HlyA also appeared to thicken bilayers to which it was added. We discuss these results in terms of models for proteolipidic pores.
INTRODUCTION
Escherichia coli remains the most common gram-negative bacterial species isolated from infections in hospitalized patients, and it is a frequent cause of extraintestinal diseases such as infections of the urinary tract, pneumonia, and meningitis, which sometimes lead to severe forms of septicemia (1). The immediate cause of these diseases is a 117-kDa protein toxin, α-hemolysin (HlyA), produced and secreted only by virulent strains of these bacteria (2).
HlyA is considered to be the prototype of a family of toxins called RTX (repeat in toxin), a series of protein toxins that contain a number of glycine- and aspartate-rich nonapeptide tandem repeats with a consensus sequence X-Leu-X-Gly-Gly-X-X-Gly-Asp-Asp-Asp near their C-terminal ends. This family includes Enterohemorrhagic O:157 hemolysin, E. coli hemolysin, the leukotoxin of Pasteurella haemolytica, the hemolysin and leukotoxin of Actinobacillus, the bifunctional adenylato-cyclase-hemolysin of Bordetella pertussis, and the hemolysins of Proteus vulgaris, Morganella morganii, and Moraxella bovis. All of these toxins have 30–50% sequence identity to E.coli α-HlyA and share genetic and structural features (3).
Comparison of hydrophobic properties of all six toxins indicates the presence of a conserved cluster of nine contiguous amphiphilic helices, located in the N-terminal half of the molecule, which would be involved in pore formation in the target membranes (4).
Lipid bilayer experiments with asolectin membranes demonstrated that HlyA increases membrane conductance by many orders of magnitude in a concentration-dependent fashion. Single-channel recordings revealed that HlyA induces formation of channels with mean conductance of ∼400 pS in NaCl or 500 pS in KCl bathing solution (5).
Menestrina et al. reported that HlyA forms pores in planar lipid bilayers composed of phosphatidylcholine/phosphatidylethanolamine (5:1) and does not require the presence of negatively charged lipids in the membrane. They suggest that either a single HlyA molecule or an aggregate reassembled in solution can form a pore. Once bound to a membrane, HlyA behaves as an intrinsic protein, and the pore formed in the planar bilayer (either in the open or in the closed configuration) is not in rapid equilibrium with the bulk solution and thus will not detach from the lipid film by thorough perfusion with toxin-free solution. The pore has a large conductance, cation selectivity, and a complex gating mechanism, fluctuating among fully open states, a low-conductance state, and a closed state (6,7).
However, an alternative proposal that HlyA disrupts membranes by either a detergent-like activity or a monolayer-specific disruption had been suggested by Soloaga et al. (8). Other results that contradict the concept of static pore formation were obtained by Moayeri and Welch (9), who observed that the degree of osmotic protection of erythrocytes afforded by protectants of varying sizes depends on the amount of the toxin applied and the duration of the assay, suggesting that HlyA creates a lesion with a very small initial size that increases in apparent diameter over time.
We found that pores formed in lipid bilayers by HlyA are not as well defined as typical proteinaceous ionic channels, and properties of these pores depend on membrane composition. These data suggest that initial HlyA pores, like pores formed by a number of other amphiphilic peptides (10), promote the local breakdown of the membrane bilayer structure, creating a pore with a surface formed partially or completely by polar lipid headgroups.
MATERIALS AND METHODS
Protein isolation, analysis, and storage
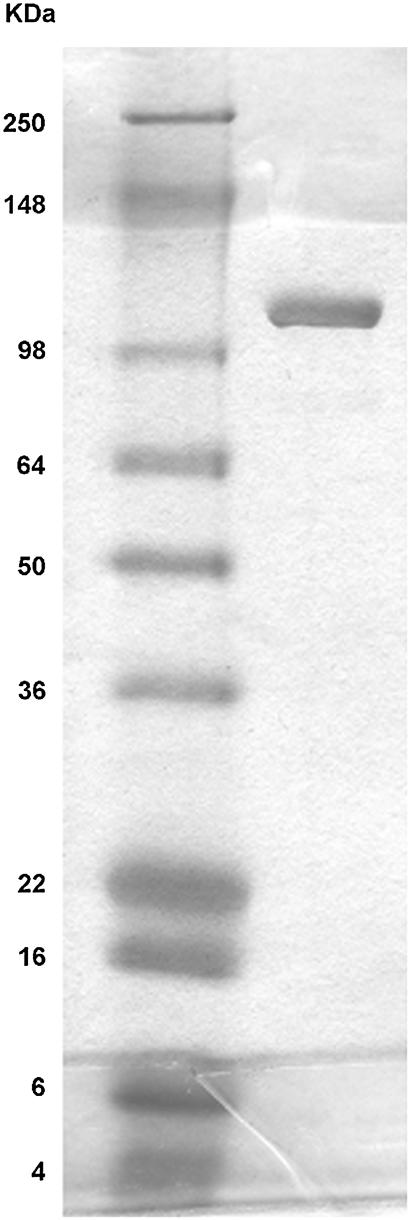
E. coli α-hemolysin (α-HlyA) was purified from culture filtrates of overproducing strains of E.coli WAM 1824, kindly provided by R. A. Welch. The cultures were grown to late phase in LB to an optical density of 0.8–1.0 at 600 nm. The cells were pelleted, and the supernatant was concentrated and partially purified by precipitation with 20% cold ethanol at the isolectric point (pH 4.5). Purity was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 4–12% gel at 135 V for 90 min with BSA as standard (Fig. 1). Proteins were visualized by stain with a sypro orange protein stain gel (Molecular Probes, Eugene, OR). Wet gel was scanned, and data were analyzed using Image Gauge software (Fuji Film, Stamford, CT). It was determined that the 117-kDa protein fraction was 85% of the total proteins in this sample. Preparation hemolytic activity was ∼104 HU/ml. Stock solution of the protein was stored frozen in tubes at −70°C.
FIGURE 1.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of a 4–12% gel run at 135 V for 90 min. (Left lane) Molecular weight markers. (Right lane) HlyA, 10 μg.
Phospholipid membrane formation, current measurements, and chemicals
We used bilayers formed from a 20 mg/ml lipid solution in decane by the Mueller-Rudin technique (11). To study membrane conductance and to measure membrane lifetimes under different voltages, we used a Delrin chamber (Warner Instruments, Hamden, CT).
For measurements of membrane specific capacitance and surface tension we used a symmetrical chamber similar to one described earlier, milled from Teflon with glass windows on both sides (12). The hole in the 0.05-mm-thick Teflon partition was 0.4 mm in diameter. A custom-made video microscope with 200× magnification was used for visual control of bilayer membrane (BLM) formation and quality. Both compartments were filled with 2.5-ml volumes of BLM bathing solution.
Ag/AgCl electrodes (In Vivo Metric, Ukiah, CA) were connected with the membrane bathing solution through 200-μl pipette tips with long thin ends filled with 2% agarose in 0.2 M KCl. An electrode placed in the cis compartment was connected to virtual ground. Because protein was added mostly to the cis compartment, we assigned the sign of the potential to the cis side. The other electrode, in the trans compartment, was connected to the input of an Axopatch 200B voltage-clamp amplifier (Axon Instruments, Union City, CA). After protein addition, the solution was stirred for 30–60 s using a miniature magnetic stirrer.
Lipids, asolectin (polar extract), dioleoylphosphatidylcholine (DOPC), dioleoylphosphatidylethanolamine (DOPE), and oleoyl-LysoPC (LPC) were purchased from Avanti Polar Lipids (Alabaster, AL). Heptane, hexadecane, and salts were purchased from Sigma Chemical (St. Louis, MO). Solutions were prepared using deionized, bidistilled water. Data were recorded on a computer disk using Axon Instruments Digidata 1322 A/D converter and pCLAMP 8.1 software.
Measurements of surface tension and membrane stability
Membrane capacitance, C, was determined from the capacitive current in response to the application of 25 V/s linear voltage ramps with an amplitude 25 mV. Specific capacitance (capacitance per square centimeter of membrane) was calculated after subtraction of chamber capacitance (measured with the hole in the Teflon filled with a large drop of lipid solution). The area of the bilayers was estimated with an accuracy of ∼5%.
Surface tension σ was found by measuring the change in membrane capacitance under the given hydrostatic pressure gradient (13).
Bilayer lifetimes, tl, were defined as the time from a step in voltage to the onset of irreversible rupture of the membranes. In purely lipidic systems, irreversible breakdown of bilayers in an electric field results from the development of lipidic pores of an overcritical radius, which tend to spontaneous expansion (14,15). The experimental dependencies of the mean tl (averaged over no less than 10 measurements) on voltage applied, U, were fit with the theoretical expression based on the general theory for lipidic pore formation and phospholipid rupture under high electrical field. The dependence of the lifetime of the membrane on the applied voltage can be described by the theoretical expression:
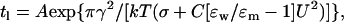 |
(1) |
where A is a preexponential factor dependent on some additional model assumptions, γ (linear tension of pore in the lipid bilayer) is the work of formation of the unit of the pore perimeter, k is the Boltzmann constant, T is the temperature in K, C is the specific capacitance of the membrane, σ is the surface tension, and ɛw = 80 and ɛm = 2 are the dielectric permittivity of water and membrane, respectively. Linear tension is a key parameter in lipidic pore development because it quantifies the work needed to form a unit of pore perimeter and gives a measure of the membrane's resistance to rupture (16). The least-squares method was applied to compute the values of γ and A, providing the best agreement between calculated and experimental lifetimes.
Differential scanning calorimetry
Multilamellar vesicles (MLV) from egg phosphatidylethanolamine (EPE) were made by vortex mixing of the dried lipid dispersed (15–20 mg/ml) in 10 mM Tris HCl, 150 mM NaCl buffer in absence or presence of HlyA at a lipid/protein ratio of 104. Dispersions were hermetically sealed in aluminum pans, and an empty double pan served as reference. The bilayer-to-hexagonal phase transition temperature of the lipid was measured using a DuPont model 910 calorimeter or DSC Polymer Laboratories equipment (Rheometric Scientific, Piscataway, NJ). The samples were analyzed at 5°C/min in a range of 20–90°C. The equipment was calibrated at a heating rate of 5°C/min using indium, lauric acid, and stearic acid (p.a.) as standards. All tests were repeated at least twice.
RESULTS
Conductance of lipid membranes treated with HlyA
Within 10–15 min after addition of 2.5 nM HlyA (final concentration) to the aqueous solution bathing a planar phospholipid BLM, the membrane conductance increased irrespective of lipid composition. In agreement with earlier studies of HlyA (5), in asolectin membranes, the time of delay between protein addition and conductance increase was significantly shorter than that in membranes formed from either PC or PE (single-component systems whose lipids comprise asolectin). Likewise, once initiated, the rate of the conductance increase was faster in asolectin than in membranes formed from either PC or PE.
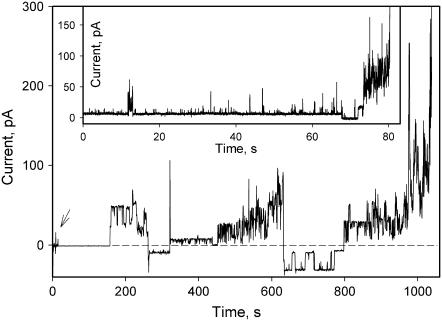
Pore formation was strongly potential dependent (Fig. 2). Although some increase in conductance was observed at all potentials, those positive on the side of protein addition caused the membrane conductance to increase much faster. Usually, one or two long-lasting conductance steps were followed by a noisier conductance increase, but quite often only noisy conductance fluctuations were observed (Fig. 2, inset).
FIGURE 2.
Effect of α-HlyA on the conductance of planar membranes. An arrow indicates addition of 2.5 nM α-HlyA to the aqueous solution bathing the cis side of a phospholipid bilayer membrane formed from asolectin. Inset shows current trace from another experiment that resulted in noisy conductance fluctuations only. The bathing solution contains 150 mM NaCl and 10 mM HEPES, pH 7.4. Membrane potential was switched several times between +50 mV and −50 mV and kept constant during positive or negative fragments of current traces. Dashed line indicates zero current.
Membranes formed from DOPC and DOPC/1% LPC were relatively stable in the presence of HlyA at positive transbilayer potentials. The records were quite noisy and, compared to asolectin, had a much smaller fraction of square “box”-like patterns reminiscent of recordings of well-defined ionic channels. Addition of HlyA to DOPE membranes also resulted in membrane breakage soon after a small number of protein molecules had presumably incorporated into membranes at positive potential, as judged by conductance increments. For all phospholipid compositions, we always observed a variety of conductance increments: step-like, short spikes, and noisy fluctuations.
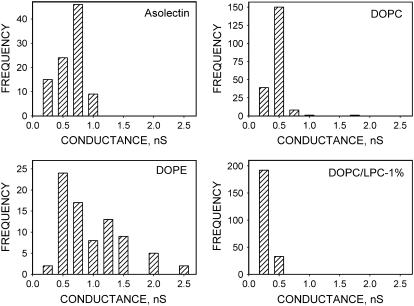
Amplitude histograms of step-like conductances depended on the lipid composition (Fig. 3), showing a broad distribution for DOPE membranes and relatively narrow peaks for DOPC, DOPC/LPC, and asolectin, membranes with maxima at 0.40, 0.34, 0.22, and 0.75 nS, respectively. An interesting observation is that the asolectin used in our assays contained 22.1% phosphatidylethanolamine, 18.4% phosphatidylinositol, 45.7% phosphatidylcholine, 6.9% phosphatidic acid, and 0% LPC as reported by Avanti Polar Lipid.
FIGURE 3.
Amplitude histograms of single-channel conductance induced by α-HlyA in membranes of various lipid composition: asolectin, DOPC, DOPE, and DOPC/LPC, 1%. Each histogram represents channels with lifetimes longer than 0.1 s collected from several experimental recordings before the record become noisy. Bathing solution contains 150 mM NaCl, 10 mM HEPES, pH 7.4. Potential on membrane +50 mV.
Monovalent cation permeability
Because of membrane instability, it was difficult to have enough time to wash out unbound protein by perfusion for conventional recording of current/voltage (I/V) dependencies in HlyA-modified membranes. However, at low HlyA concentration, the rate of conductance increase was slow, and it was possible to get almost undistorted I/V dependencies using a fast recording protocol. In asymmetric NaCl solutions, HlyA-permeabilized membranes were predominantly Na+ selective with no significant differences in selectivity for different lipid compositions (Table 1).
TABLE 1.
Na/Cl selectivity of HlyA-permeabilized membranes
| Lipid | reversal potential | PNa/PCl |
|---|---|---|
| Mean ± SD (mV) | ||
| DOPC | 18.6 ± 2.3 | 5.8 |
| DOPE | 19.4 ± 2.1 | 6.5 |
| DOPC/LPC (99/1 wt %) | 15.9 ± 4.3 | 4.1 |
Membrane stability and linear tension
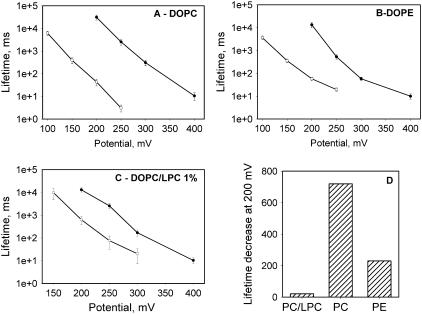
A significant decrease in the lifetimes of membranes after addition of HlyA was noted during the conductance measurements described above. To quantify this effect, we performed a series of experiments with application of different high potentials to membrane. Membranes were formed either in the presence or in the absence of HlyA at zero transmembrane potential. The potential was switched to a high level (150–400 mV) 3–5 min later, and the time between the rise of potential and the onset of BLM breakdown was measured. The decrease of membrane lifetime with HlyA treatment at different potentials was dependent on the membrane lipid composition (Fig. 4, A–C). The numerical values for membrane lifetime decrement caused by HlyA are shown in Fig. 4 D for DOPC, DOPE, and DOPC/LysoPC BLMs (at 200 mV).
FIGURE 4.
Potential dependence of α-HlyA-induced decreases in membrane lifetime. (A) Membrane formed from DOPC. (B) Membrane formed from DOPE. (C) Membrane formed from DOPC with 1% (w/w) LPC. Panels A–C show mean values ± SE of 10–15 experiments per point. Solid circles, control experiments; open circles, membranes in the presence of 2.5 nM α-HlyA membrane bathing solution: 150 mM NaCl, HEPES, pH 7.4. (D) Lifetime decrease at 200 mV for membranes formed from DOPC/LPC 1%, DOPC, and DOPE.
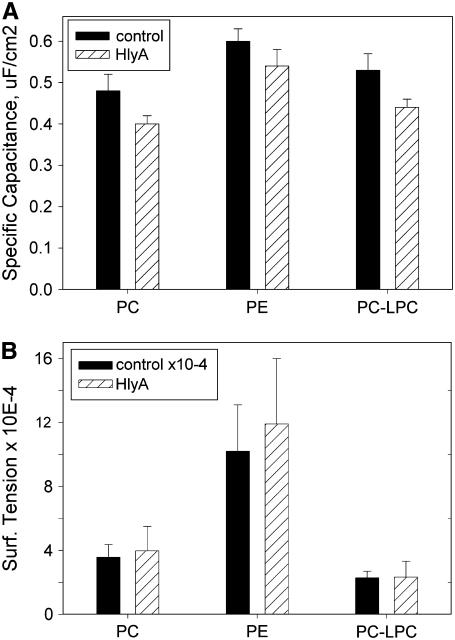
In order to evaluate the HlyA effect on BLM line tension, we also measured membrane specific capacitance and surface tension of BLM in the presence and absence of protein. We found that two-sided addition of HlyA to BLMs caused a statistically significant decrease in membrane specific capacitance but no significant change in membrane surface tension for all lipid compositions tested (Fig. 5, A and B).
FIGURE 5.
Effect of HlyA on membrane specific capacitance and surface tension. Membrane specific capacitance (A) and surface tension (B) were measured as described in Methods. Each bar represents mean ± SD of 27–36 measurements. Differences in BLM specific capacitance are statistically significant (Student's t-test, p < 0.01). Membranes were formed from DOPC, DOPE, or DOPC/LPC 1% in 0.1 M NaCl, 10 mM HEPES, pH 7.25 at 28 ± 1°C.
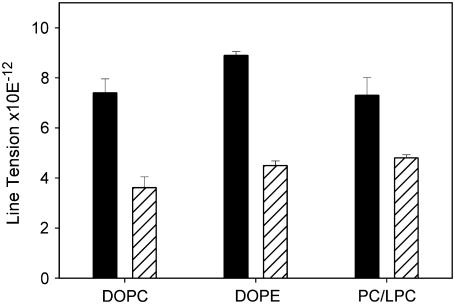
Least-square error fitting (16) of voltage dependencies shown in Fig. 4 demonstrate a significant decrease in line tension in the presence of HlyA for all membrane compositions (Fig. 6).
FIGURE 6.
Dependence of the line tension of membrane pores on lipid composition in the presence of HlyA. Black bars, control; gray bars, 2.5 nM α-HlyA.
Differential scanning calorimetry
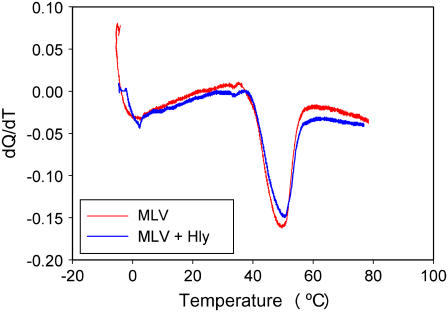
The shift in the bilayer-to-hexagonal HII phase transition temperature is taken as an indirect but sensitive measure of protein ability to enhance or reduce negative curvature strain. It is known that proteins inducing a negative curvature strain in the plane of the membrane decrease the lamellar-hexagonal phase transition temperature (TH), whereas those imposing positive curvature strain increase TH (17). Egg PE liposomes exhibited a TH of 49°C in the absence of protein. HlyA shifted TH only 3°C to a higher temperature even at high (104) lipid/protein molar ratio (Fig. 7).
FIGURE 7.
Representative DSC scans of EPE MLV. The heating scan rate was 5°C/min. Red line, control; blue line, vesicles formed in presence of HlyA.
DISCUSSION
There is substantial debate as to just how HlyA creates lesions in target cell membranes. Our data demonstrate that HlyA interaction with membranes results in formation of pores that have similarity to both typical proteinaceous ionic channels, marked by well-defined selectivity, voltage dependence, and distinct open-channel conductance (18), and to lipidic pores, which have parameters more dependent on membrane composition (19). Even in asolectin membranes, only a few step-like conductance increments were observed before noisy conductance fluctuations become responsible for the most of the increase in conductance (Fig. 2). An amplitude histogram of HlyA pores (Fig. 3) demonstrates a significant dependence of pore size on membrane phospholipid composition, indicating a possible participation of lipid in the structure of a pore. The same seems to be true for the cation/anion selectivity of HlyA-treated membranes. We found that the cation/anion selectivity of HlyA in BLM formed from noncharged lipids is little dependent on lipid composition (Table 1) but is 1.5–2 times lower than reported by Benz et al. (5) for HlyA in negatively charged asolectin membranes. Because selectivity of a water-filled pore is determined by charges on the pore wall, these data are in an agreement with the model of a lipid-lined pore. However, we cannot rule out the possibility that the pore is purely proteinaceous but sensitive to the nature of the surrounding lipids. The data on membrane lifetime measurements are certainly consistent with models featuring proteolipidic pores. Just as with other peptides and proteins studied earlier (sPB1-F2, Bax, cleaved Bcl-xl, and P828, the amphiphilic helix of HIV gp-41 (16,19–21)), HlyA induced a decrease in membrane lifetime that is adequately described by the well-established theory of lipidic pores induced by transmembrane potential (14,15). This phenomenon is not predicted by models featuring insertion and opening of a purely proteinaceous channel, which have little effect on membrane lifetimes as verified quantitatively for one example (21).
We found that HlyA is a very potent membrane destabilizer that lowers the energy barrier for pore development (Fig. 6). It is relatively easy to explain how small detergent molecules or lipids that mix with membrane lipids in high ratio decrease pore energy by altering the intrinsic curvature of the membrane monolayer; these molecules form micelles, and the edge of a lipidic pore is thought to be half a micelle rotated about the center of the pore. However, the mechanism of action of large proteins such as HlyA may be different from a simple, positive curvature-related action of small molecules. As we suggested earlier for another membrane-destabilizing protein, sPB1-F2 (21), the hydrophilic/hydrophobic mismatch in lipid bilayer packing around the protein may reduce the energy cost of subsequent pore enlargement that is equivalent to a pore line tension decrease measured experimentally. In the case of HlyA, this mechanism is supported by the finding that the thermotropically driven lamellar-to-inverted-hexagonal phase transition of PE membrane (a measure of HlyA effect on the intrinsic monolayer curvature) is not significantly changed in the presence of HlyA (Fig. 7).
Interestingly, in contrast to other known membrane-destabilizing proteins, HlyA causes a statistically significant decrease in the membrane specific capacitance for all three lipid compositions tested (Fig. 5 A). A likely explanation of this effect is an increase of the mean membrane thickness as a result of reorganization of hydrocarbon chains on interaction with HlyA (essentially straightening out chains). This 18% thickening can be compared to the much smaller changes induced by other proteolipidic pore-forming proteins: 0.43 ± 0.003 to 0.45 ± 0.005 for Bax (16) and 0.61 ± 0.01 to 0.61 ± 0.01 for PB1-F2 (21) in the absence and present of protein, respectively.
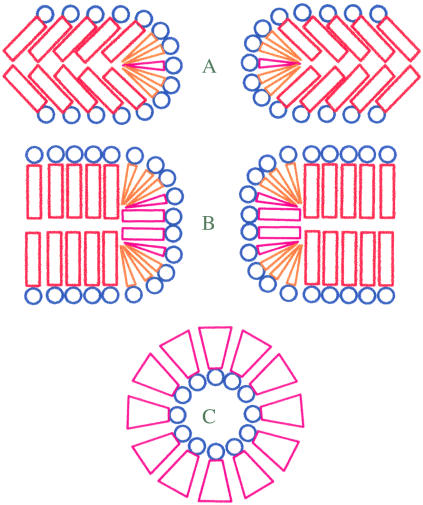
Another interesting feature of the interaction of HlyA with membranes is the increased stability of LPC-containing BLMs. Usually, LPC is a membrane-destabilizing agent (22). In the case of Bax, LPC promotes Bax-dependent liposome leakage (16). In contrast, the numerical values for membrane lifetime decrement caused by HlyA are 719 and 230 for PC and PE BLMs, respectively (at 200 mV), and only 21 for PC/LPC BLMs (Fig. 4 D). Because it was shown that HlyA binding to PC and PE liposomes is practically independent of lipid composition (23), it is unlikely that lipid dependence of HlyA binding is responsible for the observed differences in membrane stability. The most feasible explanation of this paradox in terms of molecular shape and membrane curvature seems to be related to HlyA-induced increase in membrane thickness. Generally, facilitation of pore expansion and membrane breakdown by molecules with positive spontaneous curvature (such as lysophospholipids) is explained by the decrease in energy per unit length (line tension) required to form pore edges of a short large pore whose integral geometric monolayer curvature is positive, whereas nonlamellar lipids with negative intrinsic curvature (such as diacylglycerol and PE) increase free energy (per unit length of the edge) for pore creation. In a toroid, positive curvature is found perpendicular to the plane of the membrane, but negative curvature is present in the plane of the membrane all around the pore (24,25). With the increased length of the pore, overall curvature of the pore shifts to negative values (Fig. 8), making long pores energetically less favorable in membranes that contain lipids with positive spontaneous curvature. In other words, LPC may prevent the formation of the parts of the toroid that are predominantly negative in curvature and thereby stabilize the membrane.
FIGURE 8.
Schematics of the energetically favorable localization of different lipids in short and long pores. (A and B) Cross section of short and long pores orthogonal to the plane of membrane. (C) Cross section of the middle part of the pore in plane of membrane. Lamellar lipids are distinguished by red cylinders in hydrophobic region; lipids with positive intrinsic curvature are distinguished by coral cones; lipids with negative intrinsic curvature are distinguished by magenta inverted cones.
The dependence of HlyA pores on lipid composition in planar lipid bilayers is consistent with the data on liposome permeability. PE and cholesterol promote high leakage in the presence of HlyA (26). Furthermore, LPC, which can negate the bilayer curvature effect of PE, also neutralized the lytic activity promoted by PE in liposomes (26) and inverted hexagonal (HII) phase lipid such as PE (27) and promotes pore formation by HlyA. Indeed, cholesterol and PE, lipids that promote negative spontaneous monolayer curvature, may promote HlyA pores by exactly same mechanism by which LPC inhibits HlyA pores, i.e., promoting long protein-supported lipidic pores with overall negative geometric curvature by fitting into the arcs of the pore that have predominantly negative curvature. DOPE with small headgroups favors spontaneous curvature or surface tension if the lamellar organization is imposed, as, e.g., in planar lipid bilayers. This generally affects peptide-lipid interaction (28). In the case of alamethicin, these phospholipids shift the single-channel probability distribution toward higher-conductance substates, and this is strongly correlated with spontaneous curvature or surface tension. In phosphatidylethanolamine and phosphatidylcholine binary lipid bilayers, increasing the mole fraction of the former impedes alamethicin binding to the lipids, in agreement with the larger peptide concentrations that are needed to develop similar conductances (29). This larger free energy of binding is linearly correlated with the spontaneous curvature or surface tension of the PE-rich bilayers concomitant with a local bilayer thinning that could be induced by the peptide (30).
No lamellar lipids are known to influence the avidity of alamethicin for lipid membranes, and this has been related to the effects of curvature on alamethicin channel formation (30). However, alamethicin channels are much more uniform in size and dynamic behavior than HlyA pores, and alamethicin does not affect the stability of planar phospholipid BLMs as does HlyA. A previous x-ray diffraction measurement showed that alamethicin adsorbed on the surface has the effect of thinning the bilayer in proportion to the peptide concentration. This may be because, locally, lipids whose headgroups are adjacent to alamethicin need to bend their chains below the peptide to fill in the volume of hydrocarbon under the peptide (31). A theoretical study showed that the energy cost of membrane thinning can indeed lead to peptide insertion: the thickness change with the percentage of insertion is consistent with the assumption that the hydrocarbon region of the bilayer matches the hydrophobic region of the inserted peptide, and the membrane deformation energy is the major driving force for the alamethicin insertion transition (32). However, pore formation by alamethicin, which induces membrane thinning, is inhibited by the incorporation of PE (33). This is very similar to the effect seen for magainin (34).
Several mechanisms have been proposed for amphipathic peptide-induced membrane permeabilization, and considerable controversy still exists. Alamethicin forms a “barrel-stave channel,” i.e., a bundle of membrane-spanning helices aligned with the polar side chains oriented toward the center. Cecropin and dermaseptin are suggested to disrupt bilayer organization by a “carpet-like mechanism,” where a monolayer of surface-lying peptides covers the membrane surface (35,36). Bax-type proapoptotic proteins (37), the C-terminal peptide from human immunodeficiency virus envelope glycoprotein (20), and PB1-F2 from influenza A (another proapoptotic protein) (21) form lipidic pores that are promoted by positive curvature and inhibited by negative curvature. Thus, there may be more ways to build a lipidic pore than have been imagined in the classical model.
Acknowledgments
We thank Ludmila Bezrukov for protein gel electrophoresis and Piotr Kuzmin for illuminating discussions.
L.S.B. is a member of Carrera del Investigador CIC-PBA, Argentina. V.H. is a fellow of CONICET, Argentina. This research is supported by the intramural program of the National Institute of Child Health and Human Development, National Institutes of Health.
We dedicate this paper to the memory of our great friend Gianfranco Menestrina.
References
- 1.Cavalieri, S., G. Boach, and I. Snyder. 1984. Escherichia coli hemolysin: characteristics and probable role in pathogenicity. Microb Rev. 4:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welch, R., E. P. Dellinger, B. Minshew, and S. Falkow. 1981. Haemolysin contributes to virulence of extra intestinal E. coli infections. Nature. 294:665–667. [DOI] [PubMed] [Google Scholar]
- 3.Cotte, J. G. 1992. Structural and functional relationships among the RTX toxin determinants of Gram negative bacteria FEMS. Microbiol. Rev. 88:137–162. [DOI] [PubMed] [Google Scholar]
- 4.Menestrina, G., C. Moser, S. Pellet, and R. Welch. 1994. Pore formation by Escherichia coli hemolysin (HlyA) and other member of the RTX toxins family. Toxicology. 87:249–267. [DOI] [PubMed] [Google Scholar]
- 5.Bentz, R., A. Shmid, W. Wagner, and W. Goebel. 1989. Pore formation by the Escherichia coli hemolysin evidence for an association-dissociation equilibrium of the pore-forming aggregates. Infect. Immun. 57:887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menestrina, G., N. Mackman, I. Holland, and S. Bhakdi. 1987. Escherichia coli haemolysin forms voltaje-dependent ion channels in lipid membranes. Biochim. Biophys. Acta. 905:109–117. [DOI] [PubMed] [Google Scholar]
- 7.Ropele, M., and G. Menestrina. 1989. Electrical properties and molecular architecture of the channel formed by Escherichia coli hemolysin in planar lipid membranas. Biochim. Biophys. Acta. 985:9–18. [DOI] [PubMed] [Google Scholar]
- 8.Soloaga, A., M. Veiga, L. Garcia-Segura, H. Ostolaza, R. Brasseur, and F. Goñi. 1999. Insertion of Escherichia coli alpha-haemolysin in lipid bilayers as a non-transmembrane integral protein: prediction and experiment. Mol. Microbiol. 31:1013–1024. [DOI] [PubMed] [Google Scholar]
- 9.Moayeri, M., and R. Welch. 1994. Effect of temperature, time and toxin concentration on lesion formation by the Escherichia coli hemolysin. Infec Immunol. 62:4124–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crucian, R. A., J. L. Barker, S. Durrell, G. Raghunathan, H. H. R. Guy, M. Zasloff, and E. Standley. 1992. Magainin 2, a natural antibiotic from frog skin, forms ion channels in lipid bilayer membranes. Eur. J. Pharmacol. 226:287–296. [DOI] [PubMed] [Google Scholar]
- 11.Mueller, P., D. Rudin, T. Tien, and W. Westcott. 1962. Reconstitution of cell membrane in vitro and its transformation into an excitable system. Nature. 194:979–980. [DOI] [PubMed] [Google Scholar]
- 12.Chanturiya, A., M. Whitaker, and J. Zimmerberg. 1999. Calcium Induced fusion of sea urchin secretory vesicles with planar phospholipid bilayer membranes. Mol. Membr. Biol. 16:89–94. [DOI] [PubMed] [Google Scholar]
- 13.Sukharev, S. I., V. A. Klenchin, S. M. Serov, L. V. Chernomordik, and Yu. A. Chizmadzhev. 1992. Electroporation and electrophoretic DNA transfer into cells. The effect of DNA interaction with electropores. Biophys. J. 63:1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abidor, I. G., V. B. Arakelyan, L. V. Chernomordik, Y. A. Chizmadzhev, V. F. Pastushenko, and M. R. Tarasevich. 1979. Electrical breakdown of BLM: Main experimental facts and their qualitative discussion. Bioelectrochem. Bioenerget. 6:37–52. [Google Scholar]
- 15.Chernomordik, L. V., and Yu. A. Chizmadzhev. 1989. Electroporation of bilayer lipid membranes: phenomenology and mechanism. In Electroporation and electrofusion in cell biology. E. Neumann, A. Sowers, and C. Jordan, editors. Plenum Press, New York. 181–192.
- 16.Basañez, G., A. Nechushtan, O. Drozhinin, A. Chanturiya, E. Choe, S. Tutt, K. A. Wood, Y. T. Hsu, J. Zimmerberg and R. J. Youle 1999. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc. Natl. Acad. Sci. USA. 96:5492–5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wieprecht, T., M. Dathe, R. Epand, M. Beyermann, E. Krause, W. Maloy, D. MacDonald, and M. Bienert. 1997. Influence of the angle subtended by the positively charged helix face on the membrane activity of amphipathic, antibacterial peptides. Biochemistry. 36:12869–12880. [DOI] [PubMed] [Google Scholar]
- 18.Sansom, M. S. P. 1991. The biophysics of peptide models of ion channels. Prog. Biophys. Mol. Biol. 55:139–235. [DOI] [PubMed] [Google Scholar]
- 19.Basañez, G., J. Zhang, N. Chau, G. Maksaev, I. Maksev, V. Frolov, T. Brandt, J. Burch, M. Harwick, and J. Zimmerberg. 2001. Pro-apoptotic cleavage products of Bcl-xL form cytochrome c–conducting pores in pure lipid membranes. J. Biol. Chem. 276:31084–31091. [DOI] [PubMed] [Google Scholar]
- 20.Chernomordik, L., A. Chanturiya, E. SussToby, E. Nora, and J. Zimmerberg. 1994. An amphipathic peptide from the C-terminal region of the human immunodeficiency virus envelope glycoprotein causes pore formation in membranes. J. Virol. 68:7115–7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chanturiya, A. N., G. Basañez, U. Schubert, P. Henklein, J. W. Yewdell, and Z. Zimmerberg. 2004. PB1–F2, an influenza A virus encoded pro-apoptotic mitochondrial protein, creates variably-sized pores in planar lipid membranes. J. Virol. 78:26304–26312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epand, R. 1985. Diacylglycerols, lysolecithin, or hydrocarbons markedly alter the bilayer to hexagonal phase transition temperature of phosphatidylethanolamines. Biochemistry. 24:7092–7095. [DOI] [PubMed] [Google Scholar]
- 23.Ostolaza, H., and F. Goñi. 1995. Interaction of the bacterial protein toxin alpha-haemolysin with model membranes: protein binding does not always lead to lytic activity. FEBS Lett. 371:303–306. [DOI] [PubMed] [Google Scholar]
- 24.Valcarcel, C., M. Dalla Serra, C. Potrich, I. Bernhart, M. Tejuca, D. Martinez, F. Pazos, M. Lanio, and G. Menestrina. 2001. Effects of lipid composition on membrane permeabilization by sticholysin I and II, two cytolysins of the sea anemone Stichodactyla helianthus. Biophys. J. 80:2761–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang, L., T. Weiss, R. Lehrer, and H. Huang. 2000. Crystallization of antimicrobial pores in membranes: magainin and protegrin. Biophys. J. 79:2002–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostolaza, H., B. Bartolome, I. Ortiz de Zarate, F. de la Cruz, and F. M. Goñi. 1993. Release of lipid vesicle contents by the bacterial protein toxin alpha-haemolysin. Biochim. Biophys. Acta. 1147:81–88. [DOI] [PubMed] [Google Scholar]
- 27.Cattor, R. S. 1999. The influence of membrane lateral pressures on simple geometric models of protein conformational equilibria. Chem. Phys. Lipids. 101:45–56. [DOI] [PubMed] [Google Scholar]
- 28.Cafiso, D. S. 1999. Interaction of natural and model peptides with membranes. Curr. Topics Membranes. 48:197–228. [Google Scholar]
- 29.Duclohier, H., and H. Wroblewski. 2001. Voltage-dependent pore formation and antimicrobial activity by alamethicin and analogues. J. Membr. Biol. 184:1–12. [DOI] [PubMed] [Google Scholar]
- 30.Lewis, J. R., and D. S. Cafisso. 1999. Correlation between the free energy of a channel-forming voltage-gated peptide and the spontaneous curvature of bilayer lipids. Biochemistry. 38:5932–5938. [DOI] [PubMed] [Google Scholar]
- 31.McIntosh, T., and S. Simon. 2006. Roles of bilayer material properties in function and distribution of membrane proteins. Annu. Rev. Biophys. Biomol. Struct. 35:177–198. [DOI] [PubMed] [Google Scholar]
- 32.He, K., S. Ludtke, W. Heller, and H. Huang. 1996. Mechanism of alamethicin insertion into lipid bilayers. Biophys. J. 71:2669–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heller, W., K. He, S. J. Ludtke, T. A. Harroun, and H. W. Huang. 1997. Effect of changing the size of lipid headgroup on peptide insertion into membranes. Biophys. J. 73:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuzaki, K., O. Murase, N. Fujii, and K. Miyajima. 1996. An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry. 35:11361–11368. [DOI] [PubMed] [Google Scholar]
- 35.Bechinger, B. 1997. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J. Membr. Biol. 156:197–211. [DOI] [PubMed] [Google Scholar]
- 36.Matsuzaki, K., K. Sugishita, N. Ishibe, M. Ueha, S. Nakata, K. Miyajima, and R. Epand. 1998. Relationship of membrane curvature to the formation of pores by magainin 2. Biochemistry. 37:11856–11863. [DOI] [PubMed] [Google Scholar]
- 37.Basañez, G., J. Sharpe, J. Galanis, T. Brandt, J. Hardwick, and J. Zimmerberg. 2002. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J. Biol. Chem. 277:49360–49365. [DOI] [PubMed] [Google Scholar]