Abstract
Potassium channels display a high conservation of sequence of the selectivity filter (SF), yet nature has designed a variety of channels that present a wide range of absolute rates of K+ permeation. In KcsA, the structural archetype for K channels, under physiological concentrations, two K+ ions reside in the SF in configurations 1,3 (up state) and 2,4 (down state) and ion conduction is believed to follow a throughput cycle involving a transition between these states. Using free-energy calculations of KcsA, Kv1.2, and mutant channels, we show that this transition is characterized by a channel-dependent energy barrier. This barrier is strongly influenced by the charges partitioned along the sequence of each channel. These results unveil therefore how, for similar structures of the SF, the rate of K+ turnover may be fine-tuned within the family of potassium channels.
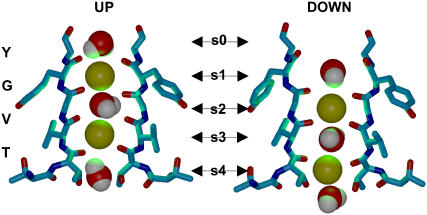
Potassium channels are proteins mediating flow of K+ ions across nerve membranes (1). In these channels, the selectivity filter (SF) is the central structural element, conserved in all K+ channels, that promotes high throughput and selection of potassium over other ionic species (2,3). The SF is a narrow pore 12 Å long, formed by a tetrameric arrangement of the carbonyl groups of a highly conserved sequence TVGYG. The construct provides five binding sites (S0–S4) that can be occupied by monovalent cations or by water molecules (3,4). Under physiological concentrations, approximately two K+ ions occupy the SF (2). They reside in specific configurations S1–S3 and S2–S4, with a water molecule intercalated between (Fig. 1). The up and down states are shown to have the same occupancy (3) and the ionic conduction through the SF is believed to follow a simple cycle involving transition between these microstates (4–7).
FIGURE 1.
Up and down states of the selectivity filter of K channels in the canonical conformations. The transition of the potassium ions (yellow) from up→down state occurring in the MD simulations of KcsA is not observed for Kv1.2.
The x-ray structures of voltage-gated channels KvAP (8) and Kv1.2 (9) confirmed that the canonical structure of the SF is conserved among K+ channels. However, potassium channels display a wide range of absolute rates of ion permeation (1,10). This raises the possibility that ion transport may imply different molecular mechanisms involving the SF. The new resolved Kv1.2 structure provides us with a unique opportunity to compare the properties of the ions in the SF to those in KcsA.
We have first carried out molecular dynamics (MD) simulations of KscA and Kv1.2 inserted in fully hydrated phospholipid bilayers. Both MD runs started with the SF occupied by two K+ ions in the up state (Fig. 1). For KcsA, a transition from up to down state occurs within 250 ps, similar to what was observed in other simulations (4,11,12). In contrast, no transition was observed for Kv1.2 during a 5 ns-simulation time (13). These equilibrium simulations for the canonical structure indicate unexpectedly that the transitions from one state to the other are channel-dependent.
The aim of this study is to thoroughly examine the energetics involved in this transition. To monitor the barrier for ion translocation, we have estimated the free energies of K+ translocations between the up and down states for KcsA, Kv1.2, and for structures where specific mutageneses of charged residues were performed. All together, our results suggest that the electrostatic environment of the selectivity filter has profound effect on this barrier.
The free-energy profiles (FEP) were computed using steered-MD simulations on the full atomistic systems, and the Jarzynski equation (14). Two pathways, e.g., dragging the upper ion from position S1 to position S2 for the up→down transition and dragging the lower ion from position S4 to position S3 for the down→up transition were considered. Since motions of the ions and the water molecules in the SF are concerted, the individual work values of the two pathways may be combined to estimate an overall FEP for the transition between the up and down states (see Supplementary Material).
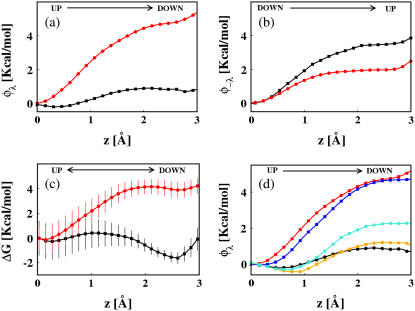
The components of the total free energy profile corresponding to each of the pathways are displayed in Fig. 2, a and b. For each channel the up→down transition and down→up transition are not equivalent. More importantly, there is a striking difference between KscA and Kv1.2 most noticeable for the up→down transition. The overall FEP for the transition between the up and down states shown in Fig. 2 c indicates that the SF, in its canonical form, presents a large stability of the up state in Kv1.2, while the down state is somewhat more favorable for KcsA.
FIGURE 2.
Free energy profiles for translocation of K+ ion pairs between up and down microstates. Decomposition of the up→down (a) and down→up (b) profiles for KcsA (black) and Kv1.2 (red). (c) Overall FEP for KcsA (black) and Kv1.2 (red). Bars represent the statistical errors. Component of the FEP for the up→down transition for Kv1.2 (red), Kv1.2 with the gating charges (Arg) turned off (blue), Kv1.2 with substitutions by residues of the KcsA sequence (orange), Kv1.2 with Val370/Glu mutation (cyan) and KcsA (black).
The analysis presented so far suggests that the sequence and the conformation of the SF are not the only factors influencing the transitions between the up and down states of the K+ ion pairs. In the following, we monitor these transitions when the electrostatic environment of the SF, through the partition of charges, is changed. We confine our investigation to the up→down transition, for which the difference between the components of FEP is the largest. We first consider the case of a mutant of Kv1.2 channel (Kv1.2a) for which the charges of one S4 helix (absent in the KcsA channel) are turned off. In this case, the barrier changed slightly compared to the wild-type (Fig. 2 d), which indicates that charge distributions around the SF may monitor to a certain extent the free-energy barrier for K+ translocation.
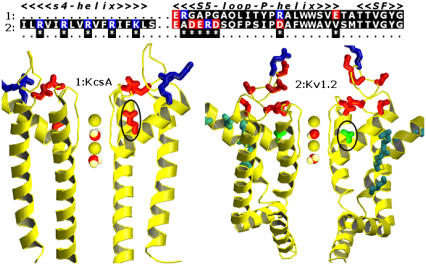
The distribution of charged residues around the SF, particularly in the fragment including the S5 loop and the P-helix (Fig. 3), shows large differences between KcsA and Kv1.2. The effect of such charge distributions on the up→down transition was investigated by considering the Kv1.2b mutant, for which charged residues in this region were substituted by those of KcsA. Fig. 2 d shows that theses mutations significantly reduce the transition barrier, leading almost to the profile of the KcsA channel. We have investigated further the effect of charges near the SF, noting that a Val370 residue in Kv1.2 corresponds to a Glu71 in KcsA (Fig. 3). The single mutation Val370/Glu in the Kv1.2 channel reduces significantly the up→down barrier. This provides compelling evidence that charge distributions in the channel mouth control the free-energy barrier for K+ translocation within the SF.
FIGURE 3.
Representation of the sequences (up) and the structures (down) of KcsA (left) and Kv1.2 (right). Stars design the position of residues considered for mutation; basic residues (blue) and acidic residues (red). Arg charges of S4 are drawn in cyan. Note that Val370 in Kv1.2, located near the TVGYG motif and highly conserved among Kv channels corresponds to Glu71 in KcsA (black circles).
So far, we have clearly identified a major factor affecting the energetic of K+ translocation: e.g., the electrostatic environment of the SF. What consequence may have the up-down free-energy barriers on the channel's conductance? A model for ion translocation through the SF, proposed by Mackinnon et al. (15), implies that conductance is maximized when the occupancies (free-energy) of the up and down states are identical. If the free-energy barrier for ion translocation is modified, the model predicts a reduction in conductance. Kinetic modeling of ion conduction in the SF predicts similarly a conductance reduction if the translocation between these states is a rate-limiting step (16). One expects therefore, based on our results, that ion channel sequences are likely to fine-tune the rate of K+ turnover within the SF.
Changes of the electrostatic environment of the SF have wide consequences on the conductive properties of K channels. For instance, KcsA conductance is strongly influenced by negative charges at the channel's intracellular mouth (17), and extracellular acidification reduces ionic currents in Kv channels (18–20). It is often suggested that alteration of K channel conductance results from the charge modulation of the accessibility of K+ ions to the SF. Here, our results point to the role of the electrostatic environment in changing the free-energy barrier for ion translocation within the SF.
Note, however, that measured conductance of a specific channel depends on several factors not considered in the present study, among which the applied transmembrane voltage, K+ concentration in the extra- and intracellular regions, and pH preclude here direct comparison to experimental observations. Note also that we have limited our investigation to the canonical conformation of the SF. Intermittent or long-lived flips of carbonyls of the SF may occur for some channels as previously observed in simulations (21,22) and in experiments (23). The study of the incidence of such flips on the conductive properties of the SF is underway.
In summary, our results indicate clearly that the immediate environment of the K+ ions provided by the carbonyls lining the selectivity filter of potassium channels does not control alone the K+ translocation between the up and down microstates, the crucial step in the conduction cycle. To a large extent, the electrostatic environment monitors this turnover, influencing therefore the conductive properties of potassium channels.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Acknowledgments
Calculations were performed at the Centre Informatique National de l'Enseignement Supérieur.
This work was supported by a Centre National de la Recherche Scientifique postdoctoral fellowship to W.T.
References
- 1.Hille, B. 1992. Ionic Channels of Excitable Membranes. Sinauer, Sunderland, MA.
- 2.Zhou, Y., and R. MacKinnon. 2003. The occupancy of ions in the K+ selectivity filter: charge balance and coupling of ion-binding to a protein conformational change underlie high conduction rates. J. Mol. Biol. 333:965–975. [DOI] [PubMed] [Google Scholar]
- 3.Zhou, M., and R. MacKinnon. 2004. A mutant KcsA K+ channel with altered conduction properties and selectivity filter ion distribution. J. Mol. Biol. 338:839–846. [DOI] [PubMed] [Google Scholar]
- 4.Bernèche, S., and B. Roux. 2000. Molecular dynamics of KcsA K+ channel in a bilayer membrane. Biophys. J. 78:2900–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernèche, S., and B. Roux. 2003. A microscopic view of ion conduction through the potassium channel. Proc. Natl. Acad. Sci. USA. 100:8644–8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domene, C., and M. S. P. Sansom. 2003. Potassium channel, ions, and water: simulation studies based on the high resolution x-ray structure of KcsA. Biophys. J. 85:2787–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giorgetti, A., and P. Carloni. 2003. Molecular modeling of ion channels: structural predictions. Curr. Opin. Chem. Biol. 7:150–156. [DOI] [PubMed] [Google Scholar]
- 8.Jiang, Y., A. Lee, J. Chen, V. Ruta, M. Cadene, B. T. Chait, and R. MacKinnon. 2003. X-ray structure of a voltage-dependent K+ channel. Nature. 423:33–41. [DOI] [PubMed] [Google Scholar]
- 9.Long, B. S., E. B. Campbell, and R. MacKinnon. 2005. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 309:897–903. [DOI] [PubMed] [Google Scholar]
- 10.Isacoff, E. Y., Y. N. Jan, and L. Y. Jan. 1991. Putative receptor for the cytoplasmic inactivation gate in the Shaker K+ channel. Nature. 353:86–90. [DOI] [PubMed] [Google Scholar]
- 11.Shrivastava, I. H., and M. S. P. Sansom. 2000. Simulations of ion permeation through a potassium channel: molecular dynamics of KcsA in a phospholipid bilayer. Biophys. J. 78:557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidoni, L., V. Torre, and P. Carloni. 2000. Water and potassium dynamics inside the KcsA K+ channel. FEBS Lett. 477:37–42. [DOI] [PubMed] [Google Scholar]
- 13.Treptow, W., and M. Tarek. 2006. Environment of the gating charges in the Kv1.2 Shaker potassium channel. Biophys. J. 90:L64–L66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park, S., F. Khalili-Araghi, E. Tajkhorshid, and K. Schulten. 2003. Free energy calculation from steered molecular dynamics simulations using Jarzynski's identity. J. Chem. Phys. 119:3559–3566. [Google Scholar]
- 15.Morais-Cabral, J. H., Y. Zhou, and R. MacKinnon. 2001. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 414:37–42. [DOI] [PubMed] [Google Scholar]
- 16.Mafé, S., J. Pellicer, and J. Cevera. 2005. Kinetic modeling of ion conduction in KcsA potassium channel. J. Chem. Phys. 122:204712. [DOI] [PubMed] [Google Scholar]
- 17.Nimigean, C. M., J. S. Chappie, and C. Miller. 2003. Electrostatic tuning of ion conductance in potassium channels. Biochemistry. 42:9263–9268. [DOI] [PubMed] [Google Scholar]
- 18.Starkus, J. G., Z. Varga, R. Schönherr, and S. H. Heinemann. 2003. Mechanisms of the inhibition of Shaker potassium channels by protons. Pflugers Arch. Eur. J. Physiol. 447:44–54. [DOI] [PubMed] [Google Scholar]
- 19.Kwan, D. C. H., D. Fedida, and S. J. Kehl. 2006. Single channel analysis reveals different modes of Kv1.5 gating behavior regulated by changes of external pH. Biophys. J. 90:1212–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kehl, S. J., C. Eduljee, D. C. H. Kwan, S. Zhang, and D. Fedida. 2002. Molecular determinants of the inhibition of Kv1.5 potassium currents by external protons and Zn2+. J. Physiol. 541:9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernèche, S., and B. Roux. 2005. A gate in the selectivity filter of potassium channels. Structure. 13:591–600. [DOI] [PubMed] [Google Scholar]
- 22.Capener, C. E., P. Proks, F. M. Ashcroft, and M. S. P. Sansom. 2003. Filter flexibility in a mammalian K channel: models and simulations of Kir6.2 mutants. Biophys. J. 84:2345–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cordero-Morales, J. F., L. G. Cuello, Y. Zhao, V. Jogini, D. M. Cortes, B. Roux, and E. Perozo. 2006. Molecular determinants of gating at the potassium-channel selectivity filter. Nat. Struct. Mol. Biol. 13:311–318. [DOI] [PubMed] [Google Scholar]