Abstract
The mechanosensitive channel of large conductance acts as a biological “emergency release valve” that protects bacterial cells from hypoosmotic stress. Although structural and functional studies and molecular dynamic simulations of this channel have led to several models for the structural transitions that occur in the gating process, inconsistencies linger and details are lacking. A previous study, using a method coined as the “in vivo SCAM”, identified several residues in the channel pore that were exposed to the aqueous environment in the closed and opening conformations. Briefly, the sulfhydryl reagent MTSET was allowed to react, in the presence or absence of hypoosmotic shock, with cells expressing mechanosensitive channel of large conductance channels that contained cysteine substitutions; channel dysfunction was assessed solely by cell viability. Here we evaluate the MTSET-induced functional modifications to these mechanosensitive channel activities by measuring single channel recordings. The observed changes in residue availability in different states, as well as channel kinetics and sensitivity, have allowed us to elucidate the microenvironment encountered for a number of pore residues, thus testing many aspects of previous models and giving a higher resolution of the pore domain and the structural transitions it undergoes from the closed to open state.
INTRODUCTION
The ability to sense and respond to mechanical stimuli is important for essentially all forms of life. It is not surprising then, that channels responding to mechanical force have now been found in a large number of organisms from archaea to vertebrates (1,2). Some of the best studied are the bacterial mechanosensitive channels (3), which gate in response to tension in the lipid membrane (4). There are three bacterial channel genes that have been identified to encode mechanosensitive activity, the mechanosensitive channel of large conductance (MscL), small conductance (MscS), and K+ regulated (MscK) (3,5,6). MscL was the first to be isolated and is to date perhaps the best studied of all mechanosensitive channels.
Early work showed that the open pore of the MscL channel is on the order of 30 Å (7). Ions, small molecules, and even some proteins can be released through the pore with little selectivity except by size. In a bacterial cell, the channel discharges small molecules to release internal pressure and protect the cell from lysis due to hypoosmotic shock (often call osmotic downshock) (5). Two transmembrane domains were postulated (3), and random mutagenesis found that mutations affecting channel gating tended to cluster on one face of the predicted alpha helical first transmembrane domain (TM1) (8). When a single residue in TM1, G22, was substituted with 19 other amino acids, it was found that mutations to more hydrophilic or charged residues were found to often cause the cell hosting the mutated MscL to have a severe slowed- or no-growth phenotype often times accompanied by a severe decrease in viability, presumably from the channel gating inappropriately and discharging the proton motive force and cell turgor (9). These studies indicated that not only was TM1 vitally important in the kinetics of the channel, but that simply adding a charge or increasing the hydrophilicity of a single residue could drastically affect channel gating and even compromise viability of the cell expressing it.
A major advance in understanding came when the Mycobacterium tuberculosis MscL was crystallized to 3.5 Å resolution (10). The crystal structure shows a homopentameric channel with two α-helical transmembrane domains. TM1 lines the pore, whereas TM2 surrounds the outside of the channel. There is a 4 Å opening in the center of the structure that is insignificant compared to the predicted open pore of 30 Å. Therefore, the authors of the crystal structure postulated that the structure was fully or mostly closed. The crystal structure gave a framework for many of the previous findings derived from both in vivo and in vitro studies of the Escherichia coli MscL. Specific attention was focused on understanding what the open-channel structure might look like and how the channel transitions to obtain an open pore. Two main theories were put forth by Sukharev and Guy (11) and by Perozo and Martinac (12,13). The former model was the first to suggest tilting of the helices as the channel opened, thus matching the thinning lipid bilayer stretched by tension (11). The proposed tilting of the helices allowed TM1 alone to form the aqueous pore of the channel, and thus correlated well with the random mutagenesis study demonstrating a clustering of substitutions that effect severe phenotypes in TM1. The model also utilized crosslinking, disulfide-trapping experiments, and computer modeling to predict the open and transitional states of the channel (14). Subsequently, Perozo and Martinac presented a model based on electron paramagnetic resonance (EPR) studies (13). These data were consistent with the tilting of the transmembrane domains and the pore lined by only the first transmembrane domains. However, the residues calculated to line the pore were entirely different. This latter model predicted that TM1 rotated in a relatively drastic clockwise manner during gating, whereas the former model indicated a counter-clockwise rotation, thus leading to an almost 180° discrepancy in the orientation of the predicted pore-lining residues.
To determine the residues exposed in the closed and opening states, we utilized the Substituted Cysteine Accessibility Method (SCAM) (15) that we adapted (16) and modified to be a more rapid in vivo assay (17). This method relied on a previously generated and extensively characterized cysteine library (18) and the observation, discussed above, that adding a charge to a single residue within or near the pore, by using the positively-charged sulfhydryl reagent MTSET, can change the gating properties of a channel and, in many instances, severely decrease viability of cells that express it. The cysteine mutants that demonstrated an MTSET-dependent decreased-viability phenotype fell into three different groups: those that strictly require in vivo channel gating, effected by an osmotic downshock, to see the phenotype, those that show some MTSET-dependent decrease in viability without an osmotic downshock but require it to see the maximal phenotype, and those that do not require any downshock to see the MTSET-dependent phenotype. The latter residues were interpreted to compose a periplasmic vestibule, whereas the two former were predicted to be fully or partially buried within the complex and exposed only upon channel gating. This in vivo SCAM study gave support for a clockwise rotation predicted in the model derived from the EPR studies, and defined a number of residues that appear to constitute the pore of the open E. coli MscL channel. However, the precise manner in which the channel activity was modified by the MTSET reagent was not determined, and thus any changes in the transition from closed to open states not determined. In this study, we examine the functional modifications effected by MTSET treatment before and subsequent to channel activation by using the patch clamp technique and have found unexpected changes in channel kinetics and aqueous availability of some residues. Taken together, the data presented confirm many of our previous predictions as well as give new insight into the structural transitions that occur upon gating.
MATERIALS AND METHODS
Strains
E. coli strain PB104 (ΔmscL∷Cm) (19), was used to host the pB10b expression constructs (8,19,20) for electrophysiological analysis. Cells were routinely grown at 37°C using Lennox Broth and retention of the plasmid was ensured by the addition of 100 μg/ml ampicillin. The wild-type E. coli MscL and cysteine substituted MscL mutants were inserted into the plasmid pB10b and expression was induced using isopropyl β-D-thiogalactoside (IPTG). The cysteine mutant library was generated by Dr. Gal Levin as described previously (18).
Spheroplast preparation
E. coli giant spheroplasts were generated as described previously (21). A culture was grown overnight in Lennox broth (LB) plus 100 μg/ml ampicillin. In the morning, it was diluted 1:100 into 10 ml of the media and allowed to grow to an OD600 0.1 ∼ 0.2. Then the culture was diluted 1:10 in a total of 30 ml of media with 60 μg/ml of cephalexin. Cells were allowed to grow until the “snakes” were roughly 50–150 μm. Expression was induced with 1 mM IPTG for 5–15 min. The cells were harvested by centrifugation at 1500 rpm for 5 min and the supernatant was aspirated. 2.5 ml 0.8 M sucrose was then used to very gently resuspend the cells without pipetting. The following reagents were added in order: 125 μl of 1 M Tris Cl (pH 8); 120 μl of lysozyme (5 mg/ml); 30 μl of DNase 1 (5 mg/ml); 150 μl of 0.125 M Na EDTA (pH 7.8). The mixture was allowed to react for 5 min at room temperature and then stopped using 1 ml of an ice cold solution containing 0.7 M sucrose, 20 mM MgCl2, and 10 mM Tris Cl. This was then layered over two 13 × 100 mm culture tubes containing 7 ml of an ice cold solution composed of 0.8 M sucrose, 10 mM MgCl2, and 10 mM Tris Cl (pH 8). The spheroplasts were harvested by centrifugation of the tubes at 4°C for 2 min at 1500 rpm. All but roughly 300 μl of the supernatant was removed and the pellet resuspended in the remaining liquid. The spheroplasts were aliquoted and stored long term at −20°C. Preparations were usually used within a week.
Electrophysiology
E. coli giant spheroplasts were generated as above and used in patch-clamp experiments as described previously (22). Inside-out patches were examined at room temperature under symmetrical conditions using a buffer comprised of 200 mM KCl, 90 mM MgCl2, 10 mM CaCl2, and 5 mM HEPES adjusted to pH 6.0. Patches were excised and recordings were performed at −20 mV. Data were acquired at a sampling rate of 50 kHz with 10 kHz filtration using an AxoPatch 200B amplifier in conjunction with Axoscope software (Axon Instruments, Union City, CA); this less-than-normal filtration was used in an attempt to resolve more rapid events. A piezoelectric pressure transducer (World Precision Instruments, Sarasota, FL) was used to measure the pressure throughout the experiments. The tension sensitivity was determined by dividing MscL pressure threshold with that of MscS, as previously described (8,19,22); also as described within these references, the open dwell times were found to be relatively constant except at very high Po, presumably because with the exception of the opening of the first substate, all subsequent events are essentially membrane tension independent (23). To be certain that membrane tension played little role in the open dwell times, as in previous studies, only patches where the probability of channel opening was relatively low were used for this analysis. For experiments utilizing [2-(trimethylammonium) ethyl]methanethiosulfonate bromide (MTSET), 1 mM final concentration was added to the bath after seal formation for cytoplasmic exposure and 2 mM was added in backfill to the pipette for periplasmic exposure. MTSET was obtained from Toronto Research Chemicals (Ontario, Canada).
RESULTS
Utilizing structural models and results from the in vivo SCAM to functionally subdivide the pore domain
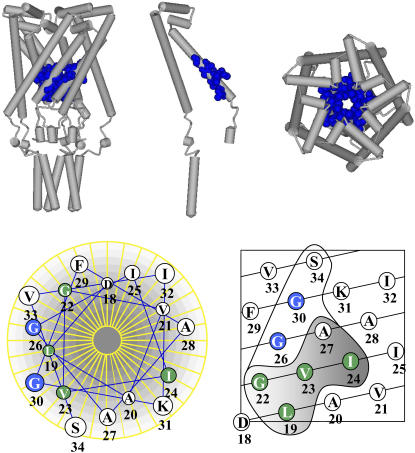
The in vivo SCAM (17) identified regions of the protein likely to be within the closed and opening pore and suggested a classification of the residues identified into three groups: those showing a phenotype in the presence of MTSET alone, those responding slightly to MTSET alone but showing maximum interaction with gating, and those strictly requiring gating to interact with MTSET. As seen in Fig. 1, this classification, combined with what is known of the structure of this region, further suggested a functional distinction. The residues that effect a phenotypic change when exposed to MTSET alone (such as G26, G30, and S34) appear to form a vestibule in the closed structure. The residues that are predicted to be buried and exposed only upon gating (L19, G22, V23, I24, and A27) are lower or more cytoplasmic in the structure. Since MTSET only reacts with cysteines exposed to the aqueous environment, these buried residues would surround the opening or fully open aqueous pore. Here, the electrophysiological analysis of the influence of MTSET on channels mutated at several of these positions was measured. From previous studies we know that the channels are functional as assayed by patch clamp (18). Although we cannot biochemically determine the number of subunits within the pentameric complex that have reacted with MTSET, we are attentive to the possibility that the complexes may be of variable saturation leading to heterogeneity of channel activity. Residues examined in this study that are predicted to be in the vestibule and those buried and exposed only upon gating are shown in Fig. 1 in blue and green, respectively; the relative bulk of the modified and unmodified side chains (R groups) are available in Supplementary Material.
FIGURE 1.
Schematic depictions of the E. coli MscL emphasizing the pore domain and specific residues that were targeted for substitutions. The upper panel shows a model for the closed MscL structure (11) based upon the crystal structure (10). The residues investigated by this study are highlighted in blue and shown in a cpk format. A side view (left), a single subunit of the pentameric complex (center), and top view are shown. The bottom panel presents an idealized helical wheel (left) and net (right) of the E. coli MscL first transmembrane domain. The residues encircled in the helical net were identified in the in vivo SCAM assay (17) as described in text. The residues within the shaded region were accessible to MTSET only upon channel gating by osmotic downshock. The residues that are further investigated by patch clamp in this study are colored dependent on whether the MTSET was accessible without (blue) or with (green) channel gating.
Modification of residues within the predicted periplasmic vestibule can effect dramatic changes in open dwell times: G26 and G30
In the in vivo SCAM, cells expressing G26C and G30C lost viability when exposed to MTSET, even if the channel was not stimulated to gate by osmotic downshock (Fig. 1 and Bartlett et al. (17)), suggesting that these residues are accessible to compounds in the periplasmic space even without channel gating. Out of the four residues that express this phenotype and are predicted to be within this periplasmic vestibule, G26C and G30C were chosen for study due to their proximity to the proposed constriction site of the closed channel. Because we utilize an inside-out excised patch configuration from native bacterial membranes, we employed a pipette back-fill approach to expose the periplasmic side of the channel to MTSET (19). Briefly, only the tip of the pipette is filled with the patch solution whereas the rest of the electrode is backfilled with the same solution containing MTSET. Therefore, the patch can be obtained and the “untreated” channel behavior observed before MTSET diffuses into the tip of the pipette and potentially reacts with the channel. The time course for this diffusion is typically ∼5–10 min. To expose the cytoplasmic side of the patch to MTSET, a concentrated solution of MTSET is simply added to the bath solution. Using these methods, the availability of a residue to the periplasmic (pipette) and cytoplasmic (bath) side of the channel can be tested.
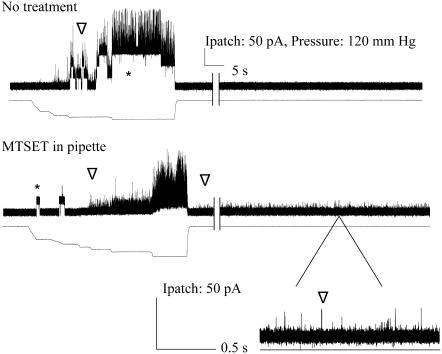
Upon formation of a giga-Ohm seal, no stimulus-independent activity is observed for either G26C or G30C. However, when MTSET was added exclusively to the periplasmic side of the G26C and G30C mutated MscLs, spontaneous openings were observed. In both instances, this gating was seen in a time-dependent manner, as expected from the back-fill procedure described above, and totally independent of any added membrane tension or other mechanical stimulation (Fig. 2, Fig. 3 bottom, and Table 1). For the G26C mutated channel, not only was spontaneous activity observed, but the open dwell time of this spontaneous activity was dramatically increased relative to the normal membrane-tension-dependent gating in the absence of MTSET. The first spontaneous channel activity in response to MTSET opened sporadically, residing in multiple substates (Fig. 2 A). With time, it “locked” into an open substate ∼4/5ths the fully open state (Fig. 2, B and C). Each subsequent channel, when resolved, appeared to do the same. In these experiments, the patch often exceeds the limit of the recording equipment after multiple openings. In contrast to G26C, the open dwell time for the G30C mutated channel decreased when exposed to MTSET on the periplasmic side and spontaneous gating was observed. (Fig. 3, bottom). For the G30C untreated mutant, the data fit well a three-component model in which the shortest τ for the open dwell constant is less than one, the second is slightly over 1, and the third is 5 ms. Although these values are less than that normally measured for wild-type MscL channels (<1, 7, and 38 ms (9)), it is greater than the spontaneous activities observed when treated with MTSET, where all measured open dwell times were significantly <1 ms, beyond the resolution of the equipment and settings used. Hence, although both residues, G26 and G30, appear to be exposed in the aqueous vestibule of the closed channel, and both channels show an increase in the probability of being open, reactivity of the cysteine substitutions at these positions have dramatically different effects on the open dwell times of the channel, with G26C locking into an open state, and G30C maintaining open states only very transiently.
FIGURE 2.
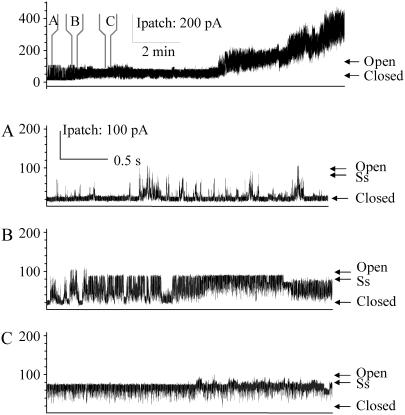
G26C locks in an open channel conformation when modified by MTSET placed on the periplasmic side. The uppermost trace shows G26C activity with MTSET added by backfilling the pipette as described in Materials and Methods, note that no pressure was applied before or during this trace. At the time points indicated by panels A, B, and C, the trace has been expanded. Panel A shows the first channel starting to open and the preference for substates and short open dwell times. Panel B shows the first channel being locked into an open state. Panel C shows the final preference of the channel for a common 4/5ths open substate (labeled as Ss).
FIGURE 3.
G30C shows gating-independent spontaneous activity when modified by MTSET placed on the periplasmic, but not bath cytoplasmic side. Single channel recordings of G30C are shown from top to bottom without treatment, with MTSET added to the bath, and with MTSET added to the periplasmic side of the patch by backfill, as described in text. Below each channel trace, the amount of pressure stimulation is shown. Note that in the top two traces, MscS activity (indicated by *) is seen before MscL activity (indicated by ∇). The arrows indicate the closed and the normal highest conducting open state for the MscL channels shown. In the final trace, G30C shows spontaneous gating with very short open dwell times; full openings are not often resolved.
TABLE 1.
Channel activity in the presence and absence of MTSET
| MscL | No treatment | MTSET in the pipette (periplasmic side) | MTSET in the bath (cytoplasmic side) |
|---|---|---|---|
| G30C | 1.62 ± 0.07 | Spontaneous; Activity independent | 1.65 ± 0.09 |
| G26C | 1.18 ± 0.04* | Spontaneous; Activity independent | Spontaneous; Activity dependent |
| I24C | 1.73 ± 0.06 | 1.23 ± 0.04; Activity independent† | 1.75 ± 0.07 |
| V23C | 0.90 ± 0.17 | Spontaneous; Activity dependent | 0.72 ± 0.07 |
| G22C‡ | 1.9 ± 0.9 | Spontaneous; Activity dependent | Spontaneous; Activity dependent |
| L19C§ | 0.78 ± 0.27 | Spontaneous; Activity dependent | Spontaneous; Activity dependent |
Shown is the threshold mean ± SE. The threshold is presented as the ratio of the pressure required to open MscL over the pressure required to open MscS in the same patch as previously described (27). This threshold ratio for wild-type MscL has been measured to be 1.55 ± 0.02 (18); a lower number indicates a more tension-sensitive channel. N ≥ 3. “Spontaneous” indicates conditions where activity is observed independent of a pressure stimulus, and changes in channel activity due to MTSET treatments are noted to be dependent or independent on channel gating.
Channel activity is not reliably observed at ambient oxidative conditions. These data (from Levin and Blount (18)) are recorded in the presence of 3–5 mM DTT.
The change in threshold was seen with the first opening of the channel.
Data from Yoshimura et al. (25).
Data from Batiza et al. (16).
G26C and G30C are not, however, accessible to the cytoplasmic side of the channel. As anticipated, when MTSET was added to the bath, the G26C MscL showed no spontaneous openings over the course of several minutes. However, immediately upon gating, the channel consistently showed a gating pattern in which it appears to gate spontaneously, shown in Fig. 4 (see also Table 1), and eventually obtains a “locked open” state, similar to that observed in Fig. 2. When MTSET is added to the bath of the G30C MscL, usually no change in the channel kinetics or pressure sensitivity was observed, even after multiple openings (Fig. 3, middle); a small amount of spontaneous activity, however, was observed under these conditions in one of eight patches (not shown). These data indicate that although neither residue is available to the cytoplasm whereas the channel is closed, only G26C is easily accessible to the aqueous pore subsequent to gating; hence, G26C is exquisitely sensitive to modification by MTSET in both the closed and open states.
FIGURE 4.
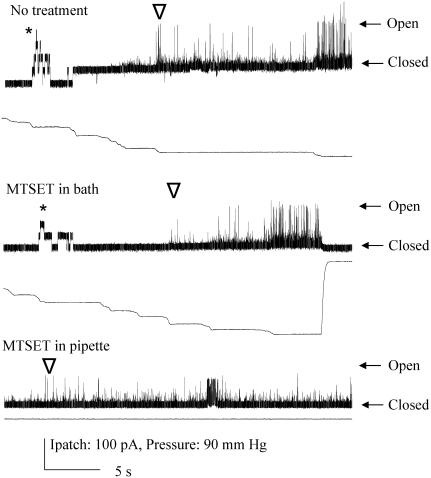
G26C is accessible from the cytoplasmic side only subsequent to channel gating. Single channel recordings of G26C were observed upon normal pressure stimulation (left; below the channel trace the amount of pressure stimulation is shown). After addition of MTSET to the bath (as indicated at arrow), no channel activity is observed, even after tens of seconds (middle of trace). Once channel gating is effected by pressure, channels are observed to gate spontaneously (right portion of trace).
Modification of residues within the predicted “buried” region of the pore can demonstrate activity-dependent changes in threshold sensitivity: V23V and I24C
The in vivo SCAM identified a number of residues postulated to be partially or fully buried within the channel. Of the five residues near the constriction point, V23 and I24 were examined here to further define this area of the channel. Cells expressing the V23C MscL mutation in the in vivo SCAM showed large differences in viability when treated with MTSET and gated by osmotic downshock. However, a slight phenotype was also observed in response to MTSET alone, suggesting that there is some reactivity of the reagent with the closed, unstimulated channel. Here, we found that when MTSET was applied to the periplasmic side of a V23C mutated channel, spontaneous substate openings were observed after stimulation (Fig. 5, bottom, and Table 1). Surprisingly, however, no activity was observed in the absence of stimulation, even after patches treated on the periplasmic side with MTSET were held for up to 20 min.
FIGURE 5.
V23C shows gating-dependent spontaneous activity when modified by MTSET placed on the periplasmic, but not cytoplasmic side. Channel traces of untreated (top) and treated (bottom) patches containing native membranes expressing V23A. In the bottom trace, treatment was effected by filling the pipette with MTSET-containing buffer. No channel activity is initially observed (left part of both traces), but can be induced by suction in the pipette (pressure is shown below each channel trace). In both traces MscS activity (indicated by *) and MscL activity (indicated by ∇) are observed (MscL rides up upon the MscS activity in the top trace). Note that in the bottom trace, subsequent to pressure-induced gating, a “flickery” channel with extremely short open dwell times is observed; this is shown in the bottom trace, which is a blowup of the indicated region. Hash marks represent ∼3–5 min removed to show durability of response.
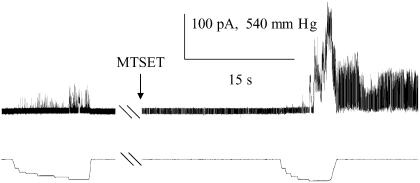
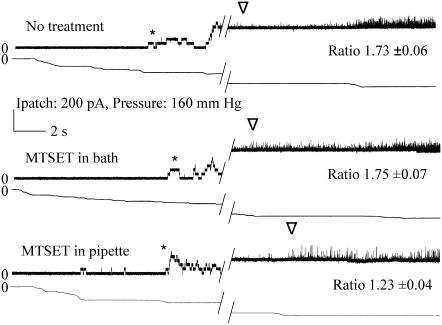
In contrast to V23, I24C absolutely required osmotic downshock in order for a phenotype to be observed (17). Here we find the threshold pressure to be significantly decreased upon the first opening of the channel; the pressure required to gate MscL compared to MscS decreased from 1.73 ± 0.06 to 1.23 ± 0.04 (Fig. 6, top and bottom, and Table 1).
FIGURE 6.
I24C shows gating-independent increased sensitivity when modified by MTSET on the periplasmic, but not cytoplasmic side. Single channel recordings of I24C illustrate the change in pressure sensitivity upon addition of MTSET on the periplasmic side. The “ratio”, which is the average mean ± SE from multiple patches, is derived from the relative pressures for opening MscL/MscS, as described in Materials and Methods; a larger ratio indicates a channel that requires more tension to open. Both the MscS (*) and MscL (∇) activities are shown. The first or topmost trace is I24C with no treatment. The second trace is the same patch with MTSET added to the cytoplasm and it has been exercised multiple times. The third trace is obtained where the pipette was backfilled with MTSET as described in Materials and Methods. Hatch marks indicate 20–30 s.
Neither V23C nor I24C appear to react with MTSET applied to the cytoplasmic side. Even when these channels were gated with MTSET present in the bath, neither kinetics nor threshold pressures changed (Fig. 6, middle and data not shown). One interpretation of these data is that these residues are exposed as the channel is opening, but is not entirely or efficiently exposed in the fully open state of the channel.
DISCUSSION
Previous studies screening randomly mutated libraries of MscL have demonstrated that mutations in and around the pore of the channel can lead to severely compromised growth and viability of cells expressing the mutated protein (8,24). In one study, electrophysiological characterization of the mutants demonstrated a correlation between the severity of the slow- or no-growth phenotype, a leftward-shift of the activation curve (the mutant channels were more sensitive to stimulus), and a decrease in the open dwell time of the channel (8). In a subsequent study, a single residue, G22, was substituted with the 19 other amino acids; this study demonstrated that the more hydrophilic the substitution in this region, the more severe the in vivo and channel phenotypes observed (9). The leftward shift of the sensitivity curve, coupled with the severely shortened open dwell times suggested that hydrophilic substitution allowed the channel to transition between a closed and open conformation more easily, which in turn led to a channel that opened at lower tensions and spent less time in the fully open conformation. At a more mechanistic level, these findings led to the proposal that at some point in the opening of the channel this residue must pass through or reside in a hydrophilic micro-environment; the residue is thus likely to be in a more hydrophobic environment in the closed position. Changing the hydrophilicity of a residue can also be accomplished posttranslationally by mutating a residue to a cysteine and then allowing it to react with a charged MTS reagent, such as MTSET. Indeed, using this approach, consistent results for the previous G22 study, discussed above, have been obtained (25). Other studies have also utilized the positively charged MTSET to identify residues within the proposed pore domain that are exposed either in the closed (presumably in a periplasmic vestibule) or opening states within a cellular context; this approach has been coined the in vivo SCAM (16,17). Here we have examined a number of mutated channels modified by this technique using electrophysiological approaches that allow us to measure the kinetics and sensitivity of the channel. Admittedly, many of the cysteine mutations themselves lead to modest changes in channel activity, which, as with all mutagenesis, makes the interpretation of the data more complicated. We cannot totally rule out the possibility that the mutation itself may alter the exposure of the residue upon gating. However, all of the mutants reported here are still gated by membrane tension and their activities in vivo function have been fully characterized, and, in general, show only modest changes ((18) and this study). In addition, channel activities between the endogenous residue, the cysteine substitution, and its subsequent modification by MTSET can all be assessed. Together, our data support many aspects of previous models of the transition states and structure of the open channel, but they also give a new higher resolution of the pore domain and its transition from the closed to open state.
Adding the charged sulfhydryl reagent MTSET at positions G30, V23, and I24 led to channel activities that not only gated at lower stimulus but also demonstrated drastically decreased open dwell times. Previously, the same modifications of two additional pore mutations, L19 and G22, yielded similar results (16,25). These findings may reflect a combination of two effects. First, the placement of a charge in these positions drastically changes the hydrophilicity of the region; the change seen in activity could be because the residues normally encounter an aqueous environment during gating, and an increase in hydrophilicity enhances the probability of this transition. Second, if more than one of the subunits within the pentameric complex is modified, one would expect electrostatic repulsion due to the proximity of these residues within the lumen of the channel. In either event, these changes could lead to either the destabilization of the closed and open states and/or stabilization of the transition states of the channel, and thus a channel with short open dwell times.
The requirements for MTSET accessibility to specific residues give clues to the microenvironment of the residue in different states of the channel. For residues L19C and G22C in the closed conformation either in vivo (17) or patch clamp (16,25), strong influences are observed subsequent to channel gating; the results strongly suggest these residues are buried in the closed state and exposed only upon channel gating (9,16). In support of the data obtained from the in vivo SCAM study, we find that G30 and G26 do not require any gating to observe dramatic changes in channel activity when treated with MTSET. In contrast, maximal effects of MTSET treatment were observed for V23 and I24 only subsequent to osmotic downshock. Interestingly, in patch clamp, channel gating was an absolute requirement for changing the channel activity of V23C, whereas the in vivo experiments suggested some accessibility independent of stimulation by hypoosmotic treatment (17). One possibility would be that there is a difference in oxidative state between the in vivo and patch clamp environments. However, as discussed more thoroughly below, G26C is much more efficient than V23C at forming disulfide bridges in patch clamp (18), yet this channel is extremely sensitive to MTSET. Perhaps a more likely explanation is that the E. coli cytoplasmic membrane has enough tension to gate V23C in vivo. Consistent with this latter interpretation, expression of a V23C mutated MscL in a cell leads to a slowed-growth phenotype (18), presumably due to promiscuous gating even in the absence of osmotic downshock. As previously noted (17), the exposure of I24 to the lumen of the pore would require a clockwise rotation of TM1 during the gating process. Again, consistent with the in vivo SCAM, we found that MTSET treatment in the presence of gating led to a channel that gated at a lower threshold. However, given the predicted “buried” nature of this residue, it is puzzling that this change in sensitivity is observed with the first opening. A clue for the resolution of this apparent paradox is obtained from another study demonstrating that the exposure of the I24 residue to the pore may occur before ion permeation. Briefly, the previous study demonstrated that an I24H mutant apparently bound to heavy metals including Ni2+ and Zn2+, which lead to a “locking” into the closed state of the channel. This would occur if the putative clockwise rotation of the TM1 domain occurred before ion permeation. Our data would be consistent with this interpretation; although channel activity is not observed in patch while the tension is subthreshold; one or more of the TM1s may be rotating as a precursor for gating, thereby exposing I24 to a position of accessibility. Together the data strongly suggest that TM1 makes a clockwise rotation to expose I24 during the normal gating process before ion permeation, and that the amount of tension in the in vivo cytoplasmic membrane is subthreshold for this motion, yet greater than the threshold for gating of the V23C mutated channel.
G26C demonstrated the most unique properties for both its accessibility to, and kinetic changes upon, modification with MTSET. This residue was first proposed to be the possible constriction point of the E. coli MscL channel when it was shown that G26C tends to form disulfide bridges and is difficult to see in patch clamp without DTT, indicating that the residues are close to each other in the closed conformation (18). A metal binding study also provided evidence that G26 residues are positioned in such a manner that they, not V23, should be the constriction point (26). Consistent with this hypothesis is the observation in this study that G26C, when modified by MTSET, resides largely in an open state. As seen in Fig. 2, this channel phenotype is not immediately observed, but instead the channels first show “flickery” spontaneous activity, then acquire a “locked” open state. These data suggest that the binding of more than one MTSET per pentameric complex is required for the open-state channel phenotype. If these residues are truly of closest proximity, then electrostatic interactions may be keeping the channel open. The fully open conductance, however, is not easily obtained, instead, the channel appears stabilized in a four-fifths subconducting state; this inability of achieving the final open state may reflect that in the higher-conducting state G26 is not as easily accessible or partially buried, and, once modified, this structure cannot be easily achieved because of steric or energetic constraints due to the charge now associated with the residue. Finally, G26C was the only residue in this study that showed accessibility, upon gating, to the cytoplasmic side of the channel. Although L19C (16) and G22C (25) have previously also been shown to be accessible from the cytoplasmic side upon gating, G26C remains the most periplasmic residue that, upon gating, is available to the cytoplasmic application of an MTS reagent. Together, these data argue for a very unique role and positioning of the G26 residue in the closed, open, and transition states of the channel.
Together, the data support a model for the sequential movements that occur in and around the lumen of the pore. The closed channel contains a periplasmic vestibule that ends at the G26 constriction point. Among the first movements upon gating, before ion permeation, is the clockwise rotation of the TM1 domain and the exposure of V23 and even I24 to the lumen of the vestibule, as has been previously proposed (12,17,26). The observation that modification of these residue locations with MTSET leads to channels with short open dwell times would be consistent with the hypothesis that a transition state, rather than an open state, is stabilized. In the fully open state, V23, I24, and G30 appear to become buried again, as indicated by the inability of these residue locations to be modified when the channel is in the open state (i.e., cytoplasmic application of MTSET and channel gating); one possible explanation for these data would be a full or partial reversal of the initial rotation of TM1 as the channel opens. G26 appears to have a unique positioning within the lumen in both the closed and nearly-fully open channel, as indicated by the availability of the G26C residue to modification by MTSET when in both of these states, and by the unique channel phenotype of a locked-open channel when modified by MTSET.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Acknowledgments
The authors thank Drs. Paul C. Moe and Irene Iscla for critical helpful discussions and critical reading of the manuscript.
This work was supported by grant I-1420 of the Welch Foundation, grant FA9550-05-1-0073 of the Air Force Office of Scientific Review, grant 0655012Y of the American Heart Association (Texas affiliate), and grant GM61028 from the National Institutes of Health.
References
- 1.Kloda, A., and B. Martinac. 2001. Mechanosensitive channels in archaea. Cell Biochem. Biophys. 34:349–381. [DOI] [PubMed] [Google Scholar]
- 2.Sukharev, S., and D. P. Corey. 2004. Mechanosensitive channels: multiplicity of families and gating paradigms. Sci. STKE. 2004:re4. [DOI] [PubMed] [Google Scholar]
- 3.Sukharev, S. I., P. Blount, B. Martinac, F. R. Blattner, and C. Kung. 1994. A large-conductance mechanosensitive channel in E. coli encoded by MscL alone. Nature. 368:265–268. [DOI] [PubMed] [Google Scholar]
- 4.Moe, P., and P. Blount. 2005. Assessment of potential stimuli for mechano-dependent gating of MscL: effects of pressure, tension, and lipid headgroups. Biochemistry. 44:12239–12244. [DOI] [PubMed] [Google Scholar]
- 5.Levina, N., S. Totemeyer, N. R. Stokes, P. Louis, M. A. Jones, and I. R. Booth. 1999. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 18:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li, Y., P. C. Moe, S. Chandrasekaran, I. R. Booth, and P. Blount. 2002. Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J. 21:5323–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruickshank, C. C., R. F. Minchin, A. C. Le Dain, and B. Martinac. 1997. Estimation of the pore size of the large-conductance mechanosensitive ion channel of Escherichia coli. Biophys. J. 73:1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ou, X., P. Blount, R. J. Hoffman, and C. Kung. 1998. One face of a transmembrane helix is crucial in mechanosensitive channel gating. Proc. Natl. Acad. Sci. USA. 95:11471–11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshimura, K., A. Batiza, M. Schroeder, P. Blount, and C. Kung. 1999. Hydrophilicity of a single residue within MscL correlates with increased channel mechanosensitivity. Biophys. J. 77:1960–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, G., R. H. Spencer, A. T. Lee, M. T. Barclay, and D. C. Rees. 1998. Structure of the MscL homolog from Mycobacterium tuberculosis: A gated mechanosensitive ion channel. Science. 282:2220–2226. [DOI] [PubMed] [Google Scholar]
- 11.Sukharev, S., S. Durell, and H. Guy. 2001. Structural models of the MscL gating mechanism. Biophys. J. 81:917–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perozo, E., D. M. Cortes, P. Sompornpisut, A. Kloda, and B. Martinac. 2002. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. 418:942–948. [DOI] [PubMed] [Google Scholar]
- 13.Perozo, E., A. Kloda, D. M. Cortes, and B. Martinac. 2001. Site-directed spin-labeling analysis of reconstituted MscL in the closed state. J. Gen. Physiol. 118:193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sukharev, S., M. Betanzos, C. Chiang, and H. Guy. 2001. The gating mechanism of the large mechanosensitive channel MscL. Nature. 409:720–724. [DOI] [PubMed] [Google Scholar]
- 15.Akabas, M. H., and A. Karlin. Substituted-cysteine accessibility method. In Methods in Enzymology. 1999. Academic Press, New York, New York. 123–144. [DOI] [PubMed]
- 16.Batiza, A. F., M. M. Kuo, K. Yoshimura, and C. Kung. 2002. Gating the bacterial mechanosensitive channel MscL in vivo. Proc. Natl. Acad. Sci. USA. 99:5643–5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartlett, J. L., G. Levin, and P. Blount. 2004. An in vivo assay identifies changes in residue accessibility on mechanosensitive channel gating. Proc. Natl. Acad. Sci. USA. 101:10161–10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin, G., and P. Blount. 2004. Cysteine scanning of MscL transmembrane domains reveals residues critical for mechanosensitive channel gating. Biophys. J. 86:2862–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blount, P., S. I. Sukharev, P. C. Moe, M. J. Schroeder, H. R. Guy, and C. Kung. 1996. Membrane topology and multimeric structure of a mechanosensitive channel protein of Escherichia coli. EMBO J. 15:4798–4805. [PMC free article] [PubMed] [Google Scholar]
- 20.Moe, P. C., G. Levin, and P. Blount. 2000. Correlating a protein structure with function of a bacterial mechanosensitive channel. J. Biol. Chem. 275:31121–31127. [DOI] [PubMed] [Google Scholar]
- 21.Martinac, B., M. Buechner, A. H. Delcour, J. Adler, and C. Kung. 1987. Pressure-sensitive ion channel in Escherichia coli. Proc. Natl. Acad. Sci. USA. 84:2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blount, P., and P. Moe. 1999. Bacterial mechanosensitive channels: integrating physiology, structure and function. Trends Microbiol. 7:420–424. [DOI] [PubMed] [Google Scholar]
- 23.Sukharev, S. I., W. J. Sigurdson, C. Kung, and F. Sachs. 1999. Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J. Gen. Physiol. 113:525–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurer, J. A., and D. A. Dougherty. 2001. A high-throughput screen for MscL channel activity and mutational phenotyping. Biochim. Biophys. Acta. 1514:165–169. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura, K., A. Batiza, and C. Kung. 2001. Chemically charging the pore constriction opens the mechanosensitive channel MscL. Biophys. J. 80:2198–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iscla, I., G. Levin, R. Wray, R. Reynolds, and P. Blount. 2004. Defining the physical gate of a mechanosensitive channel, MscL, by engineering metal-binding sites. Biophys. J. 87:3172–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blount, P., S. I. Sukharev, M. J. Schroeder, S. K. Nagle, and C. Kung. 1996. Single residue substitutions that change the gating properties of a mechanosensitive channel in Escherichia coli. Proc. Natl. Acad. Sci. USA. 93:11652–11657. [DOI] [PMC free article] [PubMed] [Google Scholar]