Abstract
Objectives
To test the hypothesis that in comparison with those with shorter risk duration, individuals with longer HIV risk duration would have reduced susceptibility to HIV-1 infection as measured by CCR5 expression, and to evaluate whether variation in CCR5 expression could be explained by known genetic polymorphisms.
Design and methods
A cross-sectional study of HIV-1 exposed but uninfected men who have sex with men. The risk duration was estimated from self-reported years since first receptive anal intercourse. CCR5 expression on peripheral blood CD4+ monocytes and T cells was determined by flow cytometry. The CCR5-Δ32 mutation and polymorphisms in the CCR5 promoter and CCR2 as well as the copy number of CCL3L1 were analyzed by polymerase chain reaction. Plasma levels of MIP-1α (CCL3), MIP-1β (CCL4) and RANTES (CCL5) were also measured. As risk duration varied with age, analyses were restricted to 67 individuals aged 30–49 years.
Results
Multiple linear regression analyses, adjusted for age and race, showed a significant negative association between HIV risk duration and CCR5 expression on monocytes (P = 0.01), and in a separate model, a similar negative association with CCR5 expression on T cells (P = 0.03). Low CCR5 expression was attributable mainly to CCR5-Δ32 heterozygosity and the CCR5-59029G allele.
Conclusions
We confirmed a role for reduced CCR5 expression in HIV-1 resistance. CCR5-Δ32 heterozygosity and the CCR5-59029G allele were significant predictors of low CCR5 expression. Individuals with high CCR5 expression who resisted infection despite long HIV risk duration form an interesting group within which to search for additional mechanisms of resistance to HIV infection.
Keywords: HIV-1 seronegative, CCR5 expression, CCR5-Δ32, CCR5-59029, CD4+ monocytes, CD4+ T cells
Introduction
The density of CCR5 chemokine receptors on CD4+ cells has been shown to affect HIV-1 transmission and disease progression [1,2]. CD4+ lymphocytes with reduced CCR5 expression from HIV-1-exposed, uninfected individuals, for example, have decreased in-vitro HIV-1 infectability [1]. Among polymorphisms associated with CCR5 expression, the CCR5-Δ32 mutation has been of particular interest because homozygous (CCR5Δ32/Δ32) individuals have no functional CCR5 receptors [3]. Heterozygous (CCR5Δ32/+) individuals have reduced receptor densities, lessened susceptibility to HIV infection, and delayed HIV disease progression [2,4,5]. The CCR5-59029G polymorphism also has been linked to decreased surface expression and reduced in-vitro infectability [6]. Additional promoter polymorphisms including CCR5-59402 and CCR5-59356 have been found to affect perinatal HIV transmission [7], although findings have been inconsistent [8]. The CCR2-64I mutation and variation in the copy number of CCL3L1, a potent ligand of CCR5, also have been correlated with HIV susceptibility, presumably by affecting surface expression or availability of the CCR5 receptor [9-11].
We undertook a cross-sectional study of men who have sex with men (MSM) to investigate the association between duration of HIV risk behaviors and CCR5 expression. We hypothesized that the duration of risk behaviors while remaining HIV-seronegative would be negatively associated with CCR5 expression on CD4+ monocytes or T cells. Furthermore, we sought to describe the genetic basis for the variability in CCR5 expression observed in this population.
Methods
Participants
This study was approved by the Institutional Review Boards of the New York University School of Medicine and the New York University Washington Square campus. Between April 2002 and May 2003, 102 sexually active MSM, aged 18 years or older, who believed they were HIV-uninfected were recruited in New York City. HIV serostatus was determined via enzyme-linked immunosorbent assay (ELISA) with western blot confirmation. Four individuals were excluded because they were HIV seropositive and two were excluded because they did not provide blood samples, leaving 96 HIV-1 seronegative MSM. We further restricted the present analyses to 67 individuals aged 30–49 years to achieve greater homogeneity in age and duration of HIV risk than was present in the source population of 96 [12].
Behavioral and demographic data were collected by audio computer-assisted interviewing. HIV risk duration was calculated by subtracting age at first unprotected receptive anal intercourse from age at interview. For four individuals who did not provide an age at first receptive anal intercourse despite reporting one or more partners in receptive anal intercourse, years since first receptive anal intercourse was imputed from regression of this variable with age in all other participants; that is, years since first receptive anal intercourse = −16.142 + 0.8402 * age.
Quantification of CCR5 expression and plasma chemokine levels
CCR5 expression on monocytes and T cells was determined by flow cytometry. Whole blood was labeled with FITC-anti-CD3, PE-anti-CCR5, PerCP-Cy5.5-anti-CD4, and APC-anti-CD14, treated with FACSLysing solution according to supplier instructions, and analyzed on a FACSCalibur (BD Biosciences, San Jose, California, USA). CCR5 expression was determined as weighted CCR5 mean fluorescence intensity (abbreviated as CCR5 FI); that is, the product of %CCR5+ cells within the CD4+CD14+ monocyte subset (or within the CD4+CD3+ T cell subset), and the mean of the CCR5 FI detected on the CCR5+ cells. Plasma concentrations of MIP-1α (CCL3), MIP-1β (CCL4) and RANTES (CCL5) were determined using Quantikine ELISA (R & D Systems, Minneapolis, Minnesota, USA) because of the potential of these chemokines to bind to CCR5 receptors and block HIV-infectability.
Genotype analyses
Genomic DNA was isolated from whole blood cells using the QIAamp DNA blood mini kit (Qiagen Inc., Valencia, California, USA). CCR5-Δ32 mutants were identified by size following polymerase chain reaction (PCR) amplification using the primer pair described by Kostrikis et al. [13]. Amplification refractory mutation system-PCR with sequence-specific primers was performed as previously described, with some modifications, for the genotyping of the CCR5-59356 (T/C) and CCR5-59402 (G/A) polymorphisms [14,15]. The 59356 forward primer was replaced with the 59402 forward primer to get more robust amplification products. PCR-restriction fragment length polymorphism was used to detect the CCR5-59029 (G/A), CCR5-59353 (C/T), and CCR2-64I polymorphisms [16-18]. CCL3L1 copy number was determined by real-time PCR [11]. Genotyping results were confirmed by direct sequencing of a subset of the samples.
Statistical analysis
Linear regression analysis was used to test for an association between number of years since first receptive anal intercourse and CCR5 expression, adjusting for age and race. Differences in the distribution of CCR5 expression by the measured factors were analyzed using the non-parametric Wilcoxon–Mann–Whitney test and linear regression. Statistical analyses were conducted using the SAS System for Windows (version 9.0; SAS Institute, Inc., Cary, North Carolina, USA).
Results
The median age of the 67 participants was 37.9 years (range 30–49 years), with 64.2% (n = 43) self-identifying as white, 15% (n = 10) as African American or black, 10.4% (n = 7) as Latino, and 10.4% (n = 7) as other or mixed race. Years since first receptive anal intercourse ranged from 0 to 43 (median = 15 years), with one man reporting never having engaged in this behavior. Lifetime numbers of sexual partners ranged from 7 to 6666 (median = 250). Lifetime numbers of sexual partners in receptive anal intercourse without a condom ranged from 0 to 800 (median = 8).
The median CCR5 FI on CD4+ monocytes was 2.5 (range, 0 to 17.5) and on CD4+ T cells was 7.6 (range, 0.4 to 44.4). In univariate analyses, CCR5 expression on monocytes did not vary by race or age, but a near-significant difference in expression (P = 0.07) by duration of risk was observed (Table 1). CCR5 expression on T cells did not vary by race or duration of risk, but variation in expression by age was near-significant (P = 0.06).
Table 1.
CCR5 expression on CD4+ monocytes and CD4+ T cells by individual characteristics.
| Characteristic | N | CCR5 FI on monocytes [median (IQRa)] | Pb | CCR5 FI on T cells [median (IQRa)] | Pb |
|---|---|---|---|---|---|
| Race/ethnicity | 0.18 | 0.29 | |||
| White | 43 | 2.4 (1.3–4.1) | 7.6 (5.8–13.7) | ||
| Black | 10 | 2.0 (0.9–4.0) | 3.8 (2.6–10.7) | ||
| Latino | 7 | 3.3 (1.6–7.1) | 9.8 (6.6–12.6) | ||
| Other | 7 | 6.7 (2.8–7.2) | 7.7 (4.0–13.9) | ||
| Age | 0.91 | 0.06 | |||
| 30–39 years | 41 | 2.4 (1.4–4.6) | 7.4 (3.1–11.7) | ||
| 40–49 years | 26 | 2.6 (1.6–4.6) | 10.5 (6.0–15.2) | ||
| Duration of risk | 0.07 | 0.90 | |||
| ≤ 15 years | 36 | 3.1 (1.7–5.9) | 7.5 (4.2–13.7) | ||
| > 15 years | 31 | 1.9 (1.3–4.0) | 7.7 (5.3–13.1) | ||
| Genotype | |||||
| CCR5-Δ32 | 0.0007 | < 0.0001 | |||
| +/+ | 52 | 3.2 (1.8–5.7) | 10.7 (6.5–14.5) | ||
| Δ32/+ | 15 | 1.3 (0.9–2.2) | 2.7 (0.8–5.8) | ||
| CCR5-59029 | 0.10 | 0.7 | |||
| GG | 15 | 1.7 (0.8–2.7) | 11.5 (6.0–13.4) | ||
| GA | 25 | 2.4 (1.3–4.6) | 7.6 (5.8–11.7) | ||
| AA | 27 | 3.7 (1.9–6.7) | 6.6 (2.6–15.2) | ||
| CCR5-59402 | 0.7 | 0.14 | |||
| AA | 31 | 3.2 (1.5–6.7) | 6.4 (2.6–14.5) | ||
| GA | 30 | 2.5 (1.4–3.9) | 7.7 (6.0–12.5) | ||
| GG | 6 | 2.6 (0.7–4.6) | 13.2 (9.8–13.4) | ||
| CCR5-59356 | 0.3 | 0.5 | |||
| CC | 63 | 2.6 (1.6–4.9) | 7.7 (5.3–13.4) | ||
| CT | 3 | 1.4 (0.9–2.7) | 3.1 (2.5–15.1) | ||
| TT | 1 | 1.3 | 4.4 | ||
| CCR2-64I | 0.004 | 0.07 | |||
| +/+ | 49 | 1.9 (1.0–3.3) | 7.0 (3.9–12.4) | ||
| +/− | 15 | 4.9 (2.4–6.7) | 10.7 (7.5–15.5) | ||
| −/− | 2 | 5.0 (3.2–6.8) | 9.8 (4.5–15.2) | ||
| CCL3L1c | 0.9 | 0.7 | |||
| < 3 | 32 | 2.7 (1.5–4.6) | 7.5 (5.9–14.0) | ||
| ≥ 3 | 35 | 2.4 (1.4–4.9) | 8.0 (4.0–12.5) |
IQR, interquartile range (25th–75th percentiles).
P-values from non-parametric Wilcoxon-Mann-Whitney test.
CCL3L1 gene copy number relative to the average of three copies. FI, weighted mean-fluorescence intensity.
Multiple linear regression analysis using the number of years since first receptive anal intercourse as the dependent variable showed a positive association with age (P < 0.0001) and a significant, negative association with CCR5 FI on CD4+ monocytes (P = 0.01), after adjusting for race. A separate analysis, also adjusted for age and race, showed a similar negative association between years since first receptive anal intercourse and CCR5 FI on CD4+ T cells (P = 0.03). Concentrations of MIP-1α, MIP-1β, and RANTES did not affect the regression estimates (data not shown).
Genotyping revealed no CCR5Δ32/Δ32 individuals, 15 (22.4%) CCR5Δ32/+, and 52 (77.6%) CCR5+/+. The proportion of individuals who were CCR5Δ32/+ among participants with long risk duration, namely greater than the median of 15 years, was 29% (9/31) compared to 16.7% (6/36) among those with short risk duration, namely less than the median of 15 years (P = 0.25). Multiple linear regression adjusted for age and race showed an 81% decrease (or an absolute decrease of 2.9) in CCR5 FI on CD4+ monocytes among CCR5-Δ32 heterozygotes compared to the predicted CCR5 FI of 3.6 in a CCR5+/+ white man at the median age of 38 (P = 0.0008). Similarly, an 81% reduction (or an absolute decrease of 9.3) in CCR5 FI was found on CD4+ T cells among CCR5-Δ32 heterozygotes compared to the predicted CCR5 FI of 11.5 in a CCR5+/+ white man at the median age of 38 (P < 0.0001). Linear regression analysis restricted to CCR5+/+ individuals continued to show a significant association between years since first receptive anal intercourse and CCR5 expression on CD4+ monocytes (P = 0.02) after adjusting for age and race. However, the previously observed association between HIV risk duration and CCR5 expression on CD4+ T cells became non-significant (P = 0.12).
Multivariable linear regression analysis controlling for age, race and CCR5-Δ32 genotype, showed a significant association between CCR5 expression on CD4+ monocytes and the CCR5-59029 genotype (P = 0.008). This relationship was not observed when CCR5 expression on T cells was considered the dependent variable. These results were not affected by excluding African Americans, among whom the CCR5-Δ32 genotype was absent. Together with the CCR5-Δ32 allele, the 59029G allele accounted for all except three of the individuals with less than the median CCR5 expression on monocytes (Fig. 1). The CCR5-59353 genotype was perfectly correlated with CCR5-59029 (data not shown). The CCR5-59402 genotype was weakly associated with CCR5 expression on monocytes (P = 0.08) after controlling for Δ32 genotype, age and race, but this association was lost upon inclusion of the 59029 genotype in the model. Similarly, a weak association (P = 0.05) was observed between the CCR2-64I genotype and CCR5 expression after adjusting for Δ32 genotype, age and race, but disappeared upon addition of the CCR5-59029 genotype to the model. No significant results were obtained from analyses involving the CCR5-59356 polymorphism or CCL3L1 copy number (data not shown).
Fig. 1.
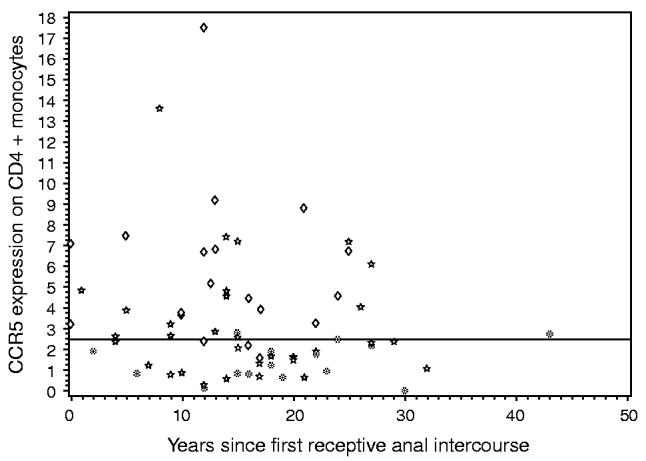
CCR5 expression on CD4+ monocytes, measured as weighted mean fluorescence intensity on CCR5+ cells, is plotted against years since first receptive anal intercourse. The CCR5-Δ32 and CCR5-59029 genotypes of each individual are indicated [CCR5Δ32/+ regardless of 59029 genotype ([unk]), CCR5+/+/59029-AG or GG (☆), CCR5+/+/59029-AA (◇)]. The horizontal line marks the median CCR5 expression level.
Discussion
In agreement with previous publications reporting reduced CCR5 levels in HIV-exposed, but uninfected individuals [1,19] and consistent with our hypothesis, we found a significant negative association between years of high-risk behavior and CCR5 expression on CD4+ monocytes and T cells. Both monocyte-derived macrophages and CD4+ T cells from HIV-exposed but uninfected individuals are resistant to infection with primary non-syncytium-inducing isolates of HIV-1 in vitro [20]. Thus, the somewhat stronger association we found between HIV-negative survival and CCR5 expression on monocytes compared to T cells is perplexing. This is especially so because activated lamina propia CD4+ T lymphocytes, rather than macrophages, have been implicated as the major HIV target in the intestinal mucosa [21,22]. The observed association of risk duration with CCR5 expression on T cells and monocytes may have been attenuated from larger, true values for several reasons: self-reported times since first receptive anal intercourse may have been poor surrogates for true cumulative risks of HIV infection; there may have been reporting biases in sexual histories; our failure to elicit lifetime residential histories may have resulted in misclassification of relative HIV risks if some participants had resided in areas with low HIV prevalence; and, by chance, some individuals may or may not have encountered HIV-infected partners during their sexual activities. Moreover, resistance mechanisms apart from reduced CCR5 receptors may exist, as suggested by the presence in the dataset of individuals with high CCR5 expression despite long durations of HIV risk-related behaviors.
The absence of CCR5-Δ32 homozygotes in this study population was surprising. The primary sources of variation in CCR5 expression in our study participants appeared to be CCR5-Δ32 heterozygosity and the CCR5-59029 mutation. Although the 59402 polymorphism also was associated with CCR5 expression in our study participants, the 59402G allele, which has been linked to decreased HIV transmission [7], occurred only in the presence of the 59029G allele. We investigated additional factors that have been associated with HIV susceptibility including the CCR2-64I polymorphism [9] and CCL3L1 copy number [11], but no significant correlations were found. The CCR2-64I allele was found only in the presence of the CCR5-59029 AA or AG genotype and therefore we conjecture that any effects of the CCR2 mutant allele on CCR5 expression can be attributed to its linkage to the 59029 genotype.
In summary, we observed a negative association between CCR5 expression and years since first receptive anal intercourse that strongly supported the selection of individuals with low levels of susceptible cells among those who have evaded HIV infection. Most low CCR5 expressors could be explained by known genetic polymorphisms. High CCR5 expression among many of the individuals who survived HIV-negative despite long periods at risk, however, suggested that additional means of HIV resistance may have been operative. Further investigation of HIV exposed but uninfected MSM is warranted.
Acknowledgements
The authors would like to thank Douglas Opler for performing the ELISAs for this study and to express their gratitude to the study volunteers for their participation.
Footnotes
Sponsorship: This work was supported in part by program project grant P01 AI057127 and Center for AIDS Research grant P30 AI27742 from the National Institute on Allergy and Infectious Diseases, National Institutes of Health, grant R01 DA15303 from the National Institute on Drug Abuse, National Institutes of Health, and General Clinical Research Center grant M01RR00096 from the National Center for Research Resources, National Institutes of Health. S.T. was supported by National Institutes of Health training grant 5T32 AI007382.
References
- 1.Paxton WA, Liu R, Kang S, Wu LJ, Gingeras TR, Landau NR, et al. Reduced HIV-1 infectability of CD4+ lymphocytes from exposed-uninfected individuals: association with low expression of CCR5 and high production of beta-chemokines. Virology. 1998;244:66–73. doi: 10.1006/viro.1998.9082. [DOI] [PubMed] [Google Scholar]
- 2.de Roda Husman AM, Blaak H, Brouwer M, Schuitemaker H. CC chemokine receptor 5 cell-surface expression in relation to CC chemokine receptor 5 genotype and the clinical course of HIV-1 infection. J Immunol. 1999;163:4597–4603. [PubMed] [Google Scholar]
- 3.Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 4.Wu L, Paxton WA, Kassam N, Ruffing N, Rottman JB, Sullivan N, et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marmor M, Sheppard HW, Donnell D, Bozeman S, Celum C, Buchbinder S, et al. Homozygous and heterozygous CCR5-Delta32 genotypes are associated with resistance to HIV infection. J Acquir Immune Defic Syndr. 2001;27:472–481. doi: 10.1097/00126334-200108150-00009. [DOI] [PubMed] [Google Scholar]
- 6.Salkowitz JR, Bruse SE, Meyerson H, Valdez H, Mosier DE, Harding CV, et al. CCR5 promoter polymorphism determines macrophage CCR5 density and magnitude of HIV-1 propagation in vitro. Clin Immunol. 2003;108:234–240. doi: 10.1016/s1521-6616(03)00147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostrikis LG. Impact of natural chemokine receptor polymorphisms on perinatal transmission of human immunodeficiency virus type 1. Teratology. 2000;61:387–390. doi: 10.1002/(SICI)1096-9926(200005)61:5<387::AID-TERA13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 8.de Souza PR, Arraes LC, de Lima Filho JL, Bruneska D, Milanese M, Crovella S. CCR5 promoter polymorphisms and HIV-1 perinatal transmission in Brazilian children. J Reprod Immunol. 2006;69:77–84. doi: 10.1016/j.jri.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Mangano A, Kopka J, Batalla M, Bologna R, Sen L. Protective effect of CCR2-64I and not of CCR5-delta32 and SDF1-3′A in pediatric HIV-1 infection. J Acquir Immune Defic Syndr. 2000;23:52–57. doi: 10.1097/00126334-200001010-00007. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama EE, Tanaka Y, Nagai Y, Iwamoto A, Shioda T. ACCR2-V64I polymorphism affects stability of CCR2A isoform. AIDS. 2004;18:729–738. doi: 10.1097/00002030-200403260-00003. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez E, Kulkarni H, Bolivar H, Mangano A, Sanchez R, Catano G, et al. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307:1434–1440. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- 12.Halkitis PN, Zade DD, Shrem M, Marmor M. Beliefs about HIV non-infection and risky sexual behavior among MSM. AIDS Educ Prev. 2004;16:448–458. doi: 10.1521/aeap.16.5.448.48739. [DOI] [PubMed] [Google Scholar]
- 13.Kostrikis LG, Tyagi S, Mhlanga MM, Ho DD, Kramer FR. Spectral genotyping of human alleles. Science. 1998;279:1228–1229. doi: 10.1126/science.279.5354.1228. [DOI] [PubMed] [Google Scholar]
- 14.John GC, Bird T, Overbaugh J, Nduati R, Mbori-Ngacha D, Rostron T, et al. CCR5 promoter polymorphisms in a Kenyan perinatal human immunodeficiency virus type 1 cohort: association with increased 2-year maternal mortality. J Infect Dis. 2001;184:89–92. doi: 10.1086/321006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Easterbrook PJ, Rostron T, Ives N, Troop M, Gazzard BG, Rowland-Jones SL. Chemokine receptor polymorphisms and human immunodeficiency virus disease progression. J Infect Dis. 1999;180:1096–1105. doi: 10.1086/314997. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima K, Tanaka Y, Nomiyama T, Ogihara T, Piao L, Sakai K, et al. Chemokine receptor genotype is associated with diabetic nephropathy in Japanese with type 2 diabetes. Diabetes. 2002;51:238–242. doi: 10.2337/diabetes.51.1.238. [DOI] [PubMed] [Google Scholar]
- 17.Clegg AO, Ashton LJ, Biti RA, Badhwar P, Williamson P, Kaldor JM, Stewart GJ. CCR5 promoter polymorphisms, CCR5 59029A and CCR5 59353C, are under represented in HIV-1-infected long-term non-progressors. The Australian Long-Term Non-Progressor Study Group. AIDS. 2000;14:103–108. doi: 10.1097/00002030-200001280-00004. [DOI] [PubMed] [Google Scholar]
- 18.Passam AM, Zafiropoulos A, Miyakis S, Zagoreos I, Stavrianeas NG, Krambovitis E, Spandidos DA. CCR2-64I and CXCL12 3′A alleles confer a favorable prognosis to AIDS patients undergoing HAART therapy. J Clin Virol. 2005;34:302–309. doi: 10.1016/j.jcv.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Hladik F, Liu H, Speelmon E, Livingston-Rosanoff D, Wilson S, Sakchalathorn P, et al. Combined effect of CCR5-Delta32 heterozygosity and the CCR5 promoter polymorphism 2459 A/G on CCR5 expression and resistance to human immunodeficiency virus type 1 transmission. J Virol. 2005;79:11677–11684. doi: 10.1128/JVI.79.18.11677-11684.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connor RI, Paxton WA, Sheridan KE, Koup RA. Macrophages and CD4+ T lymphocytes from two multiply exposed, uninfected individuals resist infection with primary non-syncytium-inducing isolates of human immunodeficiency virus type 1. J Virol. 1996;70:8758–8764. doi: 10.1128/jvi.70.12.8758-8764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith PD, Li L, Meng G. Mucosal events in the pathogenesis of human immunodeficiency virus type 1 infection. J Infect Dis. 1999;179(suppl 3):S436–S440. doi: 10.1086/314812. [DOI] [PubMed] [Google Scholar]
- 22.Smith PD, Meng G, Salazar-Gonzalez JF, Shaw GM. Macrophage HIV-1 infection and the gastrointestinal tract reservoir. J Leukoc Biol. 2003;74:642–649. doi: 10.1189/jlb.0503219. [DOI] [PubMed] [Google Scholar]


