Abstract
Background
Although described by Hippocrates in 400 B.C., pemphigus disease still needs a safe therapeutical approach, given that the currently used therapies (i.e. corticosteroids and immunosuppressive drugs) often provoke collateral effects. Here we present preliminary data on the possible use of a proteomics derived desmoglein peptide which appears promising in halting disease progression without adverse effects.
Methods
The low-similarity Dsg349–60REWVKFAKPCRE peptide was topically applied for 1 wk onto a lesion in a patient with a late-stage Pemphigus vulgaris (PV) complicated by diabetes and cataract disease. The peptide was applied as an adjuvant in combination with the standard corticosteroid-based immunosuppressive treatment.
Results
After 1 wk, the treated PV eroded lesion appeared dimensionally reduced and with an increased rate of re-epithelization when compared to adjacent non-treated lesions. Short-term benefits were: decrease of anti-Dsg antibody titer and reduction of the corticosteroid dosage. Long-term benefits: after two years following the unique 1-wk topical treatment, the decrease of anti-Dsg antibody titer persists. The patient is still at the low cortisone dosage. Adverse effects: no adverse effect could be monitored.
Conclusion
With the limits inherent to any preliminary study, this case report indicates that topical treatment with Dsg349–60REWVKFAKPCRE peptide may represent a feasible first step in the search for a simple, effective and safe treatment of PV.
Background
Pemphigus vulgaris (PV) is a rare, but severe immune-mediated blistering skin disease mediated by autoantibodies which bind to the cell surface of keratinocytes. The first recorded instance of Pemphigus disease was by Hippocrates (460–370 BC) who described pemphigoid fever as "pemphigodes pyertoi." Galen (131–201 AD) named a pustular disease of the mouth as "febris pemphigodes." In 1791, Wichmann used the term "pemphigus" to indicate a pathology characterized by flaccid bullae and painful oral ulcerations. In 1964 Beutner and Jordon reported autoantibodies in the sera of pemphigus patients, reactive with an "intercellular substance" of skin and mucosa, by using indirect immunofluorescence [1,2]. Eventually, in 1990 Amagai, Klaus-Kovtun and Stanley identified the "intercellular substance" as desmoglein-3, a 130-kDa desmosomal adhesion molecule [3]. Today the pathogenicity of anti-Dsg3 autoantibodies is a datum of fact since transfer of patient derived anti-Dsg3 serum IgG antibodies into mice induces a bullous skin disease resembling PV [4].
Histopathologically, PV is characterized by suprabasal intraepidermal bullae with acantholysis and inflammatory infiltrate of eosinophils. Immunopathologically, IgG and C3 deposits are found in intercellular/cell surface areas in skin lesions. Typically, Nikolsky's sign is present in this disease: sheetlike removal of skin by gentle pushing with a finger [5,6]. Although histologically well characterized, the course of the pemphigus pathological events and the specific pathway of the blistering process is not fully understood. In parallel, the molecular basis and the biochemical events of the pemphigus pathology remain to be clearly defined.
Therapeutically, PV treatments include corticosteroids, immunosuppressive drugs (azathioprine, cyclophosphamide, cyclosporine, and methotrexate), anti-inflammatory agents (gold, dapsone, tetracycline and nicotinamide) [5-12], plasmapheresis [13] and, more recently, intravenous immunoglobulins [14-17] and cholinergic agonists [18]. The final goal of these treatments is to reduce inflammation and/or production of the pathogenic autoreactive antibodies. There are several limitations that make current treatment protocols less than ideal: 1) no single therapy, other than high-dose steroid administration, has been reported resolutive so far; 2) prolonged immunosuppression may be associated with severe side effects, including an enhanced susceptibility to opportunistic infections; 3) the efficacy of high-dose steroid administration is transient, and relapses are the rule as soon as the steroid treatment is discontinued. Moreover, the side-effects of corticosteroid treatment are numerous and heavy, one example for all being represented by steroid-induced diabetes [19-21].
In such a context, the need for the development of alternative, effective and safe treatments for PV is unquestionable and mandatory. In our labs, we are testing the possibility of applying peptide-immunotherapy targeted to specific low-similarity protein segments, thereby treating the disease without the risk of collateral cross reactions [22-31]. Accordingly, in the present approach to PV peptide immunotherapy we have used a linear low-similarity segment of the protein autoantigen associated to PV, desmoglein-3 (Dsg3) amino acid 59–60 corresponding to the sequence REWVKFAKPCRE [32,33]. The low-similarity peptide was defined using a proteome-base computer-assisted algorithm network in order to identify Dsg3 peptide fragments potentially able to interfere with and/or stop the PV pathological event chain and, at the same time, eliminate possible collateral effects due to cross reactions. Following a series of in vitro and animal experiments [32-34], our studies have progressively focused on the Dsg349–60REWVKFAKPCRE peptide sequence that 1) is uniquely expressed in Dsg3 and, consequently, cannot induce/provoke collateral secondary autoimmune cross-reactions [22-34]; 2) is hosted in a Dsg3 domain involved in the intramolecular epitope spreading characterizing the progression of PV from mucous to muco-cutaneous stage [35]; 3) does not produce pathogenic antibodies [33].
Here we describe a case report illustrating the potential therapeutic use of the computer-designed Dsg349–60REWVKFAKPCRE peptide in PV.
Materials and methods
Peptide description
The EC1/EC2 Dsg349–60REWVKFAKPCRE peptide was synthesized using standard Fmoc (N-(9-fluorenyl)methoxycarbonyl) solid phase peptide synthesis. Peptide purity (>95%) was controlled by HPLC, and the molecular mass of purified peptide confirmed by fast atomic bombardment mass spectrometry.
Treatment design
Dsg349–60REWVKFAKPCRE peptide was administered by topical route. A cream formulation of pure vaseline containing 0.1% Dsg349–60REWVKFAKPCRE peptide was prepared under sterile conditions. Peptide immunotherapy was carried out on a late stage PV patient by using the following provisos: 1) no canonical medical treatment was discontinued and/or modified; 2) peptide immunotherapy was administered under form of topical cream; 3) the topical peptide treatment was temporally limited to one week; 4) the topical peptide treatment was locally limited to only one out of the patient's multiple erosive skin lesions; 5) the patient was hospitalized and clinically monitored in order to discontinue the treatment at the least sign of discomfort and, afterwards, remains under control for nearly two years past the topical treatment; 6) preliminarly, the possibility that the anti-Dsg349–60REWVKFAKPCRE peptide might be pathogenic (and, consequently, harmful to the patient) had been excluded [33].
Patient's history and treatment
The 1-wk topical application of the peptide cream was conducted at the Department of Dermatology, Faculty of Medicine, University of Bari in agreement to the Helsinki declaration, with patient's detailed informed consent, and applying the guidelines regulating biomedical research involving human subjects.
The patient (56-year-old, 56 kg) presented with 4-year history of mucocutaneous PV, with multiple relapses, and numerous hospitalization, during which he had been under care at the Dermatology Clinic of the Faculty of Medicine, University of Bari. The diagnosis of pemphigus had been confirmed at the onset by excisional biopsy and histology, Tzanck's cytodiagnostic method, direct immunofluorescene (DIF) and indirect immunofluorescene (IIF). The latter investigations revealed acantholytic phenomena linked to a high titer of circulating IgG AAbs (titer ≥ 1:400).
Starting in 2001, the patient had been treated with systemic corticosteroids (100 mgs/die deltacortene) plus azathioprine (100 mgs/die) and cyclosporine (300 mgs/die). When corticosteroid dosage was progressively downscaled, disease recurrence occurred at doses lower than 30 mgs/die). Because of collateral treatment effects (diabetes mellitus, arterial hypertension, corneal ulcers), the patient also underwent several sessions of plasmapheresis which led to temporary clinical improvement and reduction in the anti-Dsg3 AAb titer.
On May 2004, the subject was hospitalized because of a severe relapse. At this time the patient presented vast areas of disepithelization, especially on the back, axillary and inguino-scrotal folds plus numerous erosions in the oral mucosa. Nikolsky's sign was positive. Blood test revealed hypochromic anemia, mild hypoproteinemia and hypocalcemia as well as type II diabetes mellitus. The patient had also signs of infection of the urinary tract.
Given the severe clinical picture, the hospitalized patient was started on aggressive corticosteroid therapy (as already administered in the past), administered in 1 g sodium hydrocortisone hemisuccinate/die by i.v. route for three consecutive days. Corticosteroids were subsequently scaled down to 100 mg/die prednisone, and further lowered to topical application of clobetasol propionate cram twice a day. After 10 days, IFI decreased by 50% (AAb titer 1:200).
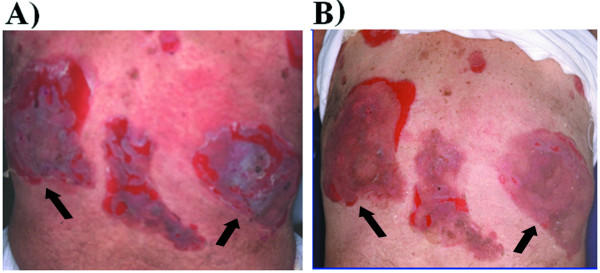
At this time, the Dsg349–60REWVKFAKPCRE peptide cream (0.1% peptide in pure vaseline) treatment was started. Specifically, two symmetrical areas hosting PV lesions (disepithelization, erosions and evident inflammation) were devised on the back (see Fig. 1A), and the peptide cream was applied twice a day to the lesion on the right, while the symmetrical eroded lesion on the left received topical application of clobetasol propionate cream. The Dsg3 peptide-cream treatment was continued for 7-days, during which the peptide cream was applied topically on the right back lesion, twice daily. Afterwards the patient remained hospitalized and under observation for a further 10 days.
Figure 1.
Clinical results following Dsg3 peptide-cream topical application. A) Patient's lesions before the peptide treatment. Arrows indicate the area selected to be treated with clobetasol propionate (on the left) or the peptide cream (on the right). B) After 1 wk of clobetasol propionate treatment (on the left) or peptide cream application (on the right).
The patient was discharged from hospital with a reduced corticosteroid prescription (50 mgs deltacortene/die), further reduced to 20 mgs/day (see Table 1) after 5 mo. Today the patient is still under control and, at nearly 2 years from that unique topical treatment, has not undergone any relapse, remains at a regimen of 20 mgs deltacortene/day and presents a clinical picture that can be defined as under control (see data in Table 1).
Table 1.
Corticosteroid dosage and anti-dsg3 AAb level. Patient's follow-up.
| Time | Deltacortene (mgs/die) | IIF Titer |
| Prior to May 2004 | 100 | 1:400 |
| May 2004* | 50 | 1:200 |
| November 2004 | 20 | 1:100 |
| February 2005 | 20 | 1:100 |
| March 2006 | 20 | 1:100 |
* Topical peptide treatment given.
Results
Scientific rationale of the Dsg349–60REWVKFAKPCRE-based treatment
Peptide immunotherapy has enormous potential to slow down the progression of malignant, autoimmune, allergic and infectious diseases [39-50]. But the consideration of safety of the treatment is paramount: in recognising the potential effectiveness of this therapy, it cannot be stressed enough that great care must be taken when attempting to suppress/modify any autoimmune diseases using peptide immunotherapy. In fact, peptides are able to cause dangerous autoimmune responses if they evoke cross-reactions with other normal housekeeping proteins and/or are pathogenic by themselves [51,52].
Given these caveats, the Dsg349–60REWVKFAKPCRE peptide appeared safe and promising of effectiveness since we have already demonstrated that the linear Dsg349–60REWVKFAKPCRE peptide sequence corresponds to a motif having a low degree of sequence similarity to host's proteome. Theoretically, this would nullify the possibility of cross-reactions between anti-Dsg349–60 REWVKFAKPCRE antibodies and other unrelated host's proteins hosting the Dsg3 motif under examination. The possibility that the linear Dsg349–60REWVKFAKPCRE peptide might be pathogenic is further to be unlikely by the fact that the Dsg1–87 portion of the Dsg protein appears not to be involved in the induction of PV. AAbs from patients at the earlier stage recognize an epitope located at Dsg387–566 but not an epitope located at the first 87 amino acids. It is noted, however, that the autoimmune humoral response of patients in the later stage of disease can be targeted towards an epitope located in the first 87 amino acids of Dsg3 [33]. Moreover, a recent work from our labs has demonstrated that IgG AAbs elicited against the Dsg349–60REWVKFAKPCRE peptide do not have pathogenic potential, providing experimental support to contradict the possibility that the peptide administration might generate pathogenic anti-Dsg3 antibodies capable of exhacerbating pemphigus pathology.
Clinical results following peptide-cream topical application
From the very first days of application, the peptide-treated lesion showed a higher rate of re-epithelization compared to the clobetasol propionate-treated area. Peptide treatment lasted 7-days. As illustrated in Fig. 1, the area treated with the peptide cream shows resolution of the dermatitis, in contrast to the contralateral lesion which still presents signs of disepithelization, mostly at the periphery. Careful clinical examination of the treated lesion put into evidence the following results when compared to non-treated lesions: a) almost immediate disappearance of the circular extra-lesion inflammation redness; b) reduction of lesion size; c) increase of the re-epithelization rate c) reduction of the erosions present in the lesion, both numerically and as area extension (Fig. 1B). There was no evidence of any adverse effect. The patient expressed well-being and highlighted the higher ease he had in moving and lying on the body part corresponding to the treated lesion.
Subsequent monitoring post-peptide cream application revealed a progressive decrease of anti-Dsg3 AAb titer, as detailed in Table 1. It is noteworthy that about 2 years after that unique peptide-therapy topical treatment, anti-Dsg3 titer still is low (1:100). As importantly, no relapse has been monitored notwithstanding the patient remains at a low regimen of corticosteroids (20 mgs/die) (Table 1).
Discussion
The "peptide vaccine" concept is based on the identification and chemical synthesis of B cell and/or T cell epitopes that are immunodominant and may induce specific immune functions (neutralization, killing, help). Peptide mimics, cyclic peptides, branched peptides, peptomers (cross-linked peptide polymers) and other complex multimeric structures, as well as peptides conjugated to other molecules (see lipopeptides), have been developed and in a few instances even tested in the attempt of identify effective peptide sequence/formulations in immunotherapeutical approaches to cancer and autoimmune disease [36-48]. The simplest peptide form in vaccine formulations is represented by linear polymers of ~8–24 amino acids. Linear amino acids sequences offer a more reductionist approach to evaluate effectiveness and results both in vivo and in vitro biomedical research [22,23]. Most importantly, preliminarily to scoring for effectiveness, a peptide vaccine must be proved to be safe and void of side effects as a first priority. Unfortunately, several peptide vaccine immunotherapeutical approaches to date have shown a concomitant sequela of harmful, sometimes lethal, autoimmune reactions due to cross reactions with not only the disease-associated-protein but also other unrelated housekeeping proteins [49-52] possibly sharing the same peptide sequence [24].
As regards PV, the HLA selective presentation of DSG3 peptides in PV has been thoroughly analyzed, starting from the premise that peptide presentation to T cells may be important in the initiation or progression of autoimmune diseases. Based on the DRB1*0402 binding motif, seven DSG3 candidate peptides were identified by Wuchepfennig et al. [53]. Accordingly, previously identified stimulatory Dsg3 peptides were docked into the binding groove of constructed atomic models of ten PV associated, non-associated and protective alleles, in order to analyze the structural aspects of allele-specific binding [54]. However, to our knowledge, none of the HLA-binding DSG3 peptides studied so far has produced possible therapeutical applications. Rather, additional studies in PV patients and healthy subjects have further confirmed that T cell recognition of Dsg3 peptides is tightly restricted by distinct HLA class II alleles, but, also, have demonstrated that T cell recognition of distinct Dsg3 peptides is independent of the development of Pemphigus vulgaris [55].
Our strategy was to use an in silico technology proteomics-based platform to identify epitopic peptide sequence(s) from disease-associated-antigens that share no similarity with the host proteome [22-34]. By identifying low/no similarity level peptide sequences, our proteomic approach offers an effective and safe tool to specifically identify motifs uniquely expressed in the disease-associated-protein, in an effort to avoid the possibility of unwanted cross-reactions.
In applying this scientific rationale to the Pemphigus vulgaris associated antigen, Dsg3, we have identified the Dsg349–60REWVKFAKPCRE peptide sequence as a fragment with low similarity to human proteome [32-34]. Based on this property, Dsg349–60REWVKFAKPCRE peptide is postulated to represent a safe and secure base for immunotherapy of pemphigus. Consequently we: 1) formulated a topical Dsg349–60REWVKFAKPCRE peptide cream and 2) elaborated a likewise safe and secure application protocol consisting of a 1 wk application time over a limited application area. This report represents an exemplar of the cautions and provisos that have to be observed for peptide immunotherapy. De facto, the Dsg349–60REWVKFAKPCRE peptide topical application has given short- and long-term positive results in terms of lesion healing and the patient's well being, with an absence of any adverse effect.
In conclusion, topical treatment with Dsg349–60REWVKFAKPCRE peptide deserves further clinical investigation in order to open the way towards a careful, cautious and safe long-term PV immunotherapy by topically applied, or even parenterally administered, Dsg349–60REWVKFAKPCRE peptide. More in general, this report promotes the concept that peptides with low level of similarity to the host's proteome might safely be used in immunotherapy of autoimmune diseases.
Authors' contributions
GA and DB carried out the clinical treatment and the patient's follow up; AL, GF, RS, AM, SS, AAS participated in the design of the study, and analyzed and controlled the data; DK conceived the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
Supported by Italian Ministry of University and Research, 60% funding (DK, SS), and Zalmin A. Arlin Cancer Fund, NY, USA (AM).
Contributor Information
Giovanni Angelini, Email: g.angelini@dermatologia.uniba.it.
Domenico Bonamonte, Email: d.bonamonte@dermatologia.uniba.it.
Alberta Lucchese, Email: alucchese@hotmail.com.
Gianfranco Favia, Email: g.favia@doc.uniba.it.
Rosario Serpico, Email: rosario.serpico@unina2.it.
Abraham Mittelman, Email: ABRAHAM_MITTELMAN@NYMC.EDU.
Simone Simone, Email: s.simone@biologia.uniba.it.
Animesh A Sinha, Email: asinha@msu.edu.
Darja Kanduc, Email: d.kanduc@biologia.uniba.it.
References
- Holubar K. Pemphigus: a disease of man and animal. Historical and other perspectives. Int J Dermatol. 1988;27:516–520. doi: 10.1111/j.1365-4362.1988.tb00937.x. [DOI] [PubMed] [Google Scholar]
- King DF, Holubar K. History of pemphigus. Clin Dermatol. 1983;1:6–12. doi: 10.1016/0738-081X(83)90019-6. [DOI] [PubMed] [Google Scholar]
- Amagai M, Klaus-Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67:869–877. doi: 10.1016/0092-8674(91)90360-B. [DOI] [PubMed] [Google Scholar]
- Mascaro JM, Jr, Espana A, Liu Z, Ding X, Swartz SJ, Fairley JA, Diaz LA. Mechanisms of acantholysis in Pemphigus vulgaris: role of IgG valence. Clin Immunol Immunopathol. 1997;85:90–96. doi: 10.1006/clin.1997.4408. [DOI] [PubMed] [Google Scholar]
- Stanley JR. Pathophysiology and therapy of pemphigus in the 21st century. J Dermatol. 2001;28:645–646. doi: 10.1111/j.1346-8138.2001.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Rogul M. Pemphigus: hard to diagnose and treat. J Am Acad Dermatol. 2004;51:S21–22. doi: 10.1016/j.jaad.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Harman KE, Albert S, Black MM. British Association of Dermatologists. Guidelines for the management of pemphigus vulgaris. Br J Dermatol. 2003;149:926–937. doi: 10.1111/j.1365-2133.2003.05665.x. [DOI] [PubMed] [Google Scholar]
- Ljubojevic S, Lipozencic J, Brenner S, Budimcic D. Pemphigus vulgaris: a review of treatment over a 19-year period. J Eur Acad Dermatol Venereol. 2002;16:599–603. doi: 10.1046/j.1468-3083.2002.00504.x. [DOI] [PubMed] [Google Scholar]
- Toth GG, van de Meer JB, Jonkman MF. Dexamethasone pulse therapy in pemphigus. J Eur Acad Dermatol Venereol. 2002;16:607–611. doi: 10.1046/j.1468-3083.2002.00413.x. [DOI] [PubMed] [Google Scholar]
- Cummins DL, Mimouni D, Anhalt GJ, Nousari CH. Oral cyclophosphamide for treatment of pemphigus vulgaris and foliaceus. J Am Acad Dermatol. 2003;49:276–280. doi: 10.1067/S0190-9622(03)00859-4. [DOI] [PubMed] [Google Scholar]
- Assmann T, Voss R, Ruzicka T, Megahed M. Therapy resistant pemphigus vulgaris. Combination therapy with methylprednisolone and doxycycline. Hautarzt. 2003;54:979–981. doi: 10.1007/s00105-003-0594-2. [DOI] [PubMed] [Google Scholar]
- Benoit Corven C, Carvalho P, Prost C, Verret JL, Saiag P, Noblesse I, Bedane C, Chosidow O, Young P, Roujeau JC, Joly P. Treatment of pemphigus vulgaris by azathioprine and low doses of prednisone (Lever scheme) Ann Dermatol Venereol. 2003;130:13–15. [PubMed] [Google Scholar]
- Mazzi G, Raineri A, Zanolli FA, Da Ponte C, De Roia D, Santarossa L, Guerra R, Orazi BM. Plasmapheresis therapy in pemphigus vulgaris and bullous pemphigoid. Transfus Apheresis Sci. 2003;28:13–18. doi: 10.1016/S1473-0502(02)00095-2. [DOI] [PubMed] [Google Scholar]
- Sami N, Bhol KC, Ahmed RA. Influence of intravenous immunoglobulin therapy on autoantibody titers to desmoglein 3 and desmoglein 1 in pemphigus vulgaris. Eur J Dermatol. 2003;13:377–381. [PubMed] [Google Scholar]
- Sami N, Qureshi A, Ruocco E, Ahmed AR. Corticosteroid-sparing effect of intravenous immunoglobulin therapy in patients with pemphigus vulgaris. Arch Dermatol. 2002;138:1158–1162. doi: 10.1001/archderm.138.9.1158. [DOI] [PubMed] [Google Scholar]
- Jolles S. High-dose intravenous immunoglobulin (hdIVIg) in the treatment of autoimmune blistering disorders. Clin Exp Immunol. 2002;129:385–389. doi: 10.1046/j.1365-2249.2002.01967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystryn JC, Jiao D, Natow S. Treatment of pemphigus with intravenous immunoglobulin. J Am Acad Dermatol. 2002;47:358–363. doi: 10.1067/mjd.2002.122735. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Arredondo J, Chernyavsky AI, Pittelkow MR, Kitajima Y, Grando SA. Pemphigus vulgaris acantholysis ameliorated by cholinergic agonists. Arch Dermatol. 2004;140:327–334. doi: 10.1001/archderm.140.3.327. [DOI] [PubMed] [Google Scholar]
- Jain R, Kumar B. Immediate and delayed complications of dexamethasone cyclophosphamide pulse(DCP) therapy. J Dermatol. 2003;30:713–718. doi: 10.1111/j.1346-8138.2003.tb00464.x. [DOI] [PubMed] [Google Scholar]
- Toth GG, Westerlaken BO, Eilders M, Laseur M, Jonkman MF, Uges DR. Dexamethasone pharmacokinetics after high-dose oral therapy for pemphigus. Ann Pharmacother. 2002;36:1108–1109. doi: 10.1345/aph.1A047. [DOI] [PubMed] [Google Scholar]
- Sakallioglu EE, Acikgoz G, Keles G, Senturk N, Karagoz F. Pemphigus vulgaris and complications of systemic corticosteroid therapy: a case report. J Oral Sci. 2003;45:165–169. doi: 10.2334/josnusd.45.165. [DOI] [PubMed] [Google Scholar]
- Kanduc D. Peptimmunology: immunogenic peptides and sequence redundancy. Curr Drug Discov Technol. 2005;2:239–244. doi: 10.2174/157016305775202946. [DOI] [PubMed] [Google Scholar]
- Kanduc D. Defining peptide sequences: from antigenicity to immunogenicity through redundancy. Curr Pharmacogenomics. 2006;4:33–37. doi: 10.2174/157016006776055374. [DOI] [Google Scholar]
- Willers J, Lucchese A, Kanduc D, Ferrone S. Molecular mimicry of phage displayed peptides mimicking GD3 ganglioside. Peptides. 1999;20:1021–1026. doi: 10.1016/S0196-9781(99)00095-9. [DOI] [PubMed] [Google Scholar]
- Natale C, Giannini T, Lucchese A, Kanduc D. Computer-assisted analysis of molecular mimicry between HPV16 E7 oncoprotein and human protein sequences. Immunol Cell Biol. 2000;78:580–585. doi: 10.1046/j.1440-1711.2000.00949.x. [DOI] [PubMed] [Google Scholar]
- Mittelman A, Lucchese A, Sinha AA, Kanduc D. Monoclonal and polyclonal humoral immune response to EC HER-2/NEU peptides with low similarity to the host's proteome. Int J Cancer. 2002;98:741–747. doi: 10.1002/ijc.10259. [DOI] [PubMed] [Google Scholar]
- Dummer R, Mittelman A, Fanizzi FP, Lucchese G, Willers J, Kanduc D. Non-self-discrimination as a driving concept in the identification of an immunodominant HMW-MAA epitopic peptide sequence by autoantibodies from melanoma cancer patients. Int J Cancer. 2004;111:720–726. doi: 10.1002/ijc.20310. [DOI] [PubMed] [Google Scholar]
- Kanduc D, Fanizzi FP, Lucchese G, Stevanovic S, Sinha AA, Mittelman A. NMR probing of in silico identification of anti-HPV16 E7 mAb linear peptide epitope. Peptides. 2004;25:243–250. doi: 10.1016/j.peptides.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Mittelman A, Tiwari R, Lucchese G, Willers J, Dummer R, Kanduc D. Identification of Monoclonal Anti-HMW-MAA Antibody Linear Peptide Epitope by Proteomic Database Mining. J Invest Dermat. 2004;123:670–675. doi: 10.1111/j.0022-202X.2004.23417.x. [DOI] [PubMed] [Google Scholar]
- Willers J, Lucchese A, Mittelman A, Dummer R, Kanduc D. Definition of anti-tyrosinase MAb T311 linear determinant by proteome-based similarity analysis. Exp Dermatol. 2005;14:543–50. doi: 10.1111/j.0906-6705.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- Lucchese A, Willers J, Mittelman A, Kanduc D, Dummer R. Proteomic scan for tyrosinase peptide antigenic pattern in vitiligo and melanoma. Role of sequence similarity and HLA-DR1 affinity. J Immunol. 2005;175:7009–7020. doi: 10.4049/jimmunol.175.10.7009. [DOI] [PubMed] [Google Scholar]
- Lucchese A, Mittelman A, Lin MS, Kanduc D, Sinha AA. Epitope definition by proteomic similarity analysis: identification of the linear determinant of the anti-Dsg3 MAb 5H10. J Transl Med. 2004;2:43. doi: 10.1186/1479-5876-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini G, Bonamonte D, Lin MS, Lucchese A, Mittelman A, Serpico R, Simone S, Sinha AA, Kanduc D. Characterization of polyclonal antibodies raised against a linear peptide determinant of desmoglein-3. J Exp Ther Oncol. 2005;5:1–7. [PubMed] [Google Scholar]
- Lucchese A, Mittelman A, Tessitore L, Serpico R, Sinha AA, Kanduc D. Proteomic definition of a desmoglein linear determinant common to Pemphigus vulgaris and Pemphigus foliaceous. J Transl Med. 2006;4:37. doi: 10.1186/1479-5876-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salato VK, Foegen MK, Lazarova Z, Fairley JA, Lin MS. The role of intra-molecular epitope spreading in the development and transition of pemphigus vulgaris. J Invest Dermatol. 2004;122:A6–Abstr 031. [Google Scholar]
- Krause I, Blank M, Shoenfeld Y. Peptide immunotherapy in autoimmune diseases. Drug News Perspect. 2000;13:78–84. doi: 10.1358/dnp.2000.13.2.858466. [DOI] [PubMed] [Google Scholar]
- Tarzi M, Larché M. Peptide immunotherapy for allergic disease. Expert Opin Biol Ther. 2003;3:617–626. doi: 10.1517/14712598.3.4.617. [DOI] [PubMed] [Google Scholar]
- Singh RR, Ebling FM, Sercarz EE, Hahn BH. Immune tolerance to autoantibody-derived peptides delays development of autoimmunity in murine lupus. J Clin Invest. 1995;96:2990–2996. doi: 10.1172/JCI118371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanne C, Avrameas S, Payelle Brogard B. A peptide derived from a polyreactive monoclonal anti-DNA natural antibody can modulate lupus development in (NZBxNZW) F1 mice. Immunology. 1999;96:333–339. doi: 10.1046/j.1365-2567.1999.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharabi A, Zinger H, Zborowsky M, Sthoeger ZM, Mozes E. A peptide based on the complementarity-determining region 1 of an autoantibody ameliorates lupus by up-regulating CD4+CD25+ cells and TGF-beta. Proc Natl Acad Sci USA. 2006;103:8810–8815. doi: 10.1073/pnas.0603201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villen J, Oliveira EdE, Nunez JI, Molina N, Sobrino F, Andreu D. Towards a multi-site synthetic vaccine to foot-and-mouth disease: addition of discontinuous site peptide mimic increases the neutralization response in immunized animals. Vaccine. 2004;22:3523–3529. doi: 10.1016/j.vaccine.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Jackson DJ, Elson CJ, Kumpel BM. Reduction of human anti-tetanus toxoid antibody in hu-PBL-SCID mice by immunodominant peptides of tetanus toxoid. Clin Exp Immunol. 2004;137:245–252. doi: 10.1111/j.1365-2249.2004.02521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselden BM, Kay AB, Larché M. Peptide-mediated immune responses in specific immunotherapy. Int Arch Allergy Immunol. 2000;122:229–237. doi: 10.1159/000024403. [DOI] [PubMed] [Google Scholar]
- von Garnier C, Astori M, Kettner A, Dufour N, Heusser C, Corradin G, Spertini F. Allergen-derived long peptide immunotherapy down-regulates specific IgE response and protects from anaphylaxis. Eur J Immunol. 2000;30:1638–1645. doi: 10.1002/1521-4141(200006)30:6<1638::AID-IMMU1638>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Wauben MH. Immunological mechanisms involved in experimental peptide immunotherapy of T-cell-mediated diseases. Crit Rev Immunol. 2000;20:451–469. [PubMed] [Google Scholar]
- Sundaram R, Lynch MP, Rawale SV, Sun Y, Kazanji M, Kaumaya PT. De novo design of peptide immunogens that mimic the coiled coil region of human T-cell leukemia virus type-1 glycoprotein 21 transmembrane subunit for induction of native protein reactive neutralizing antibodies. J Biol Chem. 2004;279:24141–24151. doi: 10.1074/jbc.M313210200. [DOI] [PubMed] [Google Scholar]
- Gorse GJ, Keefer MC, Belshe RB, Matthews TJ, Forrest BD, Hsieh RH, Koff WC, Hanson CV, Dolin R, Weinhold KJ, Frey SE, Ketter N, Fast PE. A dose-ranging study of a prototype synthetic HIV-1MN V3 branched peptide vaccine. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis. 1996;173:330–339. doi: 10.1093/infdis/173.2.330. [DOI] [PubMed] [Google Scholar]
- Langhans B, Braunschweiger I, Schweitzer S, Sauerbruch T, Spengler U. Primary immunisation of hepatitis C virus (HCV)-specific antibody producing B cells by lipidated peptides. Vaccine. 2004;22:1441–1447. doi: 10.1016/j.vaccine.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Schenk D. Opinion: amyloid-beta immunotherapy for Alzheimer's disease: the end of the beginning. Nat Rev Neurosci. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- Miller SD, Katz-Levy Y, Neville KL, Vanderlugt CL. Virus-induced autoimmunity: epitope spreading to myelin autoepitopes in Theiler's virus infection of the central nervous system. Adv Virus Res. 2001;56:199–217. doi: 10.1016/s0065-3527(01)56008-x. [DOI] [PubMed] [Google Scholar]
- Tian J, Olcott AP, Kaufman DL. Antigen-based immunotherapy drives the precocious development of autoimmunity. J Immunol. 2002;169:6564–6569. doi: 10.4049/jimmunol.169.11.6564. [DOI] [PubMed] [Google Scholar]
- Janssen EM, van Oosterhout AJ, van Rensen AJ, van Eden W, Nijkamp FP, Wauben MH. Modulation of Th2 responses by peptide analogues in a murine model of allergic asthma: amelioration or deterioration of the disease process depends on the Th1 or Th2 skewing characteristics of the therapeutic peptide. J Immunol. 2000;164:580–588. doi: 10.4049/jimmunol.164.2.580. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig KW, Yu B, Bhol K, Monos DS, Argyris E, Karr RW, Ahmed AR, Strominger JL. Structural basis for major histocompatibility complex (MHC)-linked susceptibility to autoimmunity: charged residues of a single MHC binding pocket confer selective presentation of self-peptides in pemphigus vulgaris. Proc Natl Acad Sci USA. 1995;92:11935–11939. doi: 10.1073/pnas.92.25.11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong JC, Bramson J, Kanduc D, Chow S, Sinha AA, Ranganathan S. Modeling the bound conformation of Pemphigus Vulgaris-associated peptides to MHC Class II DR and DQ Alleles. Immunome Res. 2006;2:1. doi: 10.1186/1745-7580-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman CM, Gebhard KL, Uter W, Wassmuth R, Grotzinger J, Schultz E, Hertl M. T cell recognition of desmoglein 3 peptides in patients with pemphigus vulgaris and healthy individuals. J Immunol. 2004;172:3883–3892. doi: 10.4049/jimmunol.172.6.3883. [DOI] [PubMed] [Google Scholar]