Abstract
The Arabidopsis (Arabidopsis thaliana) WRKY7 gene is induced by pathogen infection and salicylic acid (SA) treatment and may therefore play a role in plant defense responses. Here, we show that WRKY7 is localized in the nucleus, recognizes DNA molecules with the W-box (TTGAC) elements, and functions as a transcriptional repressor in plant cells. To study its biological functions directly, we have characterized both loss-of-function T-DNA insertion and RNAi mutants and gain-of-function transgenic overexpression plants for WRKY7 in Arabidopsis. The T-DNA insertion and RNAi mutant plants displayed enhanced resistance to a virulent strain of the bacterial pathogen Pseudomonas syringae as measured by significant decrease in both bacterial growth and symptom development as compared to those in wild-type plants. The enhanced resistance in the loss-of-function mutants was associated with increased induction of SA-regulated Pathogenesis-Related 1 (PR1) by the bacterial pathogen. Transgenic plants that constitutively overexpress WRKY7 have altered leaf growth and morphology strikingly similar to those observed in the previously isolated eds8 mutant plants. Like eds8 mutant plants, WRKY7-overexpressing plants supported more growth of P. syringae and developed more severe disease symptoms than wild-type plants. The enhanced susceptibility of both the WRKY7-overexpressing plants and the eds8 mutant correlated with reduced expression of defense-related genes, including PR1, but significantly increased accumulation of SA after pathogen infection, probably due to reduced negative feedback of SA synthesis. Thus, pathogen-induced WRKY7 transcription factor play a negative role in defense responses to P. syringae.
Pseudomonas syringae is a bacterial pathogen that infects a wide variety of plants (Hirano and Upper, 2000). Several strains of P. syringae infect Arabidopsis (Arabidopsis thaliana), and the establishment of the Arabidopsis-Pseudomonas model pathosystem has greatly contributed to our understanding of the molecular basis of plant-pathogen interactions (Katagiri et al., 2002). In the well-studied R gene-mediated disease resistance, recognition of specific avirulence factors from the bacterial pathogen by the products of corresponding R genes from Arabidopsis plants can trigger local plant defenses that are usually associated with programmed plant cell death, known as the hypersensitive responses (Nimchuk et al., 2003). Pathogen-induced hypersensitive responses are often associated with accumulation of salicylic acid (SA) and activation of defense mechanisms in the surrounding or even distal parts of the plants, leading to the development of systemic acquired resistance (SAR; Durrant and Dong, 2004).
A large number of Arabidopsis mutants with enhanced susceptibility to P. syringae have been isolated (Glazebrook et al., 1996; Rogers and Ausubel, 1997). Some of these mutants, including eds1 (Aarts et al., 1998), pad4 (Zhou et al., 1998), eds5 (Nawrath et al., 2002), and sid2 (Wildermuth et al., 2001), are defective in SA biosynthesis. Thus, SA-mediated defense plays a vital role in limiting P. syringae growth. In Arabidopsis, SA-induced plant defense response, including SAR, is associated with activated expression of Pathogenesis-Related (PR) genes and requires the function of the NPR1 gene (Durrant and Dong, 2004). NPR1 encodes a 66-kD protein with ankyrin repeats and some homolog with the animal IkB protein. NPR1 is localized in the cytoplasm as a large oligomeric complex in uninfected/uninduced plants. Upon SAR induction, NPR1 is reduced to form a monomer that can then translocate to the nucleus (Mou et al., 2003) and bind and enhance the DNA-binding activity of several members of the TGA/OBF transcription factor family (Zhang et al., 1999; Despres et al., 2000; Niggeweg et al., 2000; Zhou et al., 2000). Recognition of cis-acting promoters and regulation of pathogen-induced expression of Arabidopsis PR1, a commonly used molecular marker of SAR, by some of these TGA/OBF transcription factors have been demonstrated (Zhang et al., 1999; Zhou et al., 2000). Moreover, a triple deletion knockout mutant for Arabidopsis TGA2, TGA5, and TGA6 genes was found to have impaired SAR (Zhang et al., 2003).
In Arabidopsis, resistance to P. syringae can be enhanced not only by SA-mediated SAR but also by induced systemic resistance activated by biocontrol rhizobacteria (Pieterse et al., 1996, 1998; Knoester et al., 1999; van Wees et al., 1999, 2000). Induced systemic resistance is independent of SA but dependent on jasmonate (JA)/ethylene (ET) signaling (Pieterse et al., 1998). In addition, global expression phenotyping has shown that some Arabidopsis mutants with enhanced susceptibility to P. syringae, such as eds8 and pad1, are compromised in JA signaling (Glazebrook et al., 2003). Thus, defense responses mediated by JA and/or ET also play a role in Arabidopsis resistance to P. syringae. Other studies, however, have shown that SA and JA signaling pathways are mutually antagonistic. In the JA-insensitive coi1 mutant of Arabidopsis, SA-mediated gene expression and defense are elevated and resistance to P. syringae is enhanced (Kloek et al., 2001). P. syringae mutants deficient in biosynthesis of coronatine, a JA analog, are less virulent on Arabidopsis (Brooks et al., 2004). The bacterial pathogen may have evolved production of the JA analog to manipulate systemic plant defense to enhance susceptibility (Cui et al., 2005). The apparent discrepancy of the relationship between SA and JA signaling is probably a reflection of complex plant defense mechanisms. A recent study has suggested that the outcomes of the interactions between SA and JA signaling are concentration specific (Mur et al., 2006). When both signals were applied at low concentrations, there was a transient synergistic enhancement in the expression of genes associated with JA or SA. When the two signals were treated at higher concentrations or at prolonged times, they become antagonistic to each other.
A growing body of evidence suggests that WRKY proteins have regulatory functions in plant defense responses including activation of SAR (Ulker and Somssich, 2004). Pathogen infection or treatment with elicitors or SA induces WRKY genes from several plant species (Chen and Chen, 2000; Asai et al., 2002; Dong et al., 2003; Li et al., 2004; Turck et al., 2004). Several defense-regulated genes, including the regulatory NPR1 genes, contain W-box elements in their promoters that are specifically recognized by WRKY proteins and are necessary for their inducible expression (Rushton et al., 1996; Yang et al., 1999; Yu et al., 2001; Robatzek and Somssich, 2002). The involvement of WRKY protein in regulating plant SAR is further substantiated by a reported microarray study of gene-expression changes in Arabidopsis under 14 different SAR-inducing or repressing conditions (Maleck et al., 2000). The study discovered a group of 26 genes (including PR1) that were coordinately induced during SAR; these genes have higher than statistically expected frequencies of the binding sites for WRKY proteins in their promoters. Furthermore, constitutive expression of Arabidopsis WRKY18 and WRKY70 led to constitutive or enhanced expression of defense-related genes, including SA-induced PR1, and increased resistance to virulent pathogens (Chen and Chen, 2002; Li et al., 2004).
Other WRKY genes, on the other hand, may function to repress SA-mediated signaling. In a recently reported study using yeast two-hybrid screening, Arabidopsis MAP kinase 4 (MPK4), a repressor of SA-dependent resistance (Petersen et al., 2000), was found to interact with an MPK4 substrate, MKS1, which in turn interacts with Arabidopsis WRKY25 and WRKY33 (Andreasson et al., 2005). In addition, WRKY25 and WRKY33 were phosphorylated by MPK4 in vitro, and a wrky33 knockout mutant expressed elevated levels of PR1 under a short-day growth condition (Andreasson et al., 2005). These results suggest that WRKY25 and WRKY33 may function as downstream components of the MPK4-mediated signaling pathway and contribute to repression of SA-dependent disease resistance. More recently, we have shown that while overexpression of Arabidopsis WRKY18 activated SA-regulated PR1 gene expression and enhanced resistance to P. syringae, its coexpression with genes encoding its interacting partner WRKY40 or WRKY60 had opposite effects on Arabidopsis resistance to the bacterial pathogen (Xu et al., 2006).
Arabidopsis WRKY7 is induced by SA and P. syringae (Dong et al., 2003; Thilmony et al., 2006). WRKY7 is also among the genes that are coordinately induced under various SAR-inducing conditions (Maleck et al., 2000). These observations suggest a possible role of WRKY7 in SA-regulated plant defense responses. More recently, WRKY7 has been shown to bind a Ca2+-dependent calmodulin (Park et al., 2005), suggesting an additional mechanism of regulation of the WRKY protein. In this study, we show that WRKY7 is likely to be nucleus localized and functions as a DNA-binding transcriptional repressor. Loss-of-function T-DNA insertion and RNAi mutants for WRKY7 were more resistant to P. syringae and expressed higher levels of PR1 gene after pathogen infection than wild-type plants. By contrast, WRKY7-overexpresing plants displayed strikingly similar phenotypes as the eds8 mutant in leaf morphology, enhanced susceptibility to P. syringae, and reduced expression of defense-related genes. These results strongly suggest that WRKY7 is a negative regulator of plant defense against the bacterial pathogen P. syringae.
RESULTS
Protein Sequence, Subcellular Localization, and DNA Binding
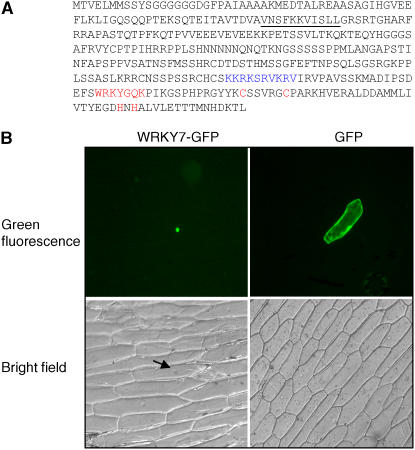
As shown in Figure 1A, Arabidopsis WRKY7 contains a single WRKY domain located at its C terminus. Inspection of the amino acid sequences of WRKY7 also revealed a typical nuclear localization sequence, with a cluster of basic residues (Fig. 1A). WRKY7 contains a number of Gly-, Glu-, or Gln-rich motifs that are often found in transcription factors with possible roles in transcriptional activation or repression (Fig. 1A). A Ser/Thr-rich region is also found at the middle of the WRKY protein (Fig. 1A), suggesting possible regulation of the transcription factor through posttranslational protein modification.
Figure 1.
Sequence and nuclear localization of WRKY7. A, Amino acids of WRKY7. The WRKY DNA-binding motif at the C terminus is indicated with the highly conserved WRKYGQK sequences and the residues forming the C2H2 zinc fingers are colored red. The putative nuclear localization signal is also indicated with a cluster of basic amino acid residues colored blue. The amino acid residues involved in binding to a Ca2+-dependent calmodulin are underlined. B, Subcellular localization of WRKY7. WRKY7 was fused to GFP to yield WRKY7-GFP; this chimeric protein is localized to the nucleus of onion epidermal cells. GFP alone is localized to both the nucleus and the cytoplasm, presumably due to its small size. A bright-field image of the onion epidermal cells is also shown below. The arrow points to the nucleus of the cell where the WRKY7-GFP fusion protein is localized.
To examine the subcellular localization of WRKY7, its coding region was fused to the 3′ end of a green fluorescent protein (GFP) reporter gene and expressed under the control of the cauliflower mosaic virus (CaMV) 35S promoter. The GFP gene alone under the control of the 35S promoter was used as a control. The GFP constructs were introduced separately by particle bombardment into onion (Allium cepa) epidermal cells. As shown in Figure 1B, this transiently expressed WRKY7-GFP fusion protein was localized exclusively to the nuclei of onion epidermal cells. The GFP protein was found in both the nuclei and cytoplasm, presumably due to its small size. This experiment suggests that WRKY7 is localized in the nucleus.
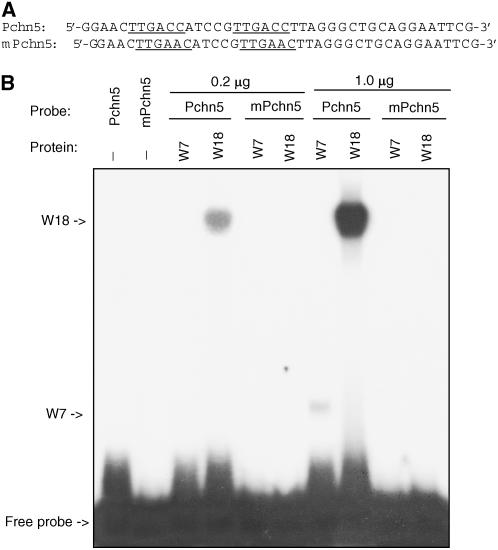
The novel type of the Cis2-His2 zinc finger of plant WRKY proteins serves as a DNA-binding domain (DBD) that specifically recognizes the TTGAC (W-box) elements (Ulker and Somssich, 2004). To examine the DNA-binding property of WRKY7, we expressed the gene in Escherichia coli, purified the recombinant proteins, and performed electrophoresis mobility shift assays (EMSA) to determine its binding to oligo DNA molecules with or without W-box elements. As a positive control, we included Arabidopsis WRKY18 recombinant protein, which is known to recognize W-box sequences (Chen and Chen, 2002). As shown in Figure 2, no shifted band was detected when a low level (0.2 μg) of WRKY7 recombinant protein was assayed in EMSA, even after a lengthy exposure. In comparison, when the same low level of WRKY18 recombinant protein was used in the assay, a retarded band was detected (Fig. 2B). When the protein level was increased to 1.0 μg in the assay, a retarded band was detected for WRKY7, but the intensity of the band was much lower than that of WRKY18 (Fig. 2B). Thus, WRKY7 bound the oligo DNA molecules (Pchn5) with two direct TTGACC repeats, but its DNA-binding activity was substantially lower that that of WRKY18. In addition, the two WRKY proteins formed DNA/protein complexes that differed in mobility on the gel (Fig. 2B). It has been recently shown that WRKY18 can self-interact to form oligomers (Xu et al., 2006) that would have a low mobility on the gel. To determine whether the TTGACC sequence is essential for the sequence-specific binding activity, we also tested a mutant probe (mPchn0) in which the two TTGACC sequences were changed to TTGAAC (Fig. 2A). As shown in Figure 2B, neither WRKY7 nor WRKY18 recognized the mutant probe.
Figure 2.
DNA-binding activity of WRKY7. A, Nucleotide sequences of probes used in EMSA. The Pchn5 probe contains two direct W-box repeats, while in the mPchn5 probe the two TTGACC W-box sequences are mutated into TTGAAC. The W-box sequences are underlined. B, EMSA of DNA-binding activity of WRKY7. Binding reactions containing recombinant WRKY7 and Pchn5 produced a DNA/protein complex with a high mobility on the gel. For comparison, binding reaction containing recombinant WRKY18 with the same probe produced a DNA/protein complex with a low mobility on the gel. Change of the TTGACC to TTGAAC in the mPchn5 probe abolished binding of both WRKY7 and WRKY18. No retarded bands were detected in the absence of the recombinant protein. The binding reactions (20 μL) contained 2 ng of labeled oligo DNA, 5 mg of poly(dIdC), and 0.2 or 1.0 μg of recombinant protein.
WRKY7 Is a Transcriptional Repressor
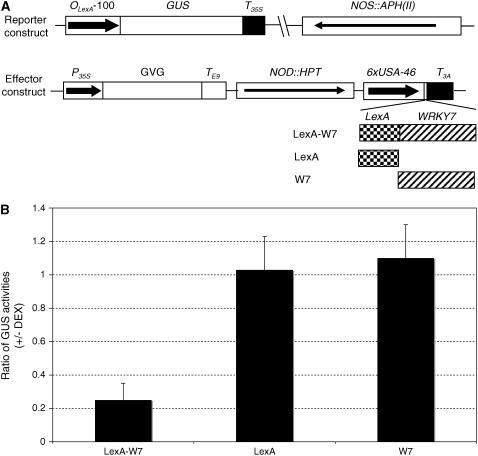
To determine the transcriptional regulatory activity of WRKY7 in planta, we developed a transgenic system in which the transcriptional regulatory activity of a protein can be determined through assays of a reporter gene in stably transformed plants. First, we generated a synthetic promoter consisting of the −100 minimal CaMV 35S promoter and eight copies of the LexA operator sequence. The promoter was fused with the β-glucuronidase (GUS) reporter gene, subcloned into a plant transformation vector, and transformed into Arabidopsis plants. Transgenic lines harboring a single insertion locus (based on kanamycin antibiotic resistance segregation) were identified, and homozygous T3 progeny lines were obtained. Due to the minimal 35S promoter used, these transgenic plants constitutively expressed similarly low levels of the GUS reporter gene, thereby making them useful for assays of transcription activation or repression by determining increase or decrease in GUS activities following coexpression of an effector protein.
To generate the WRKY7 effector, we fused its coding sequence with that of the DBD of LexA. The fusion construct was subcloned behind the steroid-inducible Gal4 promoter in pTA7002 (Aoyama and Chua, 1997) and transformed into transgenic plants that already contain the GUS reporter construct. Unfused WRKY7 and LexA DBD genes were also subcloned into pTA7002 and transformed into transgenic GUS reporter plants as controls. Transgenic plants containing both reporter and effector constructs were identified through antibiotic resistance screens. To determine how the effectors influence GUS reporter gene expression, we determined the changes of GUS activities in these transgenic plants following induction of the effector gene expression by spraying 20 μm dexamethasone (DEX), a steroid. In the transgenic plants that expressed unfused WRKY7 or LexA DBD effector, the ratios of GUS activities measured before DEX treatment to those measured after DEX treatment were close to 1 (Fig. 3B). These results indicated that induced expression of WRKY7 or LexA DBD alone had no significant effect on expression of the GUS reporter gene. In the transgenic plants harboring the LexA DBD-WRKY7 effector gene, induction of the fusion effector after DEX treatment resulted in approximately 5-fold reduction in GUS activity (Fig. 3B). These results strongly suggest that WRKY7 is a transcriptional repressor in plant cells.
Figure 3.
WRKY7 is a transcriptional repressor in plant cells. A, Constructs of reporter and effector genes. The GUS reporter gene is driven by a synthetic promoter consisting of the −100 minimal CaMV 35S promoter and eight copies of the LexA operator sequence. The effector genes were clone into pTA7002 behind the steroid-inducible promoter. The three effector genes encode LexADBA-WRKY7 fusion protein (LexA-W7), LexA DBD (LexA), and WRKY7 (W7), respectively. B, Effects on the GUS reporter gene expression by induced expression of effector genes. The ratios of GUS activities were calculated from the GUS activities in the leaves harvested prior to DEX treatment (−) over those determined in the leaves harvested 18 h after DEX treatment (+). Only those transformants that displayed induced expression of the effector genes as determined from RNA blotting following DEX treatment were used in the analyses. The GUS activities in the transformants harboring the LexA-W7 effector decreased by approximately 4- to 5-fold following induced expression of the fusion effector, whereas the GUS activities in the transformants harboring the LexA or WRKY7 effector remained largely unchanged following induction of the effector gene expression. The means and errors were calculated from at least 15 positive transformants, and the experiments were repeated; two repetitions gave very similar results.
T-DNA and RNA Silencing wrky7 Mutants
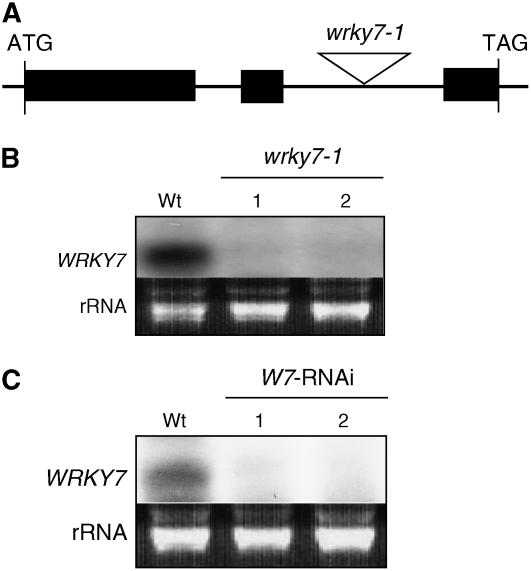
To analyze the role of WRKY7 in plant defense, we identified a T-DNA insertion mutant, wrky7-1, that carries a T-DNA insertion in the second intron of the WRKY7 gene (Fig. 4A). Northern-blot analysis revealed that SA induced WRKY7 transcripts of expected size in wild type but not in wrky7-1 (Fig. 4B). In addition, we generated RNAi mutant plants that harbor inverted repeats of a WRKY7 fragment under control of the CaMV 35S promoter. Using RNA-blot analysis, we identified several RNAi lines that accumulated little WRKY7 transcripts following SA treatment (Fig. 4C). A representative line containing a single T-DNA insertion locus (based on 3:1 antibiotic resistance segregation in T2 generation) was chosen for further investigation. The T-DNA insertion mutant and the RNAi lines grew and developed normally.
Figure 4.
Characterization of WRKY7 T-DNA insertion and RNAi mutants. A, Diagram of the WRKY7 gene and the T-DNA insertion in the wrky7-1 mutant. B, RNA-gel analysis of the wrky7-1 mutant. Wild-type (Wt) and mutant plants were treated with 2 mm SA. Four hours after the treatment, the leaves were harvested and total RNA was isolated. After separation on the gel and blotting to a nylon membrane, the blot was probed with a WRKY7 DNA fragment. C, RNA-gel analysis of the RNAi mutant plants for WRKY7. A representative transgenic RNAi line containing a single T-DNA insertion in its genome and exhibiting stable suppression of WRKY7 was chosen for the analysis. Its T3 homozygous progeny plants were used in all the experiments in the study. Wild-type (Wt) and mutant plants were treated with 2 mm SA. Four hours after the treatment, the leaves were harvested and total RNA was isolated. After separation on the gel and blotting to a nylon membrane, the blot was probed with a WRKY7 DNA fragment. Ethidium bromide-stained rRNA was used as a loading control. The experiments were repeated twice with similar results.
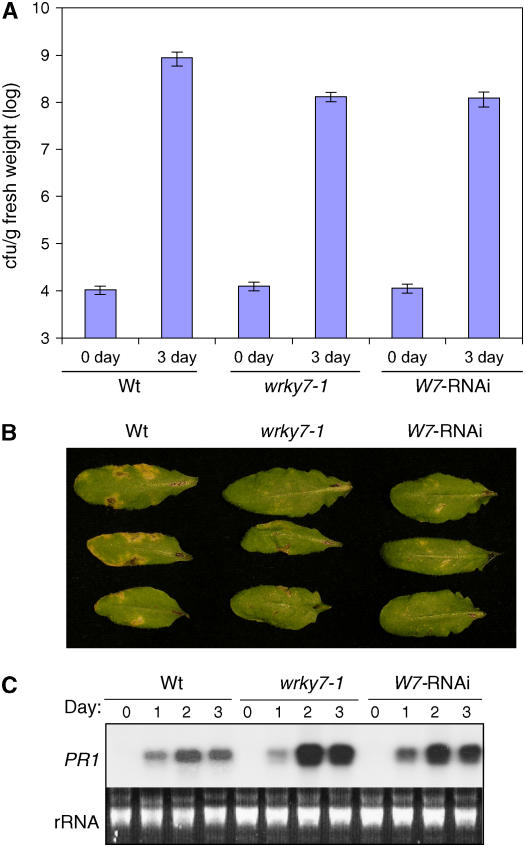
To determine the effect of disruption or suppressed expression of WRKY7 on plant disease resistance, we examined their response to P. syringae pv tomato DC3000 (PstDC3000), a strain virulent to Columbia-0 ecotype Arabidopsis plants (Whalen et al., 1991). Plants were inoculated through infiltration with the bacteria (OD600 = 0.001 in 10 mm MgCl2), and the growth of the pathogen was monitored 3 d later. As shown in Figure 5A, the bacterial growth in the T-DNA insertion and RNAi mutant plants was 6- to 7-fold lower than that in wild-type plants. The mutant plants also developed less severe disease symptoms than wild-type plants after bacterial infection (Fig. 5B). These results indicate that WRKY7 is a negative regulator of plant defense against the bacterial pathogen.
Figure 5.
Responses of WRKY7 mutants to P. syringae. A, Bacterial growth. Wild-type (Wt), wrky7-1, and RNAi (W7-RNAi) mutants were inoculated with PstDC3000 (OD600 = 0.001). Samples were taken 3 dpi to determine the bacterial titers. The means and ses were calculated from six plants. B, Disease symptom development. Wild-type (Wt), wrky7-1, and RNAi (W7-RNAi) mutants were inoculated with PstDC3000 (OD600 = 0.001). Pictures of representative inoculated leaves were taken 3 dpi. C, Pathogen-induced PR1 gene expression. Total RNA was isolated from wild-type (Wt), wrky7-1, and RNAi (W7-RNAi) mutant plants at indicated times after inoculation of PstDC3000 (OD600 = 0.001) and probed with a PR1 fragment. Ethidium bromide-stained rRNA was used as a loading control. The experiments were repeated twice with similar results.
Resistance of Arabidopsis plants to P. syringae is dependent of SA-mediated defense signaling that is often associated with enhanced expression of PR genes including PR1. To determine whether enhanced resistance of the T-DNA insertion and RNAi mutants for WRKY7 to P. syringae was associated with enhanced SA signaling, we analyzed their SA accumulation before and after bacterial infection but failed to find significant difference between the mutants and wild type. We then compared wild-type and wrky7 mutant plants for pathogen-induced PR1 expression. There was no PR1 transcript accumulation in wild type, the T-DNA insertion, or RNAi mutant plants prior to bacterial infection. At 2 and 3 d postinoculation (dpi), however, PR1 transcripts accumulated at higher levels in the mutant plants than in wild-type plants (Fig. 5C). Thus, enhanced resistance to the bacterial pathogen conferred by disruption or suppressed expression of WRKY7 correlated with enhanced induction of SA-regulated PR1 gene expression by the pathogen.
Arabidopsis WRKY70 has been shown to play a role in cross talk between SA- and JA-mediated signaling pathways (Li et al., 2004, 2006), and, therefore, we examined whether expression of WRKY7 and WRKY70 in pathogen-infected Arabidopsis plants is mutually regulated by each other. In the wrky70-1 T-DNA insertion mutant plants, we consistently observed higher levels of WRKY7 transcripts than in the wild-type plants after PstDC3000 infection (Supplemental Fig. S1). In the wrky7-1 mutant background, pathogen-induced expression of WRKY70 appeared to be normal (Supplemental Fig. S1). Thus, pathogen-regulated WRKY7 expression appeared to be negatively regulated by WRKY70.
Transgenic Plants Constitutively Expressing WRKY7
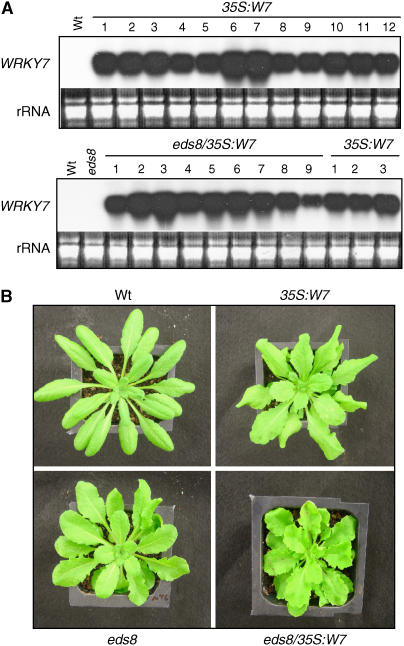
To further analyze its biological function, we overexpressed WRKY7 constitutively in transgenic Arabidopsis plants. A cDNA with the full-length coding region of WRKY7 was placed behind the CaMV 35S promoter and transformed into Arabidopsis. Northern blotting showed elevated level of WRKY7 transcripts in the transgenic plants harboring the construct (Fig. 6A). Interestingly, all those plants with substantially elevated WRKY7 transcripts have broader and more serrated leaves than wild-type plants, whereas transgenic plants with little or no elevation in WRKY7 transcripts do not exhibit such phenotypes (Fig. 6B). The altered morphology of WRKY7-overexpressing plants was strikingly similar to that of the previously isolated eds8 mutant (Fig. 6B). Because of the similarity in both leaf morphology and defense responses (see below), we also transformed the eds8 mutant plants with the 35S:WRKY7 construct, and WRKY7-overexpressing eds8 mutant plants were identified through RNA-blotting analysis (Fig. 6A). As shown in Figure 6B, when compared with the untransformed eds8 mutant plants and the WRKY7-overexpressing plants in the wild-type background, WRKY7-overexpressing eds8 mutant plants had an enhanced phenotype in leaf morphology; the sizes of these plants were also reduced, and seed setting substantially decreased. Thus, WRKY7 overexpression and mutation of EDS8 had additive effects on plant growth and development.
Figure 6.
Construction of WRKY7 overexpression lines. A, RNA-gel analysis of WRKY7 overexpression in the wild-type (35S:W7) or eds8 (eds8/35S:W7) background. RNA samples were prepared from leaves of 4.5-week-old wild-type (Wt), eds8, and transgenic plants and probed with a WRKY7 fragment. A representative transgenic WRKY7 line in the wild-type background and a representative transgenic WRKY7 line in the eds8 mutant background containing a single T-DNA insertion in their genomes and exhibiting stable WRKY7 expression were used in the analysis. Their F3 homozygous progeny plants were used in all the experiments in the study. B, Morphology of representative 5-week-old wild-type (Wt), eds8, and transgenic plants overexpressing WRKY7.
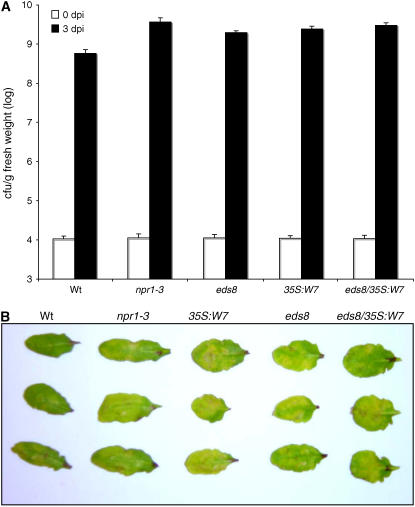
To determine the effect of WRKY7 overexpression on plant disease resistance, we examined the response of the overexpression plants to PstDC3000. For comparison, we included the npr1-3 and eds8 mutants that are known to exhibit enhanced susceptibility to the bacterial pathogen. Plants were inoculated through infiltration with the bacteria (OD600 = 0.001 in 10 mm MgCl2), and the growth of the pathogen was monitored 3 d later. As shown in Figure 7A, the bacterial growth in the npr1-3 mutant plants was approximately 7-fold higher than that in wild-type plants. The eds8 mutant also supported a significantly higher bacterial growth than wild-type plants, although the mutant was not as susceptible as the npr1 mutant based on the bacterial growth. The growth of the bacterial pathogen in the WRKY7-overexpressing plants was, at least, as high as in the eds8 mutant (Fig. 7A). When WRKY7 was overexpressed in the eds8 mutant background, the bacterial growth was further enhanced relative to those in the eds8 mutant and the WRKY7-overexpressing plants in the wild-type background. The npr1-3, eds8 mutants, and WRKY7-overexpression plants (in both the wild-type and eds8 backgrounds) also developed more severe disease symptoms than wild-type plants after infection (Fig. 7B). These results indicated that overexpression of WRKY7 had a negative effect on plant resistance to PstDC3000. It also appeared that overexpression of WRKY7 and mutation of EDS8 had an additive effect on plant susceptibility to the bacterial pathogen.
Figure 7.
Responses of WRKY7-overexpressing plants to P. syringae. A, Bacterial growth. Wild-type (Wt), npr1-3, eds8, and transgenic WRKY7-overexpressing plants in either the wild-type or eds8 background were inoculated with PstDC3000 (OD600 = 0.001). Samples were taken 3 dpi to determine the bacterial titers. The means and ses were calculated from six plants for each treatment. B, Disease symptom development. Wild-type (Wt), npr1-3, eds8, and transgenic WRKY7-overexpressing plants in either the wild-type or eds8 background were inoculated with PstDC3000 (OD600 = 0.001). Pictures of representative inoculated leaves were taken 3 dpi. The experiments were repeated twice with similar results.
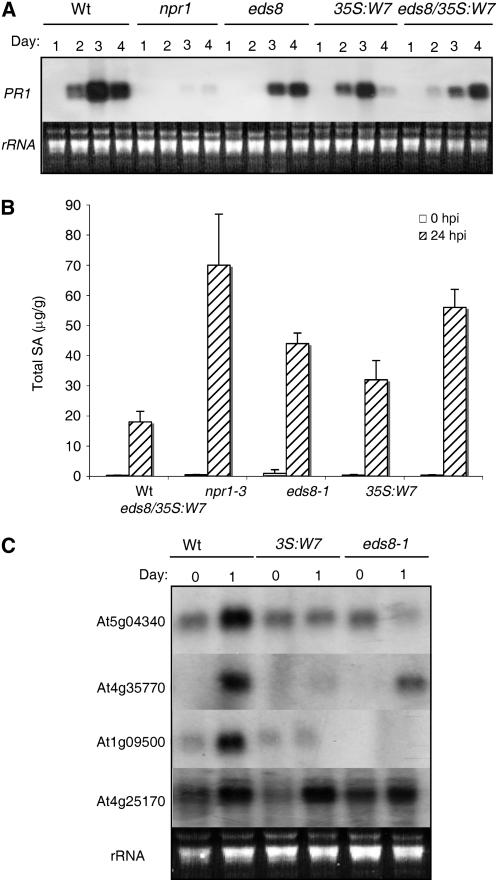
To determine whether WRKY7-induced susceptibility to PstDC3000 is associated with defects in SA signaling, we determined SA-regulated PR1 gene expression in Arabidopsis plants that overexpressed WRKY7. For comparison, we again included the npr1-3 and eds8 mutants in these analyses. As shown in Figure 8A, in wild-type plants the level of PR1 gene transcripts was elevated at 1 dpi, continued to increase at 2 dpi, and then declined at 3 dpi. By contrast, there was little induction of PR1 in the npr1-3 mutant after infection (Fig. 8A). In the eds8 mutant, PR1 transcripts were almost undetectable at 1 dpi and substantially reduced at 2 dpi as compared to those in the wild-type plants (Fig. 8A). Thus, pathogen-induced PR1 expression was both delayed and reduced in the eds8 mutant. Overexpression of WRKY7 also reduced pathogen-induced PR1 expression. In the wild-type background, WRKY7 overexpression did not appear to delay the induction based on the significant level of PR1 transcripts detected at 1 dpi in the overexpression plants (Fig. 8A). However, PR1 transcript levels were substantially lower at both 2 and 3 dpi in the transgenic overexpression plants than those in wild-type plants (Fig. 8A). In the eds8 mutant background, WRKY7 overexpression reduced PR1 transcript accumulation at both 1 and 2 dpi as compared to that in wild-type plants (Fig. 8A). It appeared that overexpression of WRKY7 in the eds8 mutant background both delayed and reduced expression of the SA-regulated defense gene.
Figure 8.
Pathogen-induced defense gene expression and SA accumulation. A, RNA-blot analysis of PR1 expression in wild-type (Wt), npr1-3, eds8, and T3 progeny of transgenic 35S:WRKY7 plants in the wild-type (35S:W7) or eds8 mutant (eds8/35S:W7) background following inoculation with PstDC3000 (OD600 = 0.001). Total RNA was isolated from inoculated leaves harvested at indicated times after inoculation and probed with a PR1 fragment. Ethidium bromide-stained rRNA was used as a loading control. B, Determination of total SA levels in wild-type (Wt), npr1-3, eds8, and T3 progeny of transgenic 35S:WRKY7 plants in the wild-type (35S:W7) or eds8 mutant (eds8/35S:W7) background following inoculation with PstDC3000 (OD600 = 0.001). Inoculated leaves were harvested at indicated times for total SA determination. The means and ses were calculated from two to three replicate samples. FW, Fresh weight. C, RNA-blot analysis of defense-related genes in wild-type (Wt), eds8, and T3 progeny of transgenic 35S:WRKY7 plants in the wild-type background (35S:W7) following inoculation with PstDC3000 (OD600 = 0.001). Total RNA was isolated from inoculated leaves harvested at indicated times after inoculation and probed with gene-specific DNA fragments. Ethidium bromide-stained rRNA was used as a loading control. The experiments were repeated twice with similar results.
We also examined the accumulation of SA in these plants. SA levels were very low in uninfected wild-type plants but increased approximately 20-fold at 24 h after infection. In the npr1-3 mutant plants, the basal SA levels were similarly low, but the induced SA levels after pathogen infection were approximately 3 to 4 times higher than those in pathogen-infected wild-type plants (Fig. 8B). The enhanced SA accumulation upon pathogen infection in the SA-signaling mutant has been attributed to negative feedback regulation of SA biosynthesis by NPR1-mediated SA signaling (Shah et al., 1997; Wildermuth et al., 2001). In the eds8 mutant, induction of SA levels after infection was also significantly higher than that in wild-type plants, although the magnitude of increase was lower than that in the npr1 mutant (Fig. 8B). There were significantly higher SA levels in WRKY7-overexpression plants than in wild-type plants (Fig. 8B). Overexpression of WRKY7 and mutation of EDS8 also had a significantly additive effect on pathogen-induced SA accumulation; the SA levels in the WRKY7-overexpressing eds8 mutant plants were significantly higher than those in the eds8 mutant and in WRKY7-overexpressing wild-type plants (Fig. 8B). Thus, pathogen-induced SA accumulation in the eds8 mutant and transgenic WRKY7 overexpression was not impaired.
Global expression phenotyping of wild-type and signaling-defective mutant plants has shown that EDS8 is involved in JA or ET signaling (Glazebrook et al., 2003). Expression of several JA/ET-regulated genes was reduced in the eds8 mutant after infection by a virulent strain of P. syringae pv maculicola (Glazebrook et al., 2003). These genes include: At5g04340 (encoding a C2H2 type transcription factor), At4g35770 (SEN1; a pathogen- and senescence-induced gene), At1g09500 (encoding a cinnamyl-alcohol dehydrogenase involved in lignin biosynthesis), and At4g25170 (encoding an unknown protein). To determine whether constitutive overexpression of WRKY7 had similar effects on expression of these JA/ET-regulated genes, we analyzed their transcript levels before and after infection of PstDC3000 in the wild-type, eds8, and WRKY7-overexpression plants. As shown in Figure 8C, expression of the four genes was induced in wild-type plants after PstDC3000 infection. In the eds8 mutant and transgenic WRKY7-overexpression plants, three of the four genes (At5g04340, At4g35770, and At1g09500) were substantially reduced in expression before and/or after pathogen infection. The fourth gene (At4g25170) expressed normally in the transgenic WRKY7-overexpression plants but expressed at a significantly reduced level in the eds8 mutant after PstDC3000 infection. Thus, mutation of EDS8 and overexpression of WRKY7 had very similar, if not identical, effects on expression of the JA/ET-regulated genes.
DISCUSSION
Interactions between Arabidopsis and the bacterial pathogen P. syringae have been extensively analyzed, and we therefore used this model system to investigate the role of WRKY7 in plant defense responses. In the T-DNA insertion and RNAi mutants for WRKY7, the growth of the bacterial pathogen and development of the disease symptom were significantly reduced when compared with those in wild-type plants (Fig. 5). Constitutive overexpression of WRKY7, on the other hand, led to enhanced susceptibility to P. syringae as manifested by enhanced bacterial growth and development of more severe disease symptom (Fig. 7). These results indicate that pathogen- and SA-induced WRKY7 plays a negative role in plant defense against the bacterial pathogen.
Arabidopsis mutants defective in SA biosynthesis or signaling, including eds1, pad4, eds5, sid2, and npr1, allow increased growth of P. syringae, indicating that SA-mediated signaling mechanisms are important for defense against P. syringae. In the T-DNA insertion and RNAi mutants for WRKY7 that exhibited enhanced resistance to P. syringae, SA-regulated PR1 gene expression was enhanced but SA accumulation was normal after infection. This observation suggests that WRKY7 might function as a negative regulator of SA signaling. In support of this hypothesis, overexpression of WRKY7 reduced SA-regulated PR1 gene expression without compromising SA accumulation. In fact, SA levels in the transgenic overexpression plants were significantly elevated after pathogen infection relative to those in wild-type plants. Enhanced SA accumulation is also observed in the SA signaling-defective npr1 mutant and is attributed to negative feedback regulation of SA biosynthesis by SA signaling (Durrant and Dong, 2004). The results from the transgenic WRKY7-overexpression plants support that WRKY7 compromises plant resistance to P. syringae, most likely by negatively regulating SA signaling. The significantly elevated SA levels in WRKY7-overexpression plants after P. syringae infection probably also resulted from reduced negative feedback regulation of SA biosynthesis by compromised SA signaling.
We have previously shown that overexpression of Arabidopsis WRKY18 led to constitutive expression of PR genes and enhanced resistance to P. syringae. Likewise, overexpression of Arabidopsis WRKY70 resulted in enhanced resistance to P. syringae and stronger induction of SA-regulated PR genes (Li et al., 2004). Additional analysis indicates that WRKY70 plays a positive role in SA signaling and functions as a negative regulator of JA-inducible genes. WRKY7, on the other hand, appears to play an opposite role in plant defense based on the phenotypes of both the overexpression plants and loss-of-function mutants. In a recent reported study using yeast two-hybrid screening, Arabidopsis MPK4, a repressor of SA-dependent resistance, was found to interact with a MPK4 substrate MKS1, which in turn interacts with Arabidopsis WRKY25 and WRKY33 (Andreasson et al., 2005). In addition, WRKY25 and WRKY33 were shown to be in vitro substrates of MPK4, and a wrky33 knockout mutant was found to express enhanced levels of PR1 gene under a short-day growth condition. These results suggest that WRKY25 and WRKY33 may function as downstream components of the MPK4-mediated signaling pathway and also act as repressors of SA-dependent disease resistance. Thus, different WRKY proteins play distinct roles in various signaling pathways of plant defense responses.
How WRKY7 negatively regulates SA-regulated PR1 gene expression and compromised plant defense against P. syringae is unclear. A number of WRKY proteins have been shown to act as positive transcriptional regulators (de Pater et al., 1996; Eulgem et al., 1999; Hara et al., 2000). Positive roles of WRKY proteins in regulation of plant defense genes are also inferred from the observations that W-box elements in the promoters of several defense-regulated genes, including NPR1, are necessary for their inducible expression (Rushton et al., 1996; Yang et al., 1999; Yu et al., 2001; Robatzek and Somssich, 2002). WRKY7, however, functions as a transcriptional repressor in plant cells and therefore might enhance plant susceptibility to P. syringae by repressing SA-regulated defense gene expression. Consistent with this possibility, SA-regulated PR1 has a W-box sequence (LS4) in its promoter that functions as a negative cis-acting element (Lebel et al., 1998). W-box sequences acting as negative regulatory sequences have also been found in the promoter of pathogen- and SA-induced Arabidopsis WRKY18 genes (Chen and Chen, 2002). Moreover, Arabidopsis WRKY6 acts as a repressor for a number of defense-related genes, including WRKY6 itself (Robatzek and Somssich, 2002). Among the defense-related genes repressed by WRKY6 is SEN1, whose expression was strongly up-regulated in the wrky6 knockout mutant (Robatzek and Somssich, 2002). Interestingly, pathogen-induced SEN1 expression was suppressed in the WRKY7-overexpressing plants (Fig. 8C). The SEN1 gene promoter contains five W-boxes within the first 1 kb of sequence (Robatzek and Somssich, 2002), and, therefore, repression of the gene by WRKY proteins may be mediated through direct binding of these W-boxes. In plants, a number of transcription factors have been identified as transcriptional repressors. For example, several class II ERF and SUPERMAN transcription factors and Aux/IAA proteins repress transcription due to a similar repression domain containing the LxLxL amino acid motif (Ohta et al., 2001; Hiratsu et al., 2002, 2004; Tiwari et al., 2004). No such LxLxL motif is found in WRKY7, and, therefore, further studies using deletion analysis would be necessary to identify its repressor sequence.
WRKY7-overexpression plants and the eds8 mutant exhibited not only similar changes in leaf morphology but also similar phenotypes in increased susceptibility to P. syringae and reduced expression of pathogen-induced PR1. In addition, there was a similar increase in the accumulation of SA in both the eds8 mutant and the WRKY7-overexpression plants. The strikingly similar phenotypes of the eds8 and transgenic WRKY7-overexpression plants raised the possibility that the two genes might function in the same signal transduction pathway. However, when WRKY7 expression was silenced in the eds8 mutant, neither the altered leaf morphology nor the enhanced disease susceptibility of eds8 was reversed (K.-C. Kim and Z. Chen, unpublished data). Thus, WRKY7 does not appear to be a downstream component of the EDS8 signaling pathway directly responsible for the mutant phenotypes of the eds8 mutant. Nevertheless, the strikingly similar phenotypes of the eds8 mutant and the WRKY7-overexpressing plants strongly suggest that they may share the same or similar molecular basis for enhanced disease susceptibility to P. syringae.
In this study, we have shown that in the eds8 mutant, induction of SA-regulated PR1 was also significantly reduced but SA accumulation was not reduced after infection by P. syringae. These observations suggested that the eds8 mutant might also be defective in SA signaling. However, global gene expression analysis has revealed that the eds8 mutant is defective in induction of genes regulated by JA, suggesting that EDS8 affects JA signaling (Glazebrook et al., 2003). Considerable evidence suggests that SA- and JA-signaling pathways have a complicated relationship of interactions including synergism and antagonism. A recent study has shown that synergism in expression of JA-regulated genes (e.g. PDF1.2 and Thi2.1) or SA-regulated genes (e.g. PR1) occurs when both signals are applied at low concentrations (Mur et al., 2006). However, when both signals are present at prolonged times or at high concentrations, antagonism between the two pathways was observed. In plants infected by P. syringae, JA signaling is activated weakly, as the JA-regulated genes such as PDF1.2 and Thi2.1 were only barely induced (Glazebrook et al., 2003). However, this weakly activated JA signaling may synergistically interact with the activated SA signaling to augment plant defense against the invading bacterial pathogen. Mutation of EDS8 may suppress this weakly activated JA signaling, compromise the synergistic interactions between SA- and JA-mediated defense signaling, and consequently cause susceptibility to the bacterial pathogen. Likewise, WRKY7 could negatively regulate plant defense against P. syringae by directly repressing SA-regulated defense genes or by repressing weakly activated JA signaling and compromising synergistic interactions between SA and JA signaling. When the cognate target genes of WRKY7 are identified in the future, they will help to distinguish between the two possible mechanisms.
MATERIALS AND METHODS
Plant Growth Conditions
The Arabidopsis (Arabidopsis thaliana) wild-type, mutant, and transgenic plants used in the study were all in the Columbia-0 genetic background and were grown in growth chambers at 22°C and 120 μE m−2 s−1 light on a 12-h-light and 12-h-dark photoperiod.
Subcellular Localization
The WRKY7 cDNA was amplified with the following primers: 5′-ATGGAATTCATGACTGTTGAGCTGATGATGAG-3′ and 5′-ATCGCCATGGAGAGTTTTGTCATGATTCATCGTCG-3′. The amplified fragment was digested with EcoR1 and NcoI and cloned into a GFP vector. The empty GFP plasmid was used as a control. The plasmid was isolated using Qiagen kits, concentrated to about 1 μg/μL, and used to coat the gold particles for bombardment experiments. Transient expression of the GFP fusion genes in onion (Allium cepa) epidermal cells through particle bombardment and subsequent localization of the proteins was performed essentially as described (Xu et al., 2006).
Production of Recombinant Protein and EMSA
To generate the WRKY7 recombinant protein, its full-length cDNA was cloned into pET32a (Novagen) and transformed into Escherichia coli strain BL21 (DE3). Induction of expression and purification of recombinant His-tagged WRKY7 protein were performed according to the protocol provided by Novagen. The purified proteins were dialyzed overnight against a nuclear extraction buffer (25 mm HEPES/KOH, pH 7.5, 40 mm KCl, 0.1 mm EDTA, 10% glycerol, 1 mm dithiothreitol, and 30 mg/L phenylmethylsulfonyl fluoride) at 4°C. Double-stranded synthetic oligonucleotides were labeled to specific activities of approximately 105 cpm/ng using the Klenow fragment of DNA polymerase I. DNA and protein complexes were allowed to form at room temperature for 30 min and resolved on a 10% polyacrylamide gel in 0.5× Tris-borate/EDTA at 4°C.
Assays of Transcriptional Regulatory Activity of WRKY7
The transcriptional regulatory activity of WRKY7 was determined in stably transformed plants through assays of a GUS reporter gene driven by a synthetic promoter consisting of the −100 minimal CaMV 35S promoter and eight copies of the LexA operator sequence. The LexA operator sequence was PCR amplified from pER8 (Zuo et al., 2000) using primers (5′-ATCGAATTCCAGCTTGGGCTGCAGGT-3′ and 5′-ATCGGATCCTAGAGTCGAGCATATTACA-3′) and cloned into the EcoRI/BamHI sites of a cloning vector. The −100 CaMV 35S minimal promoter was PCR amplified from pFF19 (Timmermans et al., 1990) using primers (5′-ATCGGATCCAAGTGGATTGATGTGATATCTCC-3′ and 5′-ATCACTAGTTCAGCGTGTCCTCTCCAA-3′) and cloned into the BamHI and SpeI downstream of the LexA operator sequence. The synthetic promoter was subcloned into a modified pOCA28 binary vector upstream of a GUS reporter gene. Arabidopsis transformation was performed by the vacuum infiltration procedure, and transformants were identified through selection in Murashige and Skoog medium containing 50 μg/mL kanamycin. A majority of the transformants contained similar but relatively low GUS activities due to the minimal 35S promoter used. A number of independent lines that contain a single T-DNA insertion (based on antibiotic resistance segregation in T2 generation) were identified and their homozygous T3 progeny were obtained. These plants were used as recipients for transformation of effector genes.
To generate effector genes, the DNA fragment for the LexA DBD was digested from the plasmid pEG202 (CLONTECH) using HindIII and EcoRI and cloned into the same sites in pBluescript. The full-length WRKY7 cDNA fragment was subsequently subcloned behind the LexA DBD to generate a translational fusion. The LexA DBD-WRKY7 fusion gene was cleaved from the plasmid with SalI/XbaI digestion and cloned into the XhoI/SpeI site of pTA2002 behind the steroid-inducible promoter (Aoyama and Chua, 1997). As controls, the unfused LexADBD and WRKY7 genes were also cloned into the same sites of PTA7002. These effector constructs were directly transformed into the transgenic GUS reporter plants and double transformants were identified through screening for antibiotic (hygromycin) resistance.
For determining activation or repression of GUS reporter gene expression by the effector proteins, one fully expanded leaf was harvested immediately prior to DEX treatment, and three additional leaves were harvested 18 h after treatment of DEX (20 μm) from each double-transformed plant (5 weeks old). Two of the three leaves harvested after DEX treatment were used for isolation of total RNA that was subsequently probed with a LexA DBD or WRKY7 DNA fragment for determining DEX-induced effector gene expression. For those double transformants with positive effector gene expression revealed from RNA blotting, the GUS activities from the third leaves harvested after DEX treatment were determined and compared with the GUS activities determined from the leaves harvested immediately before DEX treatment. GUS activity was measured through a 4-methylumbellifery-β-d-glucuronide substrate assay.
Isolation of the wrky7-1 and wrky70-1 T-DNA Insertion Mutants
The wrky7-1 mutant (GABI_356A10) contains a T-DNA insertion in the second intron of the WRKY7 gene. The T-DNA insertion in the wrky7-1 mutant was confirmed by PCR using a T-DNA-specific primer (5′-CCCATTTGGACGTGAATGTAGACAC-3′) and a WRKY7-specific primer (pW7-1, 5′-AGAGTTTTGTCATGATTCATCGTCG-3′). Homozygous wrky7-1 mutant plants were identified by PCR using a pair of primers corresponding to sequences flanking the T-DNA insertion (pW7-1 and pW7-1R, 5′-ATGACTGTTGAGCTGATGATGAG-3′). The wrky70-1 mutant (Salk_025198) contains a T-DNA insertion in the first exon of WRKY70. Homozygous wrky70-1 mutant plants were identified by PCR using a pair of primers corresponding to sequences flanking the T-DNA insertion (pW70-1, 5′-CATGTGATAACGACGGCAAG-3′ and pW70-1R, 5′-AAAGGACCTTGGGAATTTGG-3′).
Construction of Transgenic WRKY7 Overexpression and RNAi Plants
An EcoRI/HindIII fragment that contains the 35S promoter with double enhancers, multiple cloning sites, and 35S terminator was excised from pFF19 and cloned into the same sites of the transformation vector pOCA28 to generate pOCA30. To generate the 35S-WRKY7 construct, the cDNA fragment that contains full coding sequence and 3′ untranslated region of WRKY7 was excised with KpnI and SalI from a cloning plasmid and subcloned into the same restriction sites of pOCA30 in the sense orientation behind the 35S promoter.
To generate the WRKY7 RNAi construct, the first intron of the Arabidopsis Cat3 gene was first cloned between the CaMV 35S promoter and the 35S terminator of pOCA30 to generate an RNAi vector pAA1. An approximately 400-bp DNA fragment of WRKY7 was amplified with primers (5′-GACTCGAGTGGAGGAGAAGAAGCCAGAA-3′ and 5′-AGTCTAGACCTCTCGAACCAGAGAGCTG-3′) and digested with XhoI and XbaI. The XhoI/XbaI WRKY7 fragment was cloned into XbaI/SalI sites in sense orientation before the Cat3 intron in pAA1 and was cloned again into the XhoI/SpeI sites in antisense orientation behind the Cat3 intron in the resulting plasmid. The final construct contains two WRKY7 inverted repeats separated by the Cat3 intron.
Arabidopsis transformation was performed by the floral-dip procedure (Clough and Bent, 1998). The seeds were collected from the infiltrated plants and selected in Murashige and Skoog medium containing 50 μg/mL kanamycin. Kanamycin-resistant plants were transferred to soil 9 d later and grown in a growth chamber for further analysis.
Northern Blotting
For northern-blot analysis, total RNA (5 μg) was separated on agarose-formaldehyde gels and blotted to nylon membranes. Blots were hybridized with [α-32P]dATP-labeled gene-specific probes. Hybridization was performed in PerfectHyb plus hybridization buffer (Sigma) overnight at 68°C. The membrane was then washed for 10 min twice with 2× SSC and 1% SDS and 10 min with 0.1× SSC and 1% SDS at 68°C.
Pathogen Inoculation
Pathogen inoculations were performed by infiltration of leaves of at least six plants for each treatment with the PstDC3000 strain (OD600 = 0.001 in 10 mm MgCl2). Inoculated leaves were harvested 3 d after infiltration and homogenized in 10 mm MgCl2. Diluted leaf extracts were plated on King's B medium supplemented with rifampicin (100 μg/mL) and kanamycin (25 μg/mL) and incubated at 25°C for 2 d before counting the colony forming units.
Determination of SA
Total SA was extracted and quantified as described previously (Freeman et al., 2005).
Arabidopsis Genome Initiative numbers for the genes discussed in this article are as follows: WRKY7, At4g24240; PR1, At2g14610.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Pathogen-induced expression of WRKY7 and WRKY70.
Supplementary Material
Acknowledgments
We thank the Arabidopsis Resource Center at The Ohio State University and Max Planck Institute for Plant Breeding Research for Arabidopsis mutants. We also thank Jixin Dong for efforts during the initial stage of the work and John Freeman for help with SA measurement.
This work was supported by the National Science Foundation (grant no. MCB–0209819 to Z.C.). This is journal paper 2006–18006 of the Purdue Agricultural Research Program.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Zhixiang Chen (zhixiang@purdue.edu).
The online version of this article contains Web-only data.
References
- Aarts N, Metz M, Holub E, Staskawicz BJ, Daniels MJ, Parker JE (1998) Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc Natl Acad Sci USA 95: 10306–10311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson E, Jenkins T, Brodersen P, Thorgrimsen S, Petersen NH, Zhu S, Qiu JL, Micheelsen P, Rocher A, Petersen M, et al (2005) The MAP kinase substrate MKS1 is a regulator of plant defense responses. EMBO J 24: 2579–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T, Chua N-H (1997) A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J 11: 605–612 [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Brooks DM, Hernandez-Guzman G, Kloek AP, Alarcon-Chaidez F, Sreedharan A, Rangaswamy V, Penaloza-Vazquez A, Bender CL, Kunkel BN (2004) Identification and characterization of a well-defined series of coronatine biosynthetic mutants of Pseudomonas syringae pv. tomato DC3000. Mol Plant Microbe Interact 17: 162–174 [DOI] [PubMed] [Google Scholar]
- Chen C, Chen Z (2000) Isolation and characterization of two pathogen- and salicylic acid-induced genes encoding WRKY DNA-binding proteins from tobacco. Plant Mol Biol 42: 387–396 [DOI] [PubMed] [Google Scholar]
- Chen C, Chen Z (2002) Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiol 129: 706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cui J, Bahrami AK, Pringle EG, Hernandez-Guzman G, Bender CL, Pierce NE, Ausubel FM (2005) Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc Natl Acad Sci USA 102: 1791–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pater S, Greco V, Pham K, Memelink J, Kijne J (1996) Characterization of a zinc-dependent transcriptional activator from Arabidopsis. Nucleic Acids Res 24: 4624–4631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres C, DeLong C, Glaze S, Liu E, Fobert PR (2000) The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. Plant Cell 12: 279–290 [PMC free article] [PubMed] [Google Scholar]
- Dong J, Chen C, Chen Z (2003) Expression profile of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol 51: 21–37 [DOI] [PubMed] [Google Scholar]
- Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42: 185–209 [DOI] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Schmelzer E, Hahlbrock K, Somssich IE (1999) Early nuclear events in plant defence signalling: rapid gene activation by WRKY transcription factors. EMBO J 18: 4689–4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JL, Garcia D, Kim D, Hopf A, Salt DE (2005) Constitutively elevated salicylic acid signals glutathione-mediated nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Physiol 137: 1082–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J, Chen W, Estes B, Chang HS, Nawrath C, Metraux JP, Zhu T, Katagiri F (2003) Topology of the network integrating salicylate and jasmonate signal transduction derived from global expression phenotyping. Plant J 34: 217–228 [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Rogers EE, Ausubel FM (1996) Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics 143: 973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Yagi M, Kusano T, Sano H (2000) Rapid systemic accumulation of transcripts encoding a tobacco WRKY transcription factor upon wounding. Mol Gen Genet 263: 30–37 [DOI] [PubMed] [Google Scholar]
- Hirano SS, Upper CD (2000) Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae—a pathogen, ice nucleus, and epiphyte. Microbiol Mol Biol Rev 64: 624–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsu K, Mitsuda N, Matsui K, Ohme-Takagi M (2004) Identification of the minimal repression domain of SUPERMAN shows that the DLELRL hexapeptide is both necessary and sufficient for repression of transcription in Arabidopsis. Biochem Biophys Res Commun 321: 172–178 [DOI] [PubMed] [Google Scholar]
- Hiratsu K, Ohta M, Matsui K, Ohme-Takagi M (2002) The SUPERMAN protein is an active repressor whose carboxy-terminal repression domain is required for the development of normal flowers. FEBS Lett 514: 351–354 [DOI] [PubMed] [Google Scholar]
- Katagiri F, Thilmony R, He SY, editors (2002) The Arabidopsis-Pseudomonas Syringae Interaction. American Society of Plant Biologists, Rockville, MD
- Kloek AP, Verbsky ML, Sharma SB, Schoelz JE, Vogel J, Klessig DF, Kunkel BN (2001) Resistance to Pseudomonas syringae conferred by an Arabidopsis thaliana coronatine-insensitive (coi1) mutation occurs through two distinct mechanisms. Plant J 26: 509–522 [DOI] [PubMed] [Google Scholar]
- Knoester M, Pieterse CM, Bol JF, Van Loon LC (1999) Systemic resistance in Arabidopsis induced by rhizobacteria requires ethylene-dependent signaling at the site of application. Mol Plant Microbe Interact 12: 720–727 [DOI] [PubMed] [Google Scholar]
- Lebel E, Heifetz P, Thorne L, Uknes S, Ryals J, Ward E (1998) Functional analysis of regulatory sequences controlling PR-1 gene expression in Arabidopsis. Plant J 16: 223–233 [DOI] [PubMed] [Google Scholar]
- Li J, Brader G, Kariola T, Palva ET (2006) WRKY70 modulates the selection of signaling pathways in plant defense. Plant J 46: 477–491 [DOI] [PubMed] [Google Scholar]
- Li J, Brader G, Palva ET (2004) The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 16: 319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleck K, Levine A, Eulgem T, Morgan A, Schmid J, Lawton KA, Dangl JL, Dietrich RA (2000) The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat Genet 26: 403–410 [DOI] [PubMed] [Google Scholar]
- Mou Z, Fan W, Dong X (2003) Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113: 935–944 [DOI] [PubMed] [Google Scholar]
- Mur LA, Kenton P, Atzorn R, Miersch O, Wasternack C (2006) The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol 140: 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Heck S, Parinthawong N, Metraux JP (2002) EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14: 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggeweg R, Thurow C, Weigel R, Pfitzner U, Gatz C (2000) Tobacco TGA factors differ with respect to interaction with NPR1, activation potential and DNA-binding properties. Plant Mol Biol 42: 775–788 [DOI] [PubMed] [Google Scholar]
- Nimchuk Z, Eulgem T, Holt BF III, Dangl JL (2003) Recognition and response in the plant immune system. Annu Rev Genet 37: 579–609 [DOI] [PubMed] [Google Scholar]
- Ohta M, Matsui K, Hiratsu K, Shinshi H, Ohme-Takagi M (2001) Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 13: 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Lee JH, Yoo JH, Moon BC, Choi MS, Kang YH, Lee SM, Kim HS, Kang KY, Chung WS, et al (2005) WRKY group IId transcription factors interact with calmodulin. FEBS Lett 579: 1545–1550 [DOI] [PubMed] [Google Scholar]
- Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, Nielsen HB, Lacy M, Austin MJ, Parker JE, et al (2000) Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell 103: 1111–1120 [DOI] [PubMed] [Google Scholar]
- Pieterse CM, van Wees SC, Hoffland E, van Pelt JA, van Loon LC (1996) Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell 8: 1225–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CM, van Wees SC, van Pelt JA, Knoester M, Laan R, Gerrits H, Weisbeek PJ, van Loon LC (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10: 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek S, Somssich IE (2002) Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev 16: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EE, Ausubel FM (1997) Arabidopsis enhanced disease susceptibility mutants exhibit enhanced susceptibility to several bacterial pathogens and alterations in PR-1 gene expression. Plant Cell 9: 305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton PJ, Torres JT, Parniske M, Wernert P, Hahlbrock K, Somssich IE (1996) Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J 15: 5690–5700 [PMC free article] [PubMed] [Google Scholar]
- Shah J, Tsui F, Klessig DF (1997) Characterization of a salicylic acid-insensitive mutant (sai1) of Arabidopsis thaliana, identified in a selective screen utilizing the SA-inducible expression of the tms2 gene. Mol Plant Microbe Interact 10: 69–78 [DOI] [PubMed] [Google Scholar]
- Thilmony R, Underwood W, He SY (2006) Genome-wide transcriptional analysis of the Arabidopsis thaliana interaction with the plant pathogen Pseudomonas syringae pv. tomato DC3000 and the human pathogen Escherichia coli O157:H7. Plant J 46: 34–53 [DOI] [PubMed] [Google Scholar]
- Timmermans MC, Maliga P, Vieira J, Messing J (1990) The pFF plasmids: cassettes utilising CaMV sequences for expression of foreign genes in plants. J Biotechnol 14: 333–344 [DOI] [PubMed] [Google Scholar]
- Tiwari SB, Hagen G, Guilfoyle TJ (2004) Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 16: 533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turck F, Zhou A, Somssich IE (2004) Stimulus-dependent, promoter-specific binding of transcription factor WRKY1 to Its native promoter and the defense-related gene PcPR1-1 in Parsley. Plant Cell 16: 2573–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulker B, Somssich IE (2004) WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol 7: 491–498 [DOI] [PubMed] [Google Scholar]
- van Wees SC, de Swart EA, van Pelt JA, van Loon LC, Pieterse CM (2000) Enhancement of induced disease resistance by simultaneous activation of salicylate- and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc Natl Acad Sci USA 97: 8711–8716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wees SC, Luijendijk M, Smoorenburg I, van Loon LC, Pieterse CM (1999) Rhizobacteria-mediated induced systemic resistance (ISR) in Arabidopsis is not associated with a direct effect on expression of known defense-related genes but stimulates the expression of the jasmonate-inducible gene Atvsp upon challenge. Plant Mol Biol 41: 537–549 [DOI] [PubMed] [Google Scholar]
- Whalen MC, Innes RW, Bent AF, Staskawicz BJ (1991) Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell 3: 49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defense. Nature 414: 562–565 [DOI] [PubMed] [Google Scholar]
- Xu X, Chen C, Fan B, Chen Z (2006) Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 18: 1310–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Wang Z, Fan B, Chen C, Chen Z (1999) A pathogen- and salicylic acid-induced WRKY DNA-binding activity recognizes the elicitor response element of the tobacco class I chitinase gene promoter. Plant J 18: 141–149 [Google Scholar]
- Yu D, Chen C, Chen Z (2001) Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 13: 1527–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fan W, Kinkema M, Li X, Dong X (1999) Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene. Proc Natl Acad Sci USA 96: 6523–6528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tessaro MJ, Lassner M, Li X (2003) Knockout analysis of Arabidopsis transcription factors TGA2, TGA5, and TGA6 reveals their redundant and essential roles in systemic acquired resistance. Plant Cell 15: 2647–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JM, Trifa Y, Silva H, Pontier D, Lam E, Shah J, Klessig DF (2000) NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element of the PR-1 gene required for induction by salicylic acid. Mol Plant Microbe Interact 13: 191–202 [DOI] [PubMed] [Google Scholar]
- Zhou N, Tootle TL, Tsui F, Klessig DF, Glazebrook J (1998) PAD4 functions upstream from salicylic acid to control defense responses in Arabidopsis. Plant Cell 10: 1021–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Niu QW, Chua NH (2000) Technical advance: an estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J 24: 265–273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.