Apical dominance is the term used to describe the control of the shoot tip over axillary bud outgrowth (e.g. Cline, 1997). It is best demonstrated via shoot tip removal (decapitation), which leads to apical dominance. Indeed, decapitation has been widely used to study bud outgrowth. In contrast, branching may also occur in the presence of a vigorous shoot tip and be modulated by signals emanating from the root and stem. Whereas the term apical dominance can be used to describe branching phenotypes, this may not be meaningful in cases where shoot branching is not mediated predominantly by the shoot tip. Moreover, different hypotheses of branching control may be due to different experimental systems and techniques rather than divergent mechanisms of control between species. Three hypotheses continue to arise that involve a role for the plant hormone auxin. The classical hypothesis states that auxin acts to regulate shoot branching in conjunction with secondary messengers, such as cytokinin (Sachs and Thimann, 1967; Bangerth, 1994; Li et al., 1995). The auxin transport hypothesis proposes that regulatory control is exerted by auxin movement in the auxin transport stream, as opposed to the actual level of auxin (Morris, 1977; Bangerth, 1989; Li and Bangerth, 1999). The bud transition hypothesis postulates that the bud enters different developmental stages that have varying degrees of sensitivity or responses to long-distance signals, including auxin (Stafstrom and Sussex, 1992; Shimizu-Sato and Mori, 2001; Morris et al., 2005). Here, we address these hypotheses and propose that several components of each can be incorporated into one model of shoot branching.
Different shoot-branching hypotheses have arisen from experiments that have varied largely in terms of whether they focus on intact, decapitated, or in vitro systems. Although decapitated and in vitro systems are excellent tools for studying apical dominance, the differences between these and intact systems are substantial, giving good reason to consider that branching control may differ in each case. Under natural conditions, plant species with a poor response to decapitation would be likely to have a selective disadvantage. Therefore, apical dominance, however mediated, may have evolved to ensure that bud outgrowth occurs after decapitation, allowing the plant to complete its life cycle. In contrast, branches of intact plants with vigorous shoot tip growth are generally not essential for the life cycle, but rather serve to enhance vegetative proliferation and/or to generate multiple sites for seed production. Excessive branching may be costly and hence branching, especially in intact plants, is likely to be carefully modulated in response to environmental factors, such as light quality, nitrogen and carbon availability, and growth and development of other plant parts. Consequently, it is reasonable to expect that different regulatory systems may have evolved to control branching after decapitation compared with that in intact plants. It is therefore important to distinguish experimental systems that focus on apical dominance using decapitation and in vitro techniques from those that evaluate branching control using intact plants. A further consideration for decapitation and in vitro studies is that these techniques not only involve the removal of a substantial source of auxin, but also the removal of a major sink for nutrients and energy, in addition to invoking stress and biophysical responses not observed in intact plants.
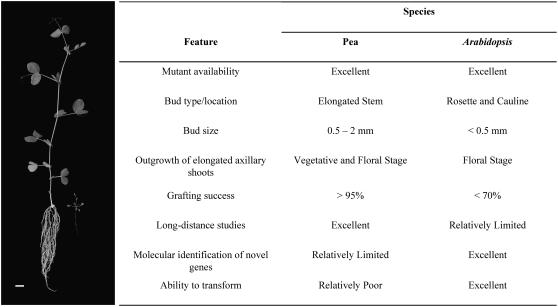
If branching in decapitated or in vitro systems is regulated somewhat differently from branching in intact plants, it is reasonable to expect that studies focused on one or the other will lead to different viewpoints (Napoli et al., 1999). Often, the experimental approach implemented (decapitated, in vitro, or intact) is dictated by the plant species studied. For example, two species that have contributed widely to our current understanding of apical dominance, pea (Pisum sativum) and Arabidopsis (Arabidopsis thaliana), have vastly different phenotypes (Fig. 1). Pea plants have long internodes separating their axillary buds from one another and the shoot tip. This phenotype has allowed for experiments using a wide range of systems, primarily during the vegetative phase. In contrast, research using Arabidopsis has predominantly focused on in vitro studies following floral transition, largely due to constraints imposed by its vegetative rosette phenotype and its weak response to exogenous auxin following decapitation (Cline, 1996; Cline et al., 2001).
Figure 1.
Comparable 3-week-old plants of pea (left) and Arabidopsis (right) exhibiting absolute apical dominance phenotypes. Important features and limitations that pertain to studies of shoot branching in these species are shown alongside. Plants were grown under an 18-h photoperiod. Bar = 2 cm.
Mutants in several species have been essential for identifying mechanisms of shoot branching in intact systems. A series of mutants in pea (rms), Arabidopsis (max), petunia (Petunia hybrida; dad), and rice (Oryza sativa; e.g. dwarf3) have been identified that show increased shoot branching in intact plants (e.g. Foo et al., 2005; Ishikawa et al., 2005; Snowden et al., 2005; Bennett et al., 2006). Branching at vegetative nodes of rms mutants of pea occurs during several stages of plant ontogeny and in the presence of a vigorous shoot tip (e.g. Beveridge et al., 2003). In contrast, elongation growth of axillary buds at rosette nodes in Arabidopsis is constrained by the floral transition. Phenotypic differences among intact max mutant and wild-type plants are not apparent at cauline nodes of the elongated inflorescence shoot (Stirnberg et al., 2002; Sorefan et al., 2003); yet, as mentioned above, physiological studies investigating Arabidopsis emphasize in vitro approaches using detached segments with cauline nodes. Typically, genetic differences revealed from observations using isolated segments of these nodes are used to develop hypotheses regarding the regulation of bud outgrowth at rosette nodes of the intact plant (e.g. Stirnberg et al., 2002; Sorefan et al., 2003; Bainbridge et al., 2005; Bennett et al., 2006).
Grafting studies using rms mutants of pea (e.g. Beveridge et al., 1994, 1997b) were the first to show that wild-type rootstocks can completely restore the branching phenotype of mutant shoots to that of the wild type. These and other studies also highlighted the point that tissues other than the shoot tip (such as the epicotyl and root) play an important role in regulating shoot branching (Napoli, 1996; Foo et al., 2001; Turnbull et al., 2002). This supports the notion argued above that the control of branching exerted by the shoot tip (apical dominance) is but one part of a regulatory system controlling branching in plants.
Several genes in different species have now been shown to affect the level of a graft-transmissible signal involved in branching inhibition (i.e. RMS1, RMS5, MAX1, MAX3, MAX4, and DAD1; for review, see McSteen and Leyser, 2005; Beveridge, 2006). For simplicity, we refer to this signal as the shoot multiplication signal (SMS; Beveridge, 2006). SMS, which is produced in the shoot and rootstock, is neither the auxin indole-3-acetic acid (IAA) nor a well-known cytokinin (Beveridge et al., 1997b; Morris et al., 2001); instead, molecular evidence indicates that it is possibly a carotenoid derivative (Sorefan et al., 2003; Schwartz et al., 2004).
CLASSICAL HYPOTHESIS
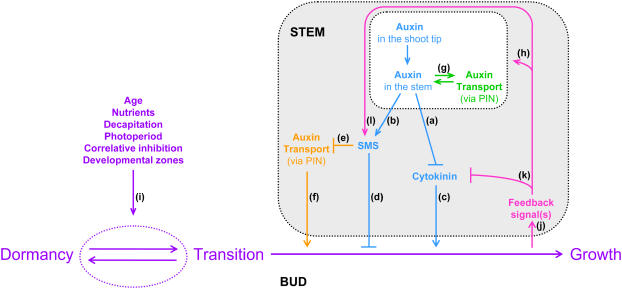
The classical hypothesis states that auxin content regulates shoot branching by influencing the levels, transport, and/or action of other signals required to inhibit bud outgrowth (Snow, 1937; Hall and Hillman, 1975; Morris, 1977; Bangerth, 1989). Candidates for this auxin-regulated signal include cytokinin (Fig. 2a; e.g. Sachs and Thimann, 1967; Li et al., 1995) and/or, as proposed more recently, SMS (Fig. 2b; Foo et al., 2005).
Figure 2.
Model for regulation of stages of bud outgrowth. The classical hypothesis is illustrated in blue (a, b, c, and d); the auxin transport hypothesis (Bennett et al., 2006) is illustrated in orange (e and f); an alternative interpretation of the auxin transport hypothesis is illustrated in green (g); hypothesized feedback interactions are illustrated in pink (h, j, k, and l); and the bud transition hypothesis is illustrated in purple, pink, blue, and green (a, b, c, d, g, h, i, j, k, and l). Arrowhead lines indicate promotion and flat-ended lines indicate inhibition. Location of cytokinin is not specified. Long-distance feedback regulation of cytokinin resulting from the perception of SMS by RMS4 (not depicted explicitly) refers to xylem-sap cytokinin, whereas auxin regulation of cytokinin occurs in shoots and roots. Feedback regulation of auxin, which may be from an independent feedback process, is not shown specifically because it may affect auxin levels or transport. The letters in the figure (a–l) are shown in the order they are referred to in the text.
The bulk of evidence supporting the classical hypothesis of auxin control comes from decapitation studies. For example, in various legumes, decapitation leads to increased levels of endogenous cytokinins in the stem and/or xylem sap (Bangerth, 1994; Li et al., 1995) and increased delivery of cytokinins to axillary buds (Turnbull et al., 1997; Tanaka et al., 2006). Tanaka et al. (2006) investigated the cytokinin content and expression of isopentenyl transferase (IPT1 and IPT2) cytokinin biosynthesis genes after decapitation in pea. These authors reported that decapitation led to increased cytokinin levels in axillary buds following an increase in PsIPT gene expression in the stem, but prior to increased PsIPT gene expression in the bud. Accordingly, cytokinins biosynthesized in the stem may be transported into the axillary buds after decapitation. The increase in cytokinin content and PsIPT expression in the stem following this removal of an endogenous auxin supply is partially prevented by auxin application (Li et al., 1995; Tanaka et al., 2006) and, indeed, elegant work by Nordstrom et al. (2004) has shown that cytokinin synthesis is directly affected by auxin. However, it is yet to be demonstrated that enhanced cytokinin supply to buds occurs prior to, and is the cause of, bud outgrowth (Turnbull et al., 1997).
Cytokinin applied directly to axillary buds or the overexpression of cytokinin biosynthesis genes often, but not always, induces bud outgrowth (Fig. 2c; King and Van Staden, 1988; Medford et al., 1989). Of the few mutants with enhanced cytokinin content, some show enhanced branching, whereas others do not. Hormone response, feedback regulation of hormone content, and hormone cross-talk may be affected differently in various mutants, leading to conflicting findings. Alternatively, perhaps cytokinin is not always the limiting factor preventing bud outgrowth or is not sufficient for bud outgrowth because other factors affect the competency for buds to respond to cytokinin.
Auxin supply from the shoot tip may also influence SMS supply in the stem (Fig. 2b). In pea, there is considerable evidence that auxin affects the production of SMS (Foo et al., 2005). Transcript levels of RMS1, which controls SMS synthesis in pea, are slightly elevated after exogenous auxin treatment to intact or decapitated plants in comparison to intact, untreated controls. Moreover, they are substantially reduced by decapitation or by treatment of intact plants with a lanolin ring containing an auxin transport inhibitor (Foo et al., 2005). Branching is inhibited less in decapitated rms1 mutant plants in response to exogenous auxin applied to the decapitated stump than it is in wild-type plants. However, inhibition of branching is restored in decapitated auxin-treated rms1 mutant shoots by grafting to wild-type rootstocks that replenish the supply of SMS to the shoot (Beveridge et al., 2000). Nevertheless, it remains unclear whether exogenous auxin inhibits branching in grafted rms1 mutant shoots by modulation of RMS1 gene expression in wild-type rootstocks and/or whether auxin affects the transport, metabolism, or action of root-derived SMS (Fig. 2d).
AUXIN TRANSPORT HYPOTHESIS
The auxin transport hypothesis is based on the theory that axillary bud outgrowth is regulated by the transport of auxin, as opposed to the quantity of auxin (Bangerth, 1989; Li and Bangerth, 1999; Leyser, 2005). In this hypothesis, the establishment of an auxin transport stream is a requirement for bud outgrowth. Typically, auxin in the shoot tip of the main stem is loaded into the polar auxin transport stream and transported in a basipetal direction. In plants where axillary bud outgrowth is inhibited entirely, this transport stream of the main stem is suggested to be full, thus limiting the flow of auxin from the axillary bud. As a result, the axillary bud is unable to establish its own auxin transport stream into the main stem and is consequently prevented from growing out. Auxin may therefore have a dual role in apical dominance by sustaining the development of outgrowing branches while inhibiting further bud outgrowth.
Bennett et al. (2006) observed that isolated inflorescence stem segments of max4 branching mutants transport greater quantities of radiolabeled IAA and have higher expression of PIN1, a gene encoding an auxin efflux carrier (Galweiler et al., 1998). This could mean that max4 mutant stems have enhanced capacity to transport auxin, which, according to the auxin transport hypothesis, would enable their buds to export auxin into the polar auxin transport stream of the main stem, thus allowing for their outgrowth (Fig. 2, e and f). Interestingly, intact rms mutant pea shoots also appear to transport a greater quantity of radiolabeled IAA compared with their wild type (Beveridge et al., 2000). This may indicate that an enhancement of transport is conserved across branching mutants of different species and may occur in both isolated and intact systems. However, from these data, we cannot yet determine whether the enhanced amounts of IAA transported are due to an increased transport capacity along the full length of the polar transport stream, causing enhanced loading into the uppermost cells of this stream, or to increased loading or transport to the top of this stream across adjacent cells. If the differences are due to the latter, the effect may be due, for example, to a reduction in the extent to which the mutants metabolize IAA upon uptake. Radiolabeled IAA, once in the polar transport stream of the stem, appears to be metabolically protected compared with other tissues such as the shoot tip, where it is readily degraded (e.g. Beveridge et al., 2000).
The enhanced movement of exogenous radiolabeled auxin in branching mutants may be indicative of enhanced content and mass flow of endogenous auxin in these plants (Fig. 2g; Morris and Johnson, 1990). This may be the case in vegetative shoot tips and internodes of intact, rms mutant pea plants, which tend to have enhanced auxin content compared with similar wild-type tissues (e.g. Beveridge et al., 1997b). Although not yet measured directly, enhanced auxin content may also occur in isolated max inflorescence stems of Arabidopsis because expression of the auxin-responsive DR5 reporter gene is elevated in these genotypes compared with the wild type (Bennett et al., 2006). Moreover, enhanced auxin levels can directly increase the auxin transport capacity via modulating the expression of PIN1 (Fig. 2g; Schrader et al., 2003), which may explain the increased PIN1 expression observed in max mutants by Bennett et al. (2006). The possibility that auxin content may be elevated in shoots of branching mutants due to a shoot-branching feedback homeostasis process (Fig. 2h; Beveridge et al., 1994) is discussed later.
To fully test the auxin transport hypothesis of shoot branching, three main points require testing. First, it needs to be determined whether the quantity of endogenous auxin transported in the polar auxin stream is at, or near, maximal capacity in nonbranching, wild-type plants. Identifying this will establish whether the auxin transport stream of a nonbranching plant is able to transport a quantity of auxin greater than its endogenous level. Second, evidence is required to demonstrate whether the inability to transport additional auxin in the stem limits the flow of auxin from the axillary bud. This will help elucidate whether the auxin transport stream of a nonbranching plant is indeed unable to incorporate additional auxin synthesized by the bud. Finally, it needs to be determined whether an enhanced flow of auxin from axillary buds occurs at a critical time or stage of bud outgrowth. This will establish whether there is a direct correlation between the movement of auxin out of a given bud and the subsequent initiation of growth of that bud.
An observation that indicates that endogenous auxin content in shoots may not have a direct correlation with branching comes from grafting studies with rms2 plants and plants treated with the auxin transport inhibitor naphthylphthalamic acid (NPA). Mutant rms2 plants have an elevated auxin content compared to intact, wild-type plants, yet bud outgrowth can be substantially suppressed in rms2 scions by grafting to wild-type rootstocks without reducing the elevated auxin content in the internodes of these rms2 scions. In this case, one can argue that enhanced auxin content may be due to a feedback regulation mechanism that enables a signal from wild-type rootstocks to inhibit branching. Furthermore, auxin transport in intact pea stems can be significantly altered, potentially affecting their available transport capacity, without inducing axillary bud outgrowth. For example, light-grown, wild-type pea treated near the shoot tip with NPA exhibited substantial depletions in their level of endogenous auxin, their transport of radiolabeled IAA supplied to the shoot tip, and their level of RMS1 gene expression, yet did not exhibit a bud outgrowth response (Foo et al., 2005; Morris et al., 2005). Decapitation, on the other hand, causes a similar or slightly greater depletion in endogenous auxin content and RMS1 gene expression, but consistently leads to bud outgrowth in wild-type plants. It might be speculated that NPA can directly inhibit bud outgrowth and that this offsets any tendency for buds to grow out from NPA-treated and, therefore, auxin-deficient stems. However, when this possibility was tested, no evidence was obtained that NPA is a direct inhibitor of bud outgrowth. Decapitated plants treated with NPA branched as much as decapitated plants treated with lanolin alone (J.J. Ross and S. Noonan, personal communication). Therefore, the lack of branching on NPA-treated plants strongly implies that auxin deficiency alone is inadequate to trigger initial bud outgrowth. Surprisingly, there are few other reports regarding the effects of apical NPA treatment on bud outgrowth using intact, light-grown plants. As discussed below, the lack of bud outgrowth following NPA treatment observed by Morris et al. (2005) may be interpreted in terms of the transition hypothesis.
BUD TRANSITION HYPOTHESIS
The bud transition hypothesis proposes that at least three stages exist at which a bud may reside: a stage of dormancy, a stage of transition, or a stage of sustained growth (Stafstrom and Sussex, 1992; Devitt and Stafstrom, 1995; Cline, 1997; Napoli et al., 1999; Shimizu-Sato and Mori, 2001; Morris et al., 2005; Beveridge, 2006). Here, dormancy is used to describe the extremely low or negligible growth rate of the axillary bud despite the fact that these buds are metabolically active. Stafstrom and Sussex (1992) found the expression of a ribosomal protein gene (rpL27) to be increased prior to visible growth in buds of decapitated pea plants. However, whereas some buds continued to sustained growth, this progression was halted in other buds at the same node. These other buds presumably reentered a stage of dormancy, reflected by a reduction in rpL27 expression to that observed in dormant buds. This and other similar studies (e.g. Madoka and Mori, 2000) support the presence of a transition stage or stages (Fig. 2) where axillary buds are more receptive than dormant buds to signals that stimulate outgrowth, yet remain able to revert to a dormant state. We suggest that a variety of factors are involved in determining the developmental stage of a bud. These factors may include the stage of whole-plant ontogenetic development, the particular node at which the bud arises, the age of the bud, genotype, light, temperature, and photoperiod (Fig. 2i; Stafstrom, 1995; Beveridge et al., 2003; Horvath et al., 2003).
Axillary buds located at different nodes exhibit varying degrees of responsiveness to signaling elements, such as cytokinins. Many studies in pea have focused on the outgrowth of axillary buds at node 2 following the application of cytokinin. However, King and Van Staden (1988) found that cytokinin application to buds at node 3 or 4 of pea plants with four fully expanded leaves did not promote outgrowth of these buds unless the plants were simultaneously decapitated. This difference in responsiveness of buds at nodes 1 and 2 compared with those at nodes 3 and 4 persists throughout ontogeny (e.g. Beveridge et al., 2003) and therefore may not simply relate to the proximity of buds to the shoot tip.
The fact that buds located at different nodes exhibit varying degrees of responsiveness to decapitation and/or cytokinin treatment suggests that the location of the bud on the stem can influence its outgrowth potential. Weberling (1989) proposed the existence of three morphological zones that influence plant growth and development: the enrichment zone, representing the region where inflorescences develop; the inhibition zone, adjacent to the enrichment zone where there is little bud outgrowth; and the innovation zone at the base of the plant, where buds either remain dormant or develop into axillary branches that more or less phenocopy the main shoot (for review, see Napoli et al., 1999). We propose that these morphological zones influence the responsiveness of axillary buds to signals such as cytokinin (King and Van Staden, 1988), auxin (Morris et al., 2005), and SMS (Beveridge et al., 2003).
Photoperiod influences the zones of branching in intact and decapitated wild type and rms mutants of pea (Beveridge et al., 2003) and intact max mutants of Arabidopsis (Stirnberg et al., 2002), with reduced branching in the basal innovation zone under long days compared with short days. Photoperiod response genes may be involved in this control because, whereas wild-type plants often branch at node 2 under short days, early-flowering day-neutral (dne) mutants under any photoperiod or wild-type plants grown under long-day floral inductive conditions only branch at this basal zone when decapitated at early stages of development (Beveridge et al., 2003). These results introduce the possibility that photoperiod and genotype (e.g. the flowering gene DNE) can shift the location of the three morphological zones suggested by Weberling (1989) and/or the responsiveness of a bud within these zones.
Given that, in different situations, nongrowing buds have different responses to various treatments that may stimulate bud outgrowth, it is useful to consider that bud outgrowth consists of several stages and that particular signals may act at some, but not all, of these stages. We suggest that auxin acts to inhibit continued growth of buds that are in a transition stage (Fig. 2, a and b). In pea, studies have focused on the long-distance regulation of branching in vegetative plants where changes in auxin supply can be induced at an apical position many centimeters away from a given axillary bud. For example, using tall plants with several internodes, Morris et al. (2005) demonstrated that initial bud outgrowth is too rapid to be regulated by IAA. These authors showed that preliminary bud outgrowth could be promoted distal to the site of decapitation prior to detectable changes in polar IAA transport or the endogenous IAA level in adjacent stem tissue. Moreover, initial growth of the axillary bud at this distal node was similar to that of decapitated controls even when exogenous auxin was supplied to the decapitated pea stump. Finally, as mentioned previously, NPA applied in a lanolin ring around the stem below the shoot tip depleted the endogenous auxin content of the stem down to that of comparable decapitated plants but failed to induce measurable bud outgrowth (Morris et al., 2005). These results indicate that auxin depletion alone, directly or indirectly, does not induce initial bud outgrowth, indicating that other factors influence the competency to respond to changes in auxin.
The results of Morris et al. (2005) indicate that decapitation causes a rapidly propagated signal that triggers a dormant bud to enter a transition stage. In this transition stage, the bud starts preliminary growth only. This is best demonstrated by the initial, but not sustained, growth that occurs in IAA-treated, decapitated wild-type pea plants (Morris et al., 2005). Furthermore, molecular markers specific to a transition stage are expressed at early time points in buds of decapitated plants, with and without auxin treatment, even though the buds of the auxin-treated plants do not continue to grow out (Stafstrom and Sussex, 1992). If we incorporate the classical hypothesis of indirect auxin action, auxin may act to inhibit progression of buds from a transition stage to sustained bud outgrowth by modulating local cytokinin biosynthesis, and possibly cytokinin transport, and/or by exerting its regulation of SMS (Fig. 2, a and b). Similarly, if auxin transport capacity is the limiting factor, then it may be important at a transition stage (Fig. 2f).
Conducting experiments at various stages of plant and/or axillary bud development may explain why bud outgrowth has been reported following some NPA treatments and not others. Tamas et al. (1989) and Chatfield et al. (2000) showed that apical application of NPA prevents the inhibitory effect of auxin on bud outgrowth in detached stem segments of Phaseolus and Arabidopsis, respectively. Consistent with Morris et al. (2005) and the transition hypothesis, buds of detached stem segments would be induced to a stage of transition as occurs in decapitated plants; a decrease in auxin would then be expected to trigger progression from a transition stage to the sustained growth stage. Of the few reports of bud outgrowth following NPA treatment in intact plant systems, Nakajima et al. (2001) showed that application of the auxin transport inhibitor NPA or 2,3,4-triiodobenzoic acid induces bud outgrowth in intact dark-grown pea seedlings. According to the transition hypothesis, the buds of these seedlings must have been in a transition stage prior to auxin transport inhibitor application. This possibility is supported by Turnbull et al. (1997), who found that axillary buds of dark-grown chickpea (Cicer arietinum) seedlings were larger than those of comparable plants grown in the light.
What is the purpose of the developmental stages of bud outgrowth and why are they important? It is likely that these stages enable multiple buds to simultaneously respond to endogenous or environmental cues for outgrowth such as decapitation, yet remain responsive to a homeostatic control system that limits the number of buds that continue to grow into leafy shoots. The implication is that feedback processes modulate the number of primary and secondary axillary shoots and/or their growth. For example, double mutants between rms1, an SMS synthesis mutant, and rms2, proposed to be involved in feedback regulation of SMS synthesis, exhibit an additive branching phenotype (Beveridge et al., 1997b).
FEEDBACK CONTROL
Feedback regulation (Fig. 2j) is commonly involved in maintaining homeostasis in systems. In terms of shoot branching, lateral bud outgrowth is balanced with the growth of other plant parts, particularly other shoots. The feedback mechanisms identified thus far for shoot branching involve auxin, cytokinin, and SMS (Fig. 2, h, k, and l; for review, see Beveridge, 2006). For example, in pea, SMS synthesis gene expression is up-regulated and xylem sap cytokinin content is down-regulated in four of five rms branching mutants (Fig. 2, l and k; Beveridge et al., 1997a; Foo et al., 2005). Feedback mechanisms may also account for the elevated expression of SMS synthesis genes in branching mutants of Arabidopsis and petunia and for the reduced xylem cytokinin content in max mutant plants (C.G.N. Turnbull and N. Young, personal communication; Bainbridge et al., 2005; Snowden et al., 2005). Similarly, it is possible that enhanced level, transport, and/or transport capacity of auxin in the stem is part of a feedback mechanism (Fig. 2, g and h; Beveridge et al., 1994) and would also influence, or be influenced by, the supply of SMS (Fig. 2, b and l; Foo et al., 2005) and cytokinin within the shoot (Fig. 2, a and k; Nordstrom et al., 2004; Tanaka et al., 2006). Importantly, we currently have little evidence to determine whether the feedback proposed in each case is caused or regulated by the same or closely related processes.
Perhaps feedback regulation is also the best explanation for the recent finding by Lazar and Goodman (2006) that the MAX1 product of Arabidopsis affects flavonoid levels. These authors suggest that flavonoids repress bud outgrowth via regulating the loading and transport of auxin in the auxin transport stream. Indeed, some flavonoid compounds have been shown to reduce polar auxin transport in zucchini (Cucurbita pepo) hypocotyls (Jacobs and Rubery, 1988). If flavonoid synthesis is suppressed due to feedback regulation, one would expect that enhanced shoot branching would be correlated with reduced flavonoid content and an enhanced transport of auxin. Horvath et al. (2005) have indeed shown reduced expression of some flavonoid biosynthesis genes following the growth induction of adventitious buds of leafy spurge. However, Bennett et al. (2006) have placed doubt over the importance of flavonoids in shoot branching by showing that the tt4 mutant of Arabidopsis, which has severely reduced flavonoid content, has only modest changes in auxin transport and does not exhibit a substantial branching phenotype. Further studies might explore the phenotype of tt4-max double mutants and seek alternate explanations for the suppressed flavonoid content in max1 mutant plants.
SUMMARY AND PERSPECTIVES
In intact plants, and to a lesser extent in decapitated plants, the location of buds, plant developmental stage, and environmental factors, such as light, carbon acquisition, and nutrients, appear to be important and may determine intrinsic or extrinsic thresholds or limitations for responses to branching signals. The number of branches that grow out in a plant is also under homeostatic control. Developmental stages of bud outgrowth are likely associated with checkpoints for feedback regulation and hence enable the homeostasis of shoot number. These stages may also account for the observation that buds at different locations show different responses to signals such as auxin, cytokinin, and SMS (Fig. 2). We do not yet have unequivocal evidence that changes in cytokinin, SMS, and auxin levels and/or transport dynamics act to trigger buds from the dormant stage to a transition stage. Available evidence does however indicate that these hormones act at a transition stage to induce the progression to sustained growth. Research needs to be targeted to identify when and where buds are in a transition stage. Correlative studies must be interpreted carefully in view of these factors/stages and of the role of feedback processes that result from, rather than induce, particular stages of bud outgrowth.
The differing architecture and anatomy of plant species used for studies of shoot branching have led to differences in experimental methodology and to alternative perspectives. In particular, differences in methodology have led to varying conclusions pertaining to apical dominance in pea (e.g. Beveridge, 2006) and Arabidopsis (e.g. Bennett et al., 2006; Fig. 1). This is despite the fact that these and other species share similar branching control mechanisms, including homologous genes required for the synthesis of SMS (e.g. MAX, RMS, and DAD genes). We consider it premature to propose that highly divergent mechanisms of bud outgrowth regulation exist in these species. In some cases, seemingly conflicting findings from different species may be the result of variations in the relative importance of feedback mechanisms and factors controlling the progression of buds to a transition stage, rather than fundamental differences in the action, interaction, and perception of signals, such as auxin, SMS, and cytokinin. We also need to consider that some observations may be the product of consequential, rather than causal, events in bud outgrowth. Moreover, to rationalize findings from different studies on shoot-branching control, we may need to account for the possibility that bud outgrowth in isolated segments and in decapitated plants represents one particular module within the branching control network. For this reason, conclusions from studies using in vitro or decapitated plants need to be followed up using intact plants to extrapolate to intact systems.
Future work will surely comprise a combination of studies using different model species that offer different technical advantages (e.g. Fig. 1). The recent discovery of comparable branching mutants in monocot species (e.g. Ishikawa et al., 2005) also provides an exciting new opportunity to reveal conserved and divergent regulatory mechanisms of branching control.
Acknowledgments
We thank Dr. John Ross, Dr. Catherine Rameau, Dr. Colin Turnbull, Dr. Eloise Foo, and Geoffrey Dun and the two reviewers for helpful comments and discussion on the manuscript, in addition to Kerry Condon and Julia Cremer for kindly providing the plants for Figure 1.
This work was supported by an Australian Postgraduate Award (scholarship to E.A.D.) and the Australian Research Council.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Christine Anne Beveridge (c.beveridge@uq.edu.au).
References
- Bainbridge K, Sorefan K, Ward S, Leyser O (2005) Hormonally controlled expression of the Arabidopsis MAX4 shoot branching regulatory gene. Plant J 44: 569–580 [DOI] [PubMed] [Google Scholar]
- Bangerth F (1989) Dominance among fruits/sinks and the search for a correlative signal. Physiol Plant 76: 608–614 [Google Scholar]
- Bangerth F (1994) Response of cytokinin concentration in the xylem exudate of bean (Phaseolus vulgaris L.) plants to decapitation and auxin treatment, and relationship to apical dominance. Planta 194: 439–442 [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Opin Plant Biol 16: 553–563 [DOI] [PubMed] [Google Scholar]
- Beveridge CA (2006) Axillary bud outgrowth: sending a message. Curr Opin Plant Biol 9: 35–40 [DOI] [PubMed] [Google Scholar]
- Beveridge CA, Murfet IC, Kerhoas L, Sotta B, Miginiac E, Rameau C (1997. a) The shoot controls zeatin riboside export from pea roots. Evidence from the branching mutant rms4. Plant J 11: 339–345 [Google Scholar]
- Beveridge CA, Ross JJ, Murfet IC (1994) Branching mutant rms-2 in Pisum sativum. Plant Physiol 104: 953–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge CA, Symons GM, Murfet IC, Ross JJ, Rameau C (1997. b) The rms1 mutant of pea has elevated indole-3-acetic acid levels and reduced root-sap zeatin riboside content but increased branching controlled by graft-transmissible signal(s). Plant Physiol 115: 1251–1258 [Google Scholar]
- Beveridge CA, Symons GM, Turnbull CGN (2000) Auxin inhibition of decapitation-induced branching is dependent on graft-transmissible signals regulated by genes Rms1 and Rms2. Plant Physiol 123: 689–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge CA, Weller JL, Singer SR, Hofer JMI (2003) Axillary meristem development. Budding relationships between networks controlling flowering, branching, and photoperiod responsiveness. Plant Physiol 131: 927–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield SP, Stirnbirg P, Forde BG, Leyser O (2000) The hormonal regulation of axillary bud growth in Arabidopsis. Plant J 24: 159–169 [DOI] [PubMed] [Google Scholar]
- Cline MG (1996) Exogenous auxin effects on lateral bud outgrowth in decapitated shoots. Ann Bot (Lond) 78: 255–266 [Google Scholar]
- Cline MG (1997) Concepts and terminology of apical dominance. Am J Bot 84: 1064–1069 [PubMed] [Google Scholar]
- Cline MG, Chatfield SP, Leyser O (2001) NAA restores apical dominance in the axr3-1 mutant of Arabidopsis thaliana. Ann Bot (Lond) 87: 61–65 [Google Scholar]
- Devitt ML, Stafstrom JP (1995) Cell cycle regulation during growth-dormancy cycles in pea axillary buds. Plant Mol Biol 29: 255–265 [DOI] [PubMed] [Google Scholar]
- Foo E, Buillier E, Goussot M, Foucher F, Rameau C, Beveridge CA (2005) The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell 17: 464–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E, Turnbull C, Beveridge CA (2001) Long distance signaling and the control of branching in the rms1 mutant of pea. Plant Physiol 126: 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Hall SM, Hillman JR (1975) Correlative inhibition of lateral bud growth in Phaseolus vulgaris L. Timing of bud growth following decapitation. Planta 123: 137–143 [DOI] [PubMed] [Google Scholar]
- Horvath DP, Anderson JV, Chao WS, Foley MR (2003) Knowing when to grow: signals regulating bud dormancy. Trends Plant Sci 8: 534–540 [DOI] [PubMed] [Google Scholar]
- Horvath DP, Soto-Suarez M, Chao WS, Jia Y, Anderson JV (2005) Transcriptome analysis of paradormancy release in root buds of leafy spurge (Euphorbia esula). Weed Sci 53: 795–801 [Google Scholar]
- Ishikawa S, Maekawa M, Arite T, Onishi K, Takamure I, Kyozuka J (2005) Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol 46: 79–86 [DOI] [PubMed] [Google Scholar]
- Jacobs M, Rubery PH (1988) Naturally occurring auxin transport regulators. Science 241: 346–349 [DOI] [PubMed] [Google Scholar]
- King RA, Van Staden J (1988) Differential responses of buds along the shoot of Pisum sativum to isopentyladenine and zeatin application. Plant Physiol Biochem 26: 253–259 [Google Scholar]
- Lazar G, Goodman HM (2006) MAX1, a regulator of the flavonoid pathway, controls vegetative axillary bud outgrowth in Arabidopsis. Proc Natl Acad Sci USA 103: 472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser O (2005) The fall and rise of apical dominance. Curr Opin Genet Dev 15: 468–471 [DOI] [PubMed] [Google Scholar]
- Li CJ, Bangerth F (1999) Autoinhibition of indoleacetic acid transport in the shoots of two-branched pea (Pisum sativum) plants and its relationship to correlative dominance. Physiol Plant 106: 415–420 [Google Scholar]
- Li CJ, Herrera GJ, Bangerth F (1995) Effect of apex excision and replacement by 1-naphthylacetic acid on cytokinin concentration and apical dominance in pea plants. Physiol Plant 94: 465–469 [Google Scholar]
- Madoka Y, Mori H (2000) Acropetal disappearance of PsAD1 protein in pea axillary buds after the release of apical dominance. Plant Cell Physiol 41: 556–564 [DOI] [PubMed] [Google Scholar]
- McSteen P, Leyser O (2005) Shoot branching. Annu Rev Plant Biol 56: 353–374 [DOI] [PubMed] [Google Scholar]
- Medford JI, Horgan R, El-Sawi Z, Klee HJ (1989) Alterations of endogenous cytokinins in transgenic plants using a chimeric isopentenyl transferase gene. Plant Cell 1: 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DA (1977) Transport of exogenous auxin in two-branched dwarf pea seedlings (Pisum sativum L.). Planta 136: 91–96 [DOI] [PubMed] [Google Scholar]
- Morris SE, Cox MCH, Ross JJ, Krisantini S, Beveridge CA (2005) Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiol 138: 1665–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Turnbull CGN, Murfet IC, Beveridge CA (2001) Mutational analysis of branching in pea. Evidence that Rms1 and Rms5 regulate the same novel signal. Plant Physiol 126: 1205–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima E, Yamada K, Kosemura S, Yamamura S, Hasegawa K (2001) Effects of the auxin-inhibiting substances raphanusanin and benzoxazolinone on apical dominance of pea seedlings. Plant Growth Regul 35: 11–15 [Google Scholar]
- Napoli C (1996) Highly branching phenotype of the Petunia dad1-1 mutant is reversed by grafting. Plant Physiol 111: 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli CA, Beveridge CA, Snowden KC (1999) Reevaluating concepts of apical dominance and the control of axillary bud outgrowth. Curr Top Dev Biol 44: 127–169 [DOI] [PubMed] [Google Scholar]
- Nordstrom A, Tarkowski P, Tarkowska D, Norbaek R, Astot C, Dolezal K, Sandberg G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc Natl Acad Sci USA 101: 8039–8044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T, Thimann KV (1967) The role of auxins and cytokinins in the release of buds from dominance. Am J Bot 54: 136–144 [Google Scholar]
- Schrader J, Baba K, May ST, Palme K, Bennett M, Bhalerao RP, Sandberg G (2003) Polar auxin transport in the wood-forming tissues of hybrid aspen is under simultaneous control of developmental and environmental signals. Proc Natl Acad Sci USA 100: 10096–10101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SH, Qin X, Loewen MC (2004) The biochemical characterization of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. J Biol Chem 279: 46940–46945 [DOI] [PubMed] [Google Scholar]
- Shimizu-Sato S, Mori H (2001) Control of outgrowth and dormancy in axillary buds. Plant Physiol 127: 1405–1413 [PMC free article] [PubMed] [Google Scholar]
- Snow R (1937) On the nature of correlative inhibition. New Phytol 36: 283–300 [Google Scholar]
- Snowden KC, Simkin AJ, Janssen BJ, Templeton KR, Loucas HM, Simons JL, Karunairetnam S, Gleave AP, Clark DG, Klee HJ (2005) The Decreased apical dominance1/Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. Plant Cell 17: 746–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorefan K, Booker J, Haurogne K, Goussot M, Bainbridge K, Foo E, Chatfield S, Ward S, Beveridge C, Rameau C, et al (2003) MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev 17: 1469–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom JP (1995) Influence of bud position and plant ontogeny on the morphology of branch shoots in pea (Pisum sativum L. cv. Alaska). Ann Bot (Lond) 76: 343–348 [Google Scholar]
- Stafstrom JP, Sussex IM (1992) Expression of a ribosomal protein gene in axillary buds of pea seedlings. Plant Physiol 100: 1494–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P, van de Sande K, Leyser HMO (2002) MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129: 1131–1141 [DOI] [PubMed] [Google Scholar]
- Tamas IA, Schlossberg-Jacobs JL, Lim R, Friedman LB, Barone CC (1989) Effect of plant growth substances on the growth of axillary buds in cultured stem segments of Phaseolus vulgaris L. Plant Growth Regul 8: 165–183 [Google Scholar]
- Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45: 1028–1036 [DOI] [PubMed] [Google Scholar]
- Turnbull CGN, Booker JP, Leyser HMO (2002) Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J 32: 255–262 [DOI] [PubMed] [Google Scholar]
- Turnbull CGN, Raymond MAA, Dodd IC, Morris SE (1997) Rapid increases in cytokinin concentration in lateral buds of chickpea (Cicer arietinum L.) during release of apical dominance. Planta 202: 271–276 [Google Scholar]
- Weberling F (1989) Morphology of Flowers and Inflorescences. Cambridge University Press, Cambridge, UK