Abstract
The fungal biocontrol agent Trichoderma asperellum has been recently shown to induce systemic resistance in plants through a mechanism that employs jasmonic acid and ethylene signal transduction pathways. Mitogen-activated protein kinase (MAPK) proteins have been implicated in the signal transduction of a wide variety of plant stress responses. Here we report the identification and characterization of a Trichoderma-induced MAPK (TIPK) gene function in cucumber (Cucumis sativus). Similar to its homologs, wound-induced protein kinase, MPK3, and MPK3a, TIPK is also induced by wounding. Normally, preinoculation of roots with Trichoderma activates plant defense mechanisms, which result in resistance to the leaf pathogen Pseudomonas syringae pv lachrymans. We used a unique attenuated virus vector, Zucchini yellow mosaic virus (ZYMV-AGII), to overexpress TIPK protein and antisense (AS) RNA. Plants overexpressing TIPK were more resistant to pathogenic bacterial attack than control plants, even in the absence of Trichoderma preinoculation. On the other hand, plants expressing TIPK-AS revealed increased sensitivity to pathogen attack. Moreover, Trichoderma preinoculation could not protect these AS plants against subsequent pathogen attack. We therefore demonstrate that Trichoderma exerts its protective effect on plants through activation of the TIPK gene, a MAPK that is involved in signal transduction pathways of defense responses.
Trichoderma spp. are effective biocontrol agents for a number of soil-borne pathogens and are also known for their ability to enhance the plant-growth response. Trichoderma antagonizes plant pathogens by producing antibiotics, competing for nutrients in the rhizosphere and exhibiting mycoparasitism (Harman et al., 2004). Trichoderma asperellum (Trichoderma harzianum 203) can penetrate the roots of cucumber (Cucumis sativus) seedlings and colonize the epidermis and outer root cortex (Yedidia et al., 1999). Recently, it has been shown that these interactions induce systemic resistance (ISR) mechanisms in plants (Yedidia et al., 2003; Shoresh et al., 2005). Studies have shown that ISR induced by certain rhizospheric bacteria is not a salicylic acid (SA)-dependent phenomenon but rather requires components of the jasmonic acid (JA) signaling pathway followed by the ethylene signaling pathway (Pieterse et al., 2003). Analysis of signal molecules involved in defense mechanisms and application of specific inhibitors of plant hormones indicated the involvement of JA and ethylene in the protective effect conferred by Trichoderma spp. against the leaf pathogen Pseudomonas syringae pv lachrymans (Psl). Moreover, examination of local and systemic gene expression in cucumber plants revealed that T. asperellum (T203) modulates the expression of genes involved in the jasmonate/ethylene signaling pathways of ISR (Shoresh et al., 2005).
Plants are exposed to a wide variety of environmental stresses and they have developed a broad range of responses to resist these stresses. Mitogen-activated protein kinase (MAPK) pathways have been implicated in signal transduction for a wide variety of stress responses, and some may be involved in JA signaling pathways (Meskiene and Hirt, 2000; Zhang and Klessig, 2001). Exposure of plants to mechanical stress, such as touch or wounding, resulted in transcript accumulation of AtMPK3 or LeMPK3, respectively (Mizoguchi et al., 1996; Mayrose et al., 2004). Wounding of tobacco (Nicotiana tabacum) leaves induced transcript accumulation and activity of wound-induced protein kinase (WIPK), both locally and systemically. In transgenic plants in which WIPK has been silenced, wounding did not induce activation of MAPK or accumulation of the wound- and JA-inducible genes PI-II and basic PR1 (Seo et al., 1995). On the other hand, WIPK-overproducing plants showed constitutive PI-II transcript accumulation and WIPK activity, and JA and methyl jasmonate (MeJA) levels were 3- to 4-fold higher than in the wild type (Seo et al., 1999). These observations demonstrated a role for WIPK in the production of jasmonate.
There are numerous examples for the involvement of MAPKs in signaling pathways of plant responses to pathogens. Direct proof of this involvement came from studies of parsley (Petroselinum crispum) cells treated with Pep13, a 13-amino acid oligopeptide fragment derived from an extracellular glycoprotein of Phytophtora sojae. By binding to a specific plasma membrane receptor, Pep13 activated plant defense responses, including activation of the MAPK MPK3a, at both the transcriptional and posttranslational levels (Ligterink et al., 1997; Kroj et al., 2003). WIPK was also activated by a fungal cell wall elicitor (Zhang et al., 1998) and by the avr9 protein from the fungal pathogen Cladosporium fulvum (Romeis et al., 1999). AtMPK3 was activated by flg22, a 22-amino acid peptide corresponding to the most conserved domain of eubacterial flagellin (Asai et al., 2002). LeMPK3 was found to be transcriptionally up-regulated by both pathogenic bacteria and fungal elicitor (Mayrose et al., 2004).
Plant RNA viruses have been shown to be an efficient tool for overexpression and knockdown expression of endogenous genes. This technology provides important new insights into the roles of specific genes in plant development and plant defense responses. Virus-induced gene silencing is a quick and efficient technique involving recombinant viruses for reverse genetics by down-regulation of target genes (Burch-Smith et al., 2004; Robertson, 2004).
Zucchini yellow mosaic virus (ZYMV) is a member of the Potyviridae family. ZYMV-AGII is a potyvirus-based vector system that has been successfully used for overexpression of various foreign genes in cucurbits (Arazi et al., 2001; Aly et al., 2005). In contrast to other known viral vectors, which cause severe disease to host plants, the AGII vector was created from an attenuated engineered ZYMV potyvirus (Arazi et al., 2001) and does not elicit the severe phenotype or developmental impairment caused by wild-type virus, and no symptoms are developed in cucumbers (Gal-On and Raccah, 2000). Therefore, it is attractive for over- or down-regulation of endogenous gene expression.
In this study, we demonstrate that in cucumber, a MAPK is activated by inoculation of the roots with the biocontrol agent T. asperellum. Moreover, activation of this gene is necessary for the plant's Trichoderma-conferred defense against bacterial pathogens; silencing of this MAPK completely eliminates this protection. We present evidence suggesting that this MAPK is also involved in other plant stress responses, including that to wounding.
RESULTS
A MAPK Is Induced by Trichoderma Inoculation of Plants
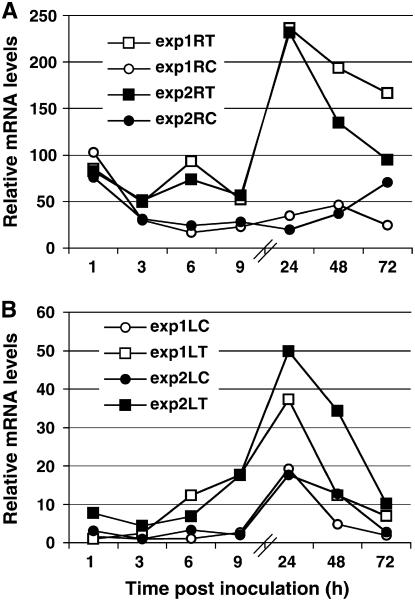
We recently demonstrated the involvement of the plant signal molecules JA and ethylene in the Trichoderma-induced plant defense response. We were therefore interested in analyzing the expression of genes involved in signal transduction during the plant-Trichoderma interaction. Because many MAPKs have been found to be involved in the plant defense response and their regulatory effects on the control of plant defense responses have been described, we decided to focus on these kinases. PCR with degenerate primers designed according to resistance-associated MAPK plant proteins allowed us to isolate a putative resistance-associated cucumber MAPK clone. We used real-time PCR analysis to examine the expression profile in reaction to Trichoderma inoculation of plant roots. The specificity of the primers was confirmed by having one clear peak of melting curve, indicating only one fragment is produced by this primer pair and by sequencing the PCR product. Six hours after Trichoderma inoculation, the MAPK mRNA levels in the roots increased, peaking at 4- to 5-fold the level in untreated plants (Fig. 1A). This was followed by another, higher peak 24 h postinoculation (hpi). In leaves, the expression levels of the gene also began to rise 6 hpi and reached a peak at 24 hpi, which was about two and one-half times higher than in noninoculated plants (Fig. 1B). Expression of this MAPK in leaves exemplifies a systemic gene response to root inoculation. Our results suggest that this Trichoderma-induced MAPK (TIPK) participates in the Trichoderma-induced defense response.
Figure 1.
Time course of TIPK gene expression. Expression was measured in roots (A) and leaves (B) of cucumber plants after Trichoderma inoculation of the root compartment (time zero) and normalized versus the control gene. Two experiments (white and black symbols) were conducted, each including approximately 20 plants per time point. Relative mRNA levels were determined by real-time PCR (see “Materials and Methods”). The internal sd values for each experiment were smaller than the size of the symbols. ▪ and □, Inoculated with Trichoderma; • and ○, control, mock-inoculated plants.
TIPK Is a Homolog of Wound-Induced MAPKs
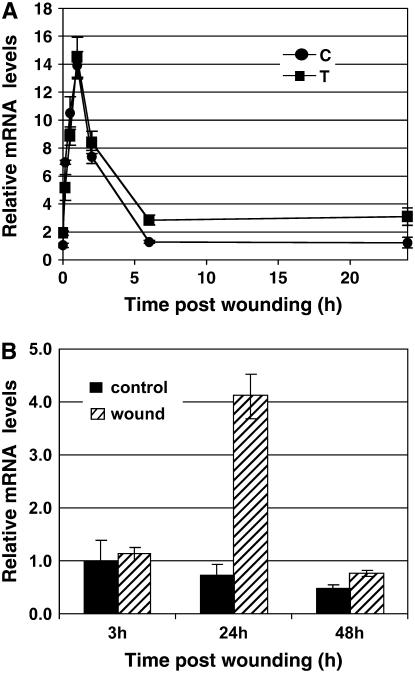
Using RACE analysis, we obtained the full-length cDNA of TIPK. Further PCR and sequence analysis enabled us to deduce the genomic organization of the gene encoding TIPK in the cucumber genome (Fig. 2A). When genomic DNA was digested with the appropriate restriction endonucleases and hybridized at high-stringent conditions with a TIPK cDNA probe, we observed unique hybridization signals, which were expected according to the deduced restriction map (Fig. 2B). This indicated that the gene encoding TIPK is apparently present as a single-copy gene in the cucumber genome. This MAPK is 84% identical and 93% similar to WIPK (tobacco), 82% identical and 92% similar to MPK3 (Arabidopsis [Arabidopsis thaliana]), and 81% identical and 91% similar to MPK3a (parsley; Fig. 3). The expressions of wipk and mpk3 have been shown to be wound induced. We therefore examined whether TIPK also responds to wounding. Wounding leaves with carborundum resulted in a rise in TIPK expression levels, starting from 10 min and peaking 1 h postwounding, with or without preinoculation with Trichoderma (Fig. 4A). TIPK was also up-regulated in systemic (nonwounded) leaves 24 h postwounding (Fig. 4B). These results demonstrate the close functional relationship of TIPK with other wound-induced MAPKs.
Figure 2.
Genomic organization of TIPK gene. A, Schematic illustration of the TIPK gene as deduced from sequence and PCR analyses. B, Southern analysis of total cucumber DNA digested by the indicated restriction enzyme using TIPK cDNA as a probe. EcoRI digested DNA was made in separate membrane to get more clear data (faint bands correspond to residuals of partial digestion).
Figure 3.
Protein-sequence alignment of the TIPK sequence (CsTIPK) with its homologs from Arabidopsis (AtMPK3; gi21431794), tobacco (NtWIPK; gi18143321), and parsley (PcMPK3a; gi2231034). Fully conserved residues are indicated by black boxes; gray and white colors represent similar and different amino acids, respectively.
Figure 4.
Time course of TIPK gene expression. A, Expression was measured in wounded leaves of cucumber plants after wounding (time zero), 96 h post Trichoderma inoculation into the root compartment, and normalized versus the control gene. Relative levels of TIPK mRNA were determined by real-time PCR (see “Materials and Methods”). Symbol at each time point represents the average of 10 plants ± se. •, Non-Trichoderma inoculated and wounded; ▪, inoculated with Trichoderma and wounded. B, Expression was measured in the nonwounded upper leaves of wounded plants (hatched boxes) and control nonwounded plants (black boxes). The presented values are means (±se, n = 4).
Effect of Plant Hormones and Their Inhibitors on TIPK Expression
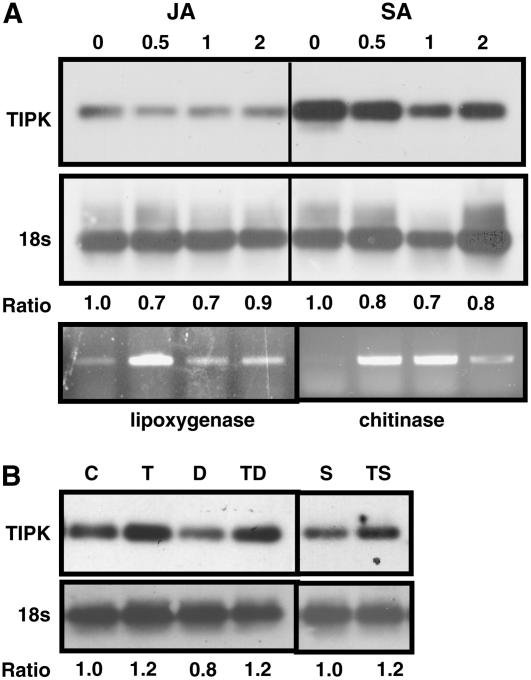
Because both Trichoderma inoculation and wounding exert their effects via the JA and ethylene signal molecules, we examined whether plant hormones or their inhibitors might affect TIPK expression levels. Treatment of cucumber roots with JA or SA did not seem to affect the TIPK expression in roots (Fig. 5A). The effect of JA and SA treatments was validated by examining the JA- and SA-inducible genes, lipoxygenase and chitinase, respectively (Fig. 5A). Because we identified a peak of TIPK expression at 24 hpi, we decided to focus on this time point for hormone treatment experiments; however, it may be that an activation of the gene occurred at a different time point under these treatments. We also examined whether inhibitors of JA production (diethyldithiocarbamic acid [DIECA]) or ethylene action (silver thiosulfate [STS]) would reduce the induction of TIPK expression in roots by Trichoderma inoculation. We noticed that treatments with JA, SA, and DIECA could repress TIPK expression when compared to control. However, using both inhibitors, induction of TIPK gene expression was similar to that in the control experiment (Fig. 5B). This suggests that TIPK may act prior to the hormones' actions.
Figure 5.
RT-PCR analysis of the TIPK gene and the control gene 18S (A) after JA or SA root treatments at concentrations of 0, 0.5, 1, and 2 mm each, and after DIECA (inhibitor of jasmonate biosynthesis) or STS (inhibitor of ethylene action) root treatments at concentrations of 100 μm and 0.25 mm, respectively (B). PCR was conducted for 20 cycles for the TIPK gene, 25 cycles for lipoxygenase and chitinase genes, and 18 cycles for the 18S gene to keep the amplification within the linear region of the reaction. For each gene, we first normalized the intensity of the band versus its corresponding 18S band. We then calculated the ratio between each hormonal treatment to the control (no hormonal treatment). The ratio, therefore, indicates the expression of each gene as compared to control treatments. C, Control; T, Trichoderma inoculation; D, DIECA treatment; TD, Trichoderma inoculation and DIECA treatment; S, STS treatment; TS, Trichoderma inoculation and STS treatment.
Expression of TIPK after Psl Infection Is Potentiated by Prior Trichoderma Inoculation
The expression of TIPK in roots of Trichoderma-inoculated and Psl-challenged plants was higher than in plants subjected to only one of those treatments (Fig. 6). Moreover, whereas leaves of Trichoderma-inoculated plants did not differ in TIPK expression level from the controls, leaves of plants inoculated with Trichoderma and challenged with Psl expressed 3- to 4-fold higher TIPK mRNA levels than plants challenged only with Psl (Fig. 6). It should also be noted that while systemic expression of TIPK decreased in Trichoderma-inoculated plants 72 hpi (Fig. 1B) and remained low at 96 hpi (Fig. 6, +T−P treatment), the high levels of TIPK transcript in leaves of the +T+P treatment 96 hpi (Fig. 6) suggest that Trichoderma inoculation prior to pathogen challenge also results in prolonged induction of the gene. This indicates that the plant's interaction with Trichoderma enables a stronger defense reaction to subsequent pathogen attack.
Figure 6.
Relative expression levels of TIPK postpathogen challenge. Expression was measured in roots (hatched boxes) and leaves (black boxes) of cucumber plants after the following treatments: −T−P, Control (mock inoculations); +T−P, Trichoderma inoculation in the root compartment, at time zero; −T+P, Psl infection of cotyledons at time 48 h; +T+P, Trichoderma inoculation at time zero and Psl infection at time 48 h. Roots and leaves for expression measurements were harvested 48 h after time of Psl infection. These experiments were repeated three times with approximately 15 plants/treatment. The presented values are means of all plants in each treatment (±se).
Construction of AGII-TIPK and AGII-TIPK Antisense
The TIPK cDNA and an antisense (AS) fragment of TIPK were inserted between the NIb and coat protein (CP) genes of the AGII virus vector using a polylinker-cloning site next to the NIa proteinase cleavage site in the NIb gene at the 3′ end of AGII (Fig. 7A). Inserted genes were designed to create an in-frame translational fusion with both flanking NIa processing sites. Proteolysis of the nascent AGII-TIPK polyprotein by NIa protease in trans was predicted to yield recombinant TIPK protein lacking the first Met and having an additional seven amino acid residues (VDTVMLQ) at the C terminus. AGII-AS was designed to produce a polyprotein that would be processed to produce all the viral proteins necessary for its reproduction in the plant, while the AS fragment embedded in the viral RNA genome was significant for the induction of TIPK silencing via virus-induced gene silencing. The presence of the intact TIPK and AS sequences was verified by reverse transcription (RT)-PCR of the viral progeny 14 and 17 d postinoculation (dpi; Fig. 7B) as well as by direct sequencing of the amplified products. As a control treatment, we used the AGII-green fluorescent protein (GFP) construct.
Figure 7.
Expression of TIPK gene via ZYMV-AGII. A, Schematic presentation of the AGII genome. AGII noncoding (stippled) and coding (white boxes) regions, and the inserted foreign sequences (TIPK, AS, and GFP) are shown. Arrows indicate NIa protease, involved in proteolysis of the foreign gene products. NIa cleavage sites are indicated by /. Amino acid sequences corresponding to the NIa protease recognition motif are indicated in bold. B, Analysis by RT-PCR of AGII-GFP, AGII-AS, and AGII-TIPK viral RNA accumulation 14 and 17 dpi. Total RNA was extracted from infected and noninfected plants and subjected to RT-PCR with primers flanking the insertion point. The expected sizes of the PCR fragments are: AGII-GFP, 1,180 bp; AGII-AS, 680 bp; and AGII-TIPK, 1,480 bp.
Expression of TIPK Is Modified by Infection with the Different AGII Constructs
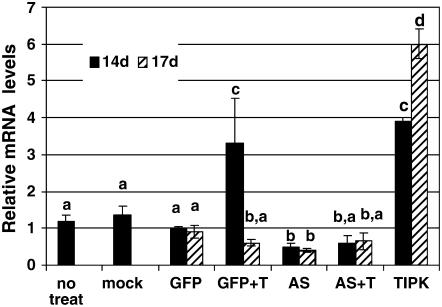
The AGII-GFP construct has been reported to be stable in cucumber plants for at least 60 dpi. Nevertheless, we verified the viral accumulation in these plants in our hydroponic system over the time interval needed for our experiments. The second leaves of systemic AGII-GFP-infected cucumbers were analyzed for GFP by visualization under UV light. Green fluorescence was observed in many regions of the leaves, but not in those that were mock inoculated (Fig. 8). Real-time PCR analysis using primers designed according to the CP gene demonstrated a consistent rise in virus quantity from 8 to 17 dpi, resulting in a 40-fold higher quantity on day 17 than on day 8 (data not shown). We then measured the endogenous RNA levels of TIPK in the AGII-GFP-, AGII-TIPK-, and AGII-AS-infected plants. The RNA levels of TIPK in the AGII-TIPK plants were 3- and 6-fold of controls, 14 dpi and 17 dpi, respectively (Fig. 9), whereas TIPK mRNA levels in the AGII-AS were 50% and 60% of controls, 14 dpi and 17 dpi, respectively (Fig. 9). Therefore, infection with the AGII constructs allowed us to modify endogenous TIPK RNA levels and produce TIPK-overexpressing and-underexpressing plants.
Figure 8.
Virus localization in leaves. Visualization of GFP fluorescence in leaves of AGII-GFP-infected plants and mock-infected plants (bottom) compared with the leaves (top), 17 d postinfection.
Figure 9.
Relative expression levels of TIPK in AGII-construct-infected plants. Expression was measured in leaves of cucumber plants 14 d (black boxes) and 17 d (hatched boxes) post-AGII-construct infection. Whenever plants were also treated with Trichoderma, it was done 24 h prior to time point of 14 dpi. The number of plants in each treatment were as follows: no treatment, 14 dpi, six plants; mock inoculation, 14 dpi, 10 plants; AGII-GFP infected (GFP), 14 dpi, 12 plants, and 17 dpi, 20 plants; AGII-GFP infected with Trichoderma inoculation (GFP + T), 14 dpi, six plants and 17 dpi, six plants; AGII-AS infected (AS), 14 dpi, 16 plants and 17 dpi, 22 plants; AGII-AS infected with Trichoderma inoculation (AS + T), 14 dpi, six plants and 17 dpi, six plants; AGII-TIPK infected (TIPK), 14 dpi, six plants and 17 dpi, six plants. The presented values are means (±se). Different letters indicate statistically significant differences between treatments by one-way ANOVA data analysis and Tukey-Kramer for comparing means (α = 0.05).
To verify the specificity of the AS construct, we cloned a fragment of another cucumber MAPK that had 97% and 96% identity to the MSK7 (alfalfa [Medicago sativa]; gi:298019) and MPK6 (Arabidopsis; gi:15224359) proteins, respectively, but only 87% identity to the TIPK protein. The RNA levels of this MAPK gene in the AGII-GFP and AGII-AS-infected plants had no significant difference at 14 dpi (means of relative RNA levels ± se and the number of repeats were as follows: AGII-GFP 14 dpi, 1.00 ± 0.21, n = 5; AGII-AS 14 dpi, 0.93 ± 0.19, n = 6). At 17 dpi, we also observed no significant difference between AGII-GFP and AGII-AS plants (AGII-GFP 17 dpi, 0.59 ± 0.19, n = 6; AGII-AS 17 dpi, 0.70 ± 0.21, n = 5).
Examination of TIPK expression in AGII-GFP plants treated with Trichoderma (Fig. 9, plants marked GFP + T) revealed the expected up-regulation of the gene at 14 dpi (which is 24 hpi) and reduction of the expression to basal level at 17 dpi (which is 96 hpi). These results are similar to TIPK expression observed in cucumber plants treated with Trichoderma (Fig. 1).
In addition, we also compared the expression of TIPK in AGII-AS plants after Trichoderma inoculation (Fig. 9, plants marked AS + T) to AGII-GFP plants treated with Trichoderma (Fig. 9, plants marked GFP + T). While in the AGII-GFP plants treated with Trichoderma, TIPK expression increased 24 hpi (14 dpi), at this time point not only the TIPK expression was not elevated in AGII-AS plants post Trichoderma inoculation, but it was decreased (Fig. 9).
Sensitivity to Pathogen Challenge Is Modified by Changes in TIPK RNA Levels
To determine whether TIPK plays a role in the Trichoderma-induced defense response, we challenged AGII-TIPK- and AGII-AS-infected plants with Psl and determined the levels of Psl proliferation in the plants' leaves. As a control, we used AGII-GFP-infected plants. Some samples varied greatly from the average of their group with respect to pathogen multiplication. Because the distribution of AGII virus in leaves was not uniform (Fig. 8), we speculate that when the pathogenic bacteria penetrate the leaf in a region where the AGII virus exists, the latter can affect the bacterial propagation based on its transgenic construct. On the other hand, when the bacteria and the virus do not colocalize, the pathogen will behave as in control leaves. For example, in the group of AGII-AS transformants, one plant had 150-fold fewer bacteria than the average, another transformant in this group had 5-fold less bacteria. We assume that in these transformants, the virus and the Psl did not colocalize, or the viral infection was not efficient. In the AGII-TIPK transformants, we had four plants with levels of bacteria that were more similar to the AGII-GFP control plants. This could also result from noncolocalization of the virus and the Psl or inefficient viral infection. Nevertheless, the AGII-TIPK transformants group was significantly different from the AGII-GFP plants (Fig. 10). In the AGII-AS plants treated with Trichoderma, we had five plants with 5- to 10-fold higher Psl counts than all other plants. In these plants, the virus either colocalized with the bacteria perfectly, or the efficiency of the AS inhibition was greater. In either case, it fits well with our hypothesis that inhibition of TIPK expression decreases plant defense even in the presence of Trichoderma.
Figure 10.
Quantification of Psl multiplication in AGII-construct-treated plants. Cotyledons of 10-d-old cucumbers grown in the hydroponic system were mechanically inoculated with AGII-virus construct and after 13 d inoculated with Trichoderma. Challenge was performed 48 h post Trichoderma inoculation. Leaves were harvested 72 h postchallenge with Psl. The treatments and the number of repeats in each treatment were as follows: AGII-AS challenged with Psl, n = 12; AGII-AS preinoculated with Trichoderma and challenged with Psl, n = 20; AGII-GFP challenged with Psl, n = 23; AGII-GFP preinoculated with Trichoderma and challenged with Psl, n = 26; AGII-TIPK challenged with Psl, n = 18. The presented values are means (±se). Different letters indicate statistically significant differences between treatments by one-way ANOVA data analysis and Tukey-Kramer for comparing means (α = 0.05).
Psl multiplication was significantly lower at 72 dpi in the AGII-TIPK (29 × 106, compare with μ/g fresh weight [FW]) plants than in AGII-GFP control plants (181 × 106, compare with μ/g FW; Fig. 10). In fact, Psl multiplication in AGII-TIPK plants was similar to that observed in AGII-GFP plants that had been inoculated with Trichoderma prior to pathogen challenge (37 × 106, compare with μ/g FW). This demonstrates that overexpression of the TIPK gene can confer the same level of protection as Trichoderma inoculation. Although Psl multiplication was higher in AGII-AS plants (303 ×106, compare with μ/g FW) than in AGII-GFP control plants (181 × 106, compare with μ/g FW), these differences were not statistically significant (Fig. 10). More importantly, the significantly higher Psl multiplication in plants infected with AGII-AS and preinoculated with Trichoderma (940 × 106, compare with μ/g FW) than in AGII-GFP control plants (Fig. 10) demonstrates that reduced TIPK expression abolishes the protective effect normally conferred by Trichoderma.
DISCUSSION
In recent years, it has become clear that MAPK signaling pathways are involved in plant resistance. We isolated and characterized a MAPK gene that is activated by root inoculation with the biocontrol fungus T. asperellum (Fig. 1). Sequence analysis demonstrated that the TIPK is homologous to MPK3a, WIPK, and MPK3 (Fig. 3), genes which have been shown to be up-regulated by pathogenic bacterial and fungal elicitors (Ligterink et al., 1997; Zhang et al., 2000; Schenk et al., 2003; Mayrose et al., 2004). Moreover, similar to these genes, TIPK is also induced by wounding. The transcriptional response is fast and transient, with transcript levels starting to accumulate 10 min after wounding and reaching a maximum at 1 h (Fig. 4A). This fast and transient accumulation is very similar to the reaction of AtMPK3, LeMPK3, and WIPK to mechanical stress and wounding (Seo et al., 1995; Mizoguchi et al., 1996; Mayrose et al., 2004). The kinetics of the accumulation of TIPK mRNA in response to Trichoderma root inoculation was slower than that in response to wounding, reaching a maximum at 24 hpi (Fig. 1A). This demonstrates that TIPK is induced with distinct activation kinetics by different stimuli and suggests that TIPK may be a convergence point for different stimuli and that different induction kinetics may play a role in the types of downstream processes it mediates.
However, we also observed systemic expression of the gene in leaves post Trichoderma inoculation (Fig. 1B) and postwounding (Fig. 4B). Moreover, the systemic expression post Trichoderma inoculation was much higher and more prolonged when plants were inoculated with Trichoderma prior to pathogen challenge (Fig. 6). The potentiation effect of Trichoderma on plant defense-related gene expression has been recently demonstrated (Yedidia et al., 2003; Shoresh et al., 2005). It has also been shown that plants preinoculated with Trichoderma are more resistant to subsequent Psl challenge (Shoresh et al., 2005). Together with the results presented here, the plant's interaction with Trichoderma prior to pathogen challenge appears to enable a stronger defense response.
The expression of TIPK in wounded plant leaves post Trichoderma root inoculation did not differ from that in wounded leaves from noninoculated plants (Fig. 4A), and no potentiation effect was observed postwounding in plants preinoculated with Trichoderma (data not shown). This demonstrates that TIPK potentiation post Trichoderma inoculation is specific to the plant's response to pathogen challenge.
We have recently demonstrated the involvement of the JA signaling pathway in the Trichoderma-induced plant response (Shoresh et al., 2005). WIPK-overproducing plants show 3- to 4-fold higher JA and MeJA levels than wild-type plants (Seo et al., 1999). Similarly, rice (Oryza sativa) plants transformed with MK1, a homolog of WIPK from pepper (Capsicum annuum), showed a 3-fold higher level of JA than the wild type (Lee et al., 2004). On the other hand, wipk-silenced plants produced much less JA and MeJA after wounding than wild-type plants (Seo et al., 1995). We found that JA or SA could not activate transcription of TIPK, even at high concentrations (Fig. 5A). In fact, the hormones seem to inhibit the basal expression of TIPK, suggesting that there may be some modulation of the gene activity by those hormones. But this modulation of gene expression is different from what we observe with the Trichoderma inoculation. JA is also unable to induce expression of LeMPK3 (Mayrose et al., 2004) or activate WIPK or its alfalfa homolog, SAMK (Bögre et al., 1997; Kumar and Klessig, 2000). We previously demonstrated that root treatments with either STS or DIECA do not affect root inoculation by Trichoderma (Shoresh et al., 2005). While a JA-production inhibitor (DIECA) can decrease Trichoderma's protective effect on plants against the pathogen (Shoresh et al., 2005), it did not affect TIPK-induced expression post Trichoderma root inoculation (Fig. 5B). We also found that while an ethylene-action inhibitor (STS) can decrease Trichoderma's protective effect on plants against the pathogen (Shoresh et al., 2005), it does not inhibit TIPK induction by Trichoderma (Fig. 5B). This is consistent with the observation that WIPK is not activated by ethylene (Kumar and Klessig, 2000). These results together with previous studies of TIPK homologs suggest a role for TIPK upstream of these signaling molecules during plant reaction to Trichoderma inoculation.
If signaling through the MAPK cascade is the primary or only route by which Trichoderma-interaction information can be transmitted to trigger a defense response, then interfering with the cascade by reducing TIPK expression should reduce the plant's resistance to subsequent pathogen challenge, even in the presence of Trichoderma. Therefore, we modified the expression levels of TIPK by employing an attenuated potyvirus vector, ZYMV-AGII (Arazi et al., 2001). This vector represents a unique system for gene expression in cucurbit cytosol. Because it was engineered from an attenuated strain, it is nonpathogenic and does not impair growth of the host plant (Arazi et al., 2001). The AGII-GFP construct has been reported to be stable in cucumber plants for at least 60 dpi (Arazi et al., 2001), and we also validated the propagation of the virus during our experimental period. We determined the stability of the viral constructs throughout the experiments (Fig. 7B). The distribution of AGII virus in leaves was not uniform (Fig. 8). Similar observation of nonuniform expression via the AGII vector was observed in other studies while using this vector (Arazi et al., 2001; Aly et al., 2005). Nevertheless, the AGII vector provides a good systemic expression system for a gene of interest.
Introducing the coding region of TIPK into the AGII viral genome allowed us to create TIPK-overproducing plants. In these plants, 14 d after AGII-TIPK infection, the level of TIPK RNA was 3-fold higher than in control AGII-GFP plants, and 17 dpi, the levels of TIPK were 6-fold that in controls (Fig. 9). When AGII-GFP plants were inoculated with Trichoderma, we observed a 3-fold elevation in TIPK mRNA levels (Fig. 9), which is on the same order of magnitude as in TIPK-overproducing plants. Challenging the latter with a bacterial pathogen resulted in pathogen resistance that was at least as effective as with the Trichoderma root treatment of AGII-GFP control plants (Fig. 10). In a recent study, transformation of rice plants with the gene MK1, the pepper homolog of WIPK, resulted in expression of the transgene at both the RNA and protein levels, as well as increased resistance to rice blast disease (Lee et al., 2004). Together, this demonstrates that plant resistance to subsequent pathogens is conferred by overexpression of TIPK.
Southern analysis of DNA digested by several restriction enzymes revealed that TIPK is present as a single-copy gene (Fig. 2), thus simplifying the interpretation of the AS experiments. Similarly, the TIPK homologs WIPK and MPK3a have been shown to be single-copy genes (Seo et al., 1995; Ligterink et al., 1997).
When we infected cucumber plants with AGII virus harboring an AS fragment of TIPK, a reduction of approximately 50% in TIPK mRNA levels was obtained (Fig. 9). It is important to note that the primers used to detect these mRNA levels did not overlap with the AS fragment cloned into the AGII-AS construct, thus enabling us to detect endogenous mRNA levels of TIPK gene. Virus-derived siRNA has been shown to accumulate in plants infected with Potyvirus (Xie et al., 2004; A. Gal-On, unpublished data; in ZYMV-infected plants). This indicates that the silencing process is initiated in the cells of the plant despite the presence of the potyviral suppressor HC-Pro. We assume that in plants infected with AGII-AS, ZYMV siRNAs are produced and include the TIPK AS sequence. The TIPK siRNAs may target, and cause cleavage of, the TIPK mRNA through the RISC complex. Additionally, the TIPK siRNA could serve as a primer for plant RdRp-mediated TIPK dsRNA amplification, which would then be degraded by DICER-like proteins as part of the plant-silencing mechanism. The mRNA levels of a homologous MAPK were not decreased in the AGII-AS plants as compared to AGII-GFP plants, indicating the specificity of the AS. TIPK expression was also silenced in AGII-AS plants inoculated with Trichoderma (Fig. 9). More importantly, Psl multiplication in TIPK-silenced plants preinoculated with Trichoderma was higher than in AGII-GFP control plants, while in AGII-GFP plants preinoculated with Trichoderma, Psl multiplication was largely reduced. Therefore, in our TIPK-silenced plants, Trichoderma treatment failed to protect the plants from subsequent pathogenic challenge (Fig. 10). Altogether, this clearly demonstrates that TIPK is a crucial component in the pathway of signals being transferred from the interaction site of Trichoderma with the plant. Moreover, it is shown clearly that Trichoderma exerts its protective effect on plants through activation of the TIPK gene. So it appears that without the ability to activate this gene, the plant cannot be protected by Trichoderma.
MATERIALS AND METHODS
Plant Material
Seeds of cucumber (Cucumis sativus L. cv Kfir) from Gedera Seeds were used in this experiment. Plant growth medium (PGM) was prepared according to Yedidia et al. (1999).
Axenic Growth System
Seeds were surface sterilized in 2.0% (v/v) NaOCl for 2 min and thoroughly washed with sterile distilled water. Seeds (25/box) were placed on a sterile gauze sheet, which was then placed in an axenic hydroponic growth system (Yedidia et al., 1999). Plants were grown in a controlled environment: 26°C, 80% relative humidity, light 300 μE m−2 s−1, and a circadian cycle of 16 h light and 8 h darkness.
Fungal Material
Trichoderma asperellum (Trichoderma harzianum strain T203) was grown on potato (Solanum tuberosum) dextrose agar (Difco). Synthetic medium for T. asperellum was prepared according to Yedidia et al. (1999). The inoculum consisted of 1 mL (109 spores, as counted by hemocytometer) of 10-d-old T. asperellum cultured on potato dextrose agar added to a 250-mL flask containing 100 mL synthetic medium. The flask was shaken at 150 rpm for 16 to 18 h at 30°C to allow spore germination. The inoculum was then separated from the growth medium by centrifugation at 5,000 rpm at 4°C, followed by two washes with 100 mL distilled water.
Trichoderma Plant Inoculation
Inoculum was added under aseptic conditions to the PGM of 7-d-old seedlings to a final concentration of ±105 germinated spores/mL (Yedidia et al., 1999). Control plants were treated with sterile distilled water.
Plants were harvested at 1, 3, 6, 9, 24, 48, and 72 hpi. The induced expression of defense-related genes was examined in roots and leaves. These experiments were repeated twice, and each time point represents approximately 20 plants/experiment.
Treatment with Plant Hormones and Plant-Hormone Inhibitors
Plants were grown in a hydroponic growth system for 12 d and then transferred to 50-mL tubes in closed chambers, five plants per tube. Each tube contained hydroponic medium ± the hormone. Hormone concentrations (both JA and SA) were: 0 (control), 0.5, 1, and 2 mm. Plants were harvested at 24 h postexposure to the hormones (five plants per treatment at each time point).
DIECA (Sigma), a potent inhibitor of jasmonate biosynthesis (Menke et al., 1999), was added to the root compartment at a final concentration of 100 μm, 1 h after Trichoderma inoculation.
STS, an inhibitor of ethylene action (Abeles et al., 1992), was prepared by mixing solutions of 0.1 m sodium thiosulfate with 0.1 m silver nitrate in a 4:1 (v/v) ratio. STS was added to the root compartment at a final concentration of 0.25 mm, 3 hpi. Plants roots were harvested 24 hpi.
The treatments and the controls were: −T−inhibitor; −T+inhibitor; +T−inhibitor; +T+inhibitor.
Wound Treatments
Plants were grown in a hydroponic growth system for 11 d. The roots were inoculated with germinated Trichoderma (T203) spores (as already described). After 96 h, leaves were wounded by rubbing with carborundum and then harvested from each treatment at the following time points: 0 min, 10 min, 30 min, 1 h, 2 h, 6 h, and 24 h postwounding, with 10 plants/time point.
RNA Isolation
For RNA analysis, roots and leaves were harvested and placed immediately in liquid nitrogen and then stored at −70°C until use (1–2 weeks). Total RNA was extracted using the EZ-RNA Total RNA Isolation kit (Biological Industries). RNA was treated with RNase-free DNase I in 40 mm Tris-HCl, pH 7.9, 10 mm NaCl, 6 mm MgCl2, and 1 mm CaCl2 for 30 min at 37°C (Roche). This was followed by a phenol/chloroform and chloroform extraction and a subsequent ethanolic precipitation.
Cloning and Sequencing
Degenerate primers designed according to several known MAPK plant genes were used to isolate the MAPK gene using the Expand High Fidelity PCR system (Roche). Primer sequences used to clone TIPK were: forward 5′-GG(C/T)GCTTA(T/C)GG(T/A/C)AT(T/G)GT(C/T)TGT-3′, reverse 5′-ACC(A/G)AC(A/T)GACCA(A/T)ATATCAA-3′. Primer sequences used to clone MPK6 were: forward 5′-C(A/C/T)TT(T/C)AA(T/C)GATGT(G/T)TA(C/T)AT(T/C)GC(A/G)TA-3′, reverse 5′-TCTGA(A/T)GG(T/A)GT(G/T/A)CC(A/T)AT(C/G)A(G/A)CTCCA-3′.
PCR fragments were cloned in pGEM-T Easy Vector (Promega) and both strands were sequenced.
RACE and Genomic Walk Analysis
A 5′/3′ RACE kit (Roche) was used to isolate 5′ and 3′ sequences according to the manufacturer's instructions. The gene-specific primers used were: reverse 5′-CAAGGCACTCTAATTCGAAGATGCGGT-3′, forward 5′-TGTGACAAGATGGTACAGAGCACCTGA-3′. The universal Genome Walker kit (CLONTECH) was used to isolate 5′ upstream sequences to the gene. The gene-specific primer used was: 5′-AGGCATAATCGGAGGACGATATTTGGA-3′.
Comparison of TIPK cDNA to genomic sequences of homologous genes was performed to design primers surrounding the postulated introns, and the corresponding introns were isolated. The GenBank accession number of the TIPK sequence is DQ118734. Accession number of the second cucumber MAPK is DQ841553.
Southern Analysis
Genomic DNA of cucumber was digested with EcoRI, SacI, PstI, and XhoI, separated on an agarose gel, and blotted onto a nylon membrane. A fragment of 1.2 kb from the cDNA was labeled with 32P and used as a probe. ULTRAhyb (Ambion) was used as a hybridization solution at 42°C. Membrane was washed according to manufacturer's instructions with one modification: stringent washing was done at 45°C.
RT
After treatment with DNase I, 1 μg of total RNA was used for a RT reaction using Superscript II (Invitrogen) according to the manufacturer's instructions.
Quantitative PCR
The size of all amplified fragments was 200 bp, and the annealing temperature of all primers was 60°C. The sequences of the primers used were: TIPK, forward 5′-CCGTCATGCATTCATTTTCAGAA-3′, reverse 5′-TCCGCTCCAACCAAAGTTTATC-3′; 18S, forward 5′-GTTGCTTTAAGGACTCCGCCA-3′, reverse 5′-AGGGGTACCTCCGCATAGCTAG-3′ (gi|7595414); MPK6, forward 5′-CCAGATACTTCGTGGATTGAAG-3′, reverse 5′-AGACATCAATAGCTGCAGTG-3′.
The specificity of the primers to the genes they were designed for was tested by using melting curve analysis of the PCR reaction (standard protocol of the real-time PCR machine), as well as sequence analysis of the PCR product amplified. The primers described above are those that passed these tests. PCR was carried out in 96-well plates (20 μL/well) in a reaction buffer containing 1× SYBR Green PCR Master mix (Perkin-Elmer Applied Biosystems), 350 nm primers (for each forward and reverse primer), and 1/40 of the RT reaction for TIPK detection or 1/1,000 for 18S detection. Quantitative analysis was performed using the GeneAmp7000 Sequence Detection system (Perkin-Elmer Applied Biosystems) with PCR conditions of 95°C for 15 s and 60°C for 1 min for 40 cycles. The absence of primer-dimer formation was examined in no-template controls. Specificity of primers to cucumber genes was examined by using Trichoderma DNA and reverse-transcribed RNA as templates. The 18S ribosomal cDNA was used as a control reference. Each sample was examined in triplicate, using relative quantification analysis. This method normalizes the expression of the specific gene versus the control reference with the formula 2−ΔΔCT, where ΔCT = CT specific gene − CT reference gene; ΔΔCT = ΔCT − arbitrary constant (the highest ΔCT; for further elaboration, see Perkin-Elmer Applied Biosystems Sequence Detector user bulletin no. 2). The CT (threshold cycle) value is defined as the PCR cycle number that crosses an arbitrarily placed threshold line.
For gel visualization of quantitative PCR, we used the same conditions and primers but with a standard PCR instrument for 20 cycles (for TIPK) or 18 cycles (for 18S) and ran 10 μL on the gel. Gels were then blotted and hybridized with a probe of TIPK cDNA or 18S DNA, respectively, using standard protocols (Sambrook et al., 1989). Images were quantified using ImageJ 1.36. For verification of hormone treatments, we performed PCR for 25 cycles using primers designed to amplify chitinase and lipoxygenase genes as described in Shoresh et al. (2005).
Insertion of TIPK Gene and TIPK-AS into the AGII Genome
ZYMV-AGII (AGII) is a potyvirus-based vector system that has recently been developed for the expression of foreign genes in cucurbits (Arazi et al., 2001). To construct AGII-TIPK and AGII-AS, the TIPK gene (1,200 bp) and a 300-bp AS fragment of TIPK were amplified from the TIPK cDNA clone by PCR. For AGII-TIPK, the primers used were: forward 5′-ATACTGCAGGCTGATGTTGGTCAGAACAAC-3′, reverse 5′-ATAGTCGACTGCAAATTCTGGATTGAGTGC-3′, with the added PstI and SalI sites, respectively (underlined). For AGII-AS, the primers used were: forward 5′-ATACTGCAGAGTAAGCTTAATCTCACGGAACG-3′, reverse 5′-ATAGTCGACGAAGGCGTTTCTGAGCAAGG-3′, with the added PstI and SalI sites, respectively (underlined). The amplified fragments were double digested with the respective enzymes and cloned into the AGII genome between the CP and the NIb-coding regions. Sequence analysis was used to verify that no mutations were inserted by the PCR. We also used the AGII-GFP construct described in Arazi et al. (2001).
Plant Growth Conditions and Virus Inoculation
Potted squash (Cucurbita pepo L. cv Ma'ayan) was grown in a greenhouse. Particle bombardment was used to propel microprojectiles containing plasmid with the AGII-TIPK, AGII-AS, or AGII-GFP constructs into the fully expanded cotyledons of each plant as described in Gal-On et al. (1997). Infected squash leaves (10–14 dpi) were extracted with ice-cold water (1 g/5 mL) and centrifuged at 4,000 rpm for 5 min. Cotyledons of 10-d-old cucumbers grown in the hydroponic system were mechanically inoculated by rubbing sap extract on them with a sterile gauze sheet. Trichoderma inoculation of AGII-infected plants was performed 13 d post-AGII infection.
RT-PCR Analysis of Recombinant Virus Progeny
The second leaf from each plant was harvested. RNA extraction and RT were conducted as already described. The PCR was performed using AGII polylinker flanking primers: 5′-AAGGGAGCGGATACAAGTGA-3′ and 5′-TGATGAGACGCTCGTGTGTT-3′. PCR conditions were: 95°C for 15 s, 56°C for 30 s, and 72°C for 1 min, for 40 cycles.
Bacterial Inoculum
Psl was grown in Tryptic Soy Broth (Difco) overnight at 30°C. Bacterial cells were pelleted at 5,000 rpm and resuspended in sterile saline-phosphate buffer (5 mm, pH 7.2). Challenge was performed 48 hpi to the PGM. Psl bacterial suspension (20 μL; optical density 0.5) containing 0.01% (v/v) surfactant (Tween 20) was applied to the surface of the second leaf and gently smeared with a sterile tip. Bacterial inoculation was performed under aseptic conditions. Psl multiplication in the leaves was assessed 72 h postbacterial challenge. These experiments were repeated three times. Leaves were weighed and each leaf was homogenized in a sterile solution of 10 mm phosphate-saline buffer (1 mL/leaf). Ten-fold dilutions were plated onto Pseudomonas-selective King's B agar supplemented with 1 mL/L of 9 mg/mL basic fuchsin, 200 mg/mL cycloheximide, 10 mg/mL nitrofurantoin, and 23 mg/mL nalidixic acid. After incubation at 28°C for 2 d, the number of Psl colony-forming units per gram of infected tissue was determined. We also harvested the upper leaf for real-time analysis and to confirm the AGII virus's presence via RT-PCR analysis (as already described).
Statistical Analysis
Statistical analysis was performed using STATISTICA 7 software. Data were subjected to one-way ANOVA analysis and Tukey-Kramer honestly significant difference for comparison of means.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers DQ118734 and DQ841553.
This work was supported by the U.S.-Israel Binational Agricultural Research and Development Fund (grant no. 3507–04), by the Dr. Alexander and Myrna Strelinger Endowment Fund, and by the Fienberg Graduate School of the Weizmann Institute of Science (postdoctoral fellowship to M.S.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Michal Shoresh (ms534@cornell.edu).
References
- Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in Plant Biology, Ed 2. Academic Press, San Diego
- Aly R, Mansour F, Abo Much F, Edelstein M, Gal-On A (2005) A novel approach to spider mite control based on expression of sarcotoxin IA peptide via ZYMV-AGII vector in squash plants. Phytoparasitica 33: 177–186 [Google Scholar]
- Arazi T, Shiboleth YM, Gal-On A (2001) A nonviral peptide can replace the entire N terminus of zucchini yellow mosaic potyvirus coat protein and permits viral systemic infection. J Virol 75: 6329–6336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Bögre L, Ligterink W, Meskiene I, Barker PJ, Heberle-Bors E, Huskisson NS, Hirt H (1997) Wounding induces the rapid and transient activation of a specific MAP kinase pathway. Plant Cell 9: 75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith TM, Anderson JC, Martin GB, Dinesh-Kumar SP (2004) Applications and advantages of virus induced gene silencing for gene function studies in plants. Plant J 39: 734–746 [DOI] [PubMed] [Google Scholar]
- Gal-On A, Meiri E, Elman C, Gray DJ, Gaba V (1997) Simple hand-held devices for the efficient infection of plants with viral-encoding constructs by particle bombardment. J Virol Methods 64: 103–110 [DOI] [PubMed] [Google Scholar]
- Gal-On A, Raccah B (2000) A point mutation in the FRNK motif of the potyvirus HC-Pro gene alters symptom expression in cucurbits and elicits protection against the severe homologous virus. Phytopathology 90: 467–473 [DOI] [PubMed] [Google Scholar]
- Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species: opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2: 43–56 [DOI] [PubMed] [Google Scholar]
- Kroj T, Rudd JJ, Nurnberger T, Gabler Y, Lee J, Scheel DJ (2003) Mitogen-activated protein kinases play an essential role in oxidative burst-independent expression of pathogenesis-related genes in parsley. Biol Chem 278: 2256–2264 [DOI] [PubMed] [Google Scholar]
- Kumar D, Klessig DF (2000) Differential induction of tobacco MAP kinases by the defense signals nitric oxide, salicylic acid, ethylene, and jasmonic acid. Mol Plant Microbe Interact 13: 347–351 [DOI] [PubMed] [Google Scholar]
- Lee DE, Lee IJ, Han O, Baik MG, Han SS, Back K (2004) Pathogen resistance of transgenic rice plants expressing mitogen-activated protein kinase 1, MK1, from Capsicum annuum. Mol Cells 17: 81–85 [PubMed] [Google Scholar]
- Ligterink W, Kroj T, zur Nieden U, Hirt H, Scheel D (1997) Receptor-mediated activation of a MAP kinase in pathogen defense of plants. Science 276: 2054–2057 [DOI] [PubMed] [Google Scholar]
- Mayrose M, Bonshtien A, Sessa GJ (2004) LeMPK3 is a mitogen-activated protein kinase with dual specificity induced during tomato defense and wounding responses. J Biol Chem 279: 14819–14827 [DOI] [PubMed] [Google Scholar]
- Menke FL, Parchmann S, Mueller MJ, Kijne JW, Memelink J (1999) Involvement of the octadecanoid pathway and protein phosphorylation in fungal elicitor-induced expression of terpenoid indole alkaloid biosynthetic genes in Catharanthus roseus. Plant Physiol 119: 1289–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meskiene I, Hirt H (2000) MAP kinase pathways: molecular plug-and-play chips for the cell. Plant Mol Biol 42: 791–806 [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Irie K, Hirayama T, Hayashida N, Yamaguchi-Shinozaki K, Matsumoto K, Shinozaki K (1996) A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana. Proc Natl Acad Sci USA 93: 765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Van Pelt JA, Verhagen BWM, Ton J, Van Wees SCM, Leon-Kloosterziel KM, Van Loon LC (2003) Induced systemic resistance by plant growth-promoting rhizobacteria. Symbiosis 35: 39–54 [Google Scholar]
- Robertson D (2004) VIGS vectors for gene silencing: many targets, many tools. Annu Rev Plant Biol 55: 495–519 [DOI] [PubMed] [Google Scholar]
- Romeis T, Piedras P, Zhang S, Klessig DF, Hirt H, Jones JD (1999) Rapid Avr9- and Cf-9-dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell 11: 273–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schenk PM, Kazan K, Manners JM, Anderson JP, Simpson RS, Wilson IW, Somerville SC, Maclean DJ (2003) Systemic gene expression in Arabidopsis during an incompatible interaction with Alternaria brassicicola. Plant Physiol 132: 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y (1995) Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science 270: 1988–1992 [DOI] [PubMed] [Google Scholar]
- Seo S, Sano H, Ohashi Y (1999) Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell 11: 289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoresh M, Yedidia I, Chet I (2005) Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 95: 76–84 [DOI] [PubMed] [Google Scholar]
- Xie Z, Johansen LK, Gustafson AM, Kasschau KD, Lellis AD, Zilberman D, Jacobsen SE, Carrington JC (2004) Genetic and functional diversification of small RNA pathways in plants. PLoS Biol 2: 642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedidia I, Benhamou N, Chet I (1999) Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl Environ Microbiol 65: 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedidia I, Shoresh M, Kerem Z, Benhamou N, Kapulnik Y, Chet I (2003) Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl Environ Microbiol 69: 7343–7353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Du H, Klessig DF (1998) Activation of the tobacco SIP kinase by both a cell wall-derived carbohydrate elicitor and purified proteinaceous elicitins from Phytophthora spp. Plant Cell 10: 435–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Klessig DF (2001) MAPK cascades in plant defense signaling. Trends Plant Sci 6: 520–527 [DOI] [PubMed] [Google Scholar]
- Zhang S, Liu Y, Klessig DF (2000) Multiple levels of tobacco WIPK activation during the induction of cell death by fungal elicitins. Plant J 23: 339–347 [DOI] [PubMed] [Google Scholar]