Abstract
The amino acids glutamate (Glu) and glycine (Gly) trigger large, rapid rises in cytosolic Ca2+ concentration and a concomitant rise in membrane potential (depolarization) in plants. The possibility that plant homologs of neuronal ionotropic glutamate receptors mediate these neuron-like ionic responses was tested in Arabidopsis (Arabidopsis thaliana) seedlings using a combination of Ca2+ measurements, electrophysiology, and reverse genetics. The membrane depolarization triggered by Glu was greatly reduced or completely blocked in some conditions by mutations in GLR3.3, one of the 20 GLR genes in Arabidopsis. The same mutations completely blocked the associated rise in cytosolic Ca2+. These results genetically demonstrate the participation of a glutamate receptor in the rapid ionic responses to an amino acid. The GLR3.3-independent component of the depolarization required Glu concentrations above 25 μm, did not display desensitization, and was strongly suppressed by increasing extracellular pH. It is suggested to result from H+-amino acid symport. Six amino acids commonly present in soils (Glu, Gly, alanine, serine, asparagine, and cysteine) as well as the tripeptide glutathione (γ-glutamyl-cysteinyl-Gly) were found to be strong agonists of the GLR3.3-mediated responses. All other amino acids induced a small depolarization similar to the non-GLR, putative symporter component and in most cases evoked little or no Ca2+ rise. From these results it may be concluded that sensing of six amino acids in the rhizosphere and perhaps extracellular peptides is coupled to Ca2+ signaling through a GLR-dependent mechanism homologous to a fundamental component of neuronal signaling.
A transient rise in the cytosolic concentration of Ca2+ is an early step in the process by which many stimuli are transduced into a physiological or developmental response. In plants such stimuli include light, hormones, temperature, microbes, and touch (Sanders et al., 2002; Hetherington and Brownlee, 2004; Hepler, 2005). Calcium transients may result from Ca2+ influx across the plasma membrane and/or Ca2+ release to the cytoplasm from internal stores. Recently, the TPC1 channel of Arabidopsis (Arabidopsis thaliana) was shown to conduct Ca2+ from the vacuole to the cytoplasm (Peiter et al., 2005). However, almost nothing is known at the molecular level about Ca2+ entry across the plasma membrane, a fundamental issue in plant cell physiology. Cyclic-nucleotide-gated channels are candidates for Ca2+-influx channels at the plasma membrane (Véry and Sentenac, 2002; White et al., 2002; Lemtiri-Chlieh and Berkowitz, 2004), but little evidence to date supports a role for them in the generation of a cytosolic Ca2+ signal. Glutamate receptors are also candidates for an influx pathway (Lacombe et al., 2001; Véry and Sentenac, 2002; White et al., 2002). The Arabidopsis genome contains a family of 20 GLR genes homologous with the ionotropic (ion-conducting) glutamate receptors that mediate synaptic transmission and generate Ca2+ signals in the mammalian central nervous system (Chiu et al., 1999; Lacombe et al., 2001; Davenport, 2002). At synapses, Glu released by the presynaptic cell opens glutamate-receptor channels in the postsynaptic cell, causing influx of Ca2+, K+, and Na+ (Dingledine et al., 1999; Madden, 2002). The resulting membrane depolarization propagates the impulse and the rise in Ca2+ influences many postsynaptic processes, including the synaptic conditioning that underpins learning (Ghosh and Greenberg, 1995).
In Arabidopsis, Glu and Gly trigger very large and fast changes in cytosolic Ca2+ (Dennison and Spalding, 2000; Dubos et al., 2003; Meyerhoff et al., 2005). The rise in Ca2+ triggered by Glu is accompanied by a large, transient membrane depolarization that is due at least in part to Ca2+ influx across the plasma membrane (Dennison and Spalding, 2000; Meyerhoff et al., 2005). A patch-clamp study concluded that Glu activated nonspecific cation channels in root cells, which may be the conductances responsible for these ionic responses (Demidchik et al., 2004). Overexpression of GLR3.2 led to poor plant health that was ameliorated by treatment with Ca2+, and to hypersensitivity to K+ and Na+ (Kim et al., 2001). Glu was shown to depolarize the membrane, depolymerize cortical microtubules, and slow root growth within minutes (Sivaguru et al., 2003). All of these observations are consistent with the action of ionotropic glutamate receptors at the plant plasma membrane. However, no evidence more direct than the effects of animal iGluR inhibitors links the Arabidopsis GLR genes to the transport of Ca2+ or any electrophysiological response (Dennison and Spalding, 2000; Dubos et al., 2003; Meyerhoff et al., 2005). Needed is a genetic test of the connection between the GLR genes and the ionic responses that quickly follow treatment with amino acids.
When depolarizations in response to amino acids in plants were first described by Etherton and colleagues, the results were interpreted in terms of multiple amino acid transporters and consequent active proton extrusion (Etherton and Rubinstein, 1978; Kinraide and Etherton, 1980; Kinraide and Etherton, 1982). No genetic or molecular evidence to date precludes this interpretation of ionic responses to amino acids. It is possible that plant glutamate receptors have evolved a different physiological function and that they do not form ligand-gated ion channels similar to their neuronal counterparts. Indeed, the amino acid sequence in the predicted pore region of the plant GLRs differs significantly from that of animal ionotropic glutamate receptors, which may be evidence of a very different or even null ion transport function (Davenport, 2002). If, on the other hand, it could be shown that a GLR gene is responsible for a component of the ionic responses to Glu in plants, a fundamental aspect of neuronal signaling will have been demonstrated to operate in a complex, multicellular, but aneural organism. Any link between a plant GLR gene and the Ca2+ transient triggered by Glu would add much-needed molecular information to the topic of how Ca2+ signals are generated in plants. The present research addresses the function of plant GLR molecules with a combination of reverse genetic and cell physiological techniques.
RESULTS
Desensitization of Ionic Responses to Glu
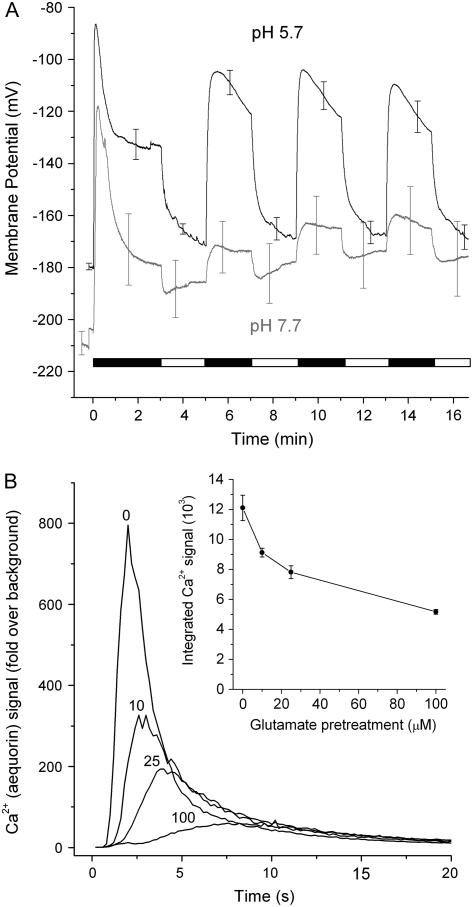
Desensitization is an important mode of regulation of ligand-gated channels that participate in Ca2+ signaling at synapses in the central nervous system (Jones and Westbrook, 1996). Glu-induced membrane depolarizations in leaf mesophyll cells display desensitization (Meyerhoff et al., 2005), consistent with the possibility that ligand-gated channels are responsible for the electrical effects of amino acids. If the electrical responses to Glu and the cytosolic Ca2+ rise triggered by Glu are manifestations of the same mechanism (e.g. the activation of ligand-gated channels), then both should display desensitization. This was investigated in Arabidopsis seedlings by experiments employing aequorin-expressing seedlings to measure Ca2+ changes and intracellular microelectrodes to measure membrane potential. Figure 1A shows that Glu triggered a large, rapid, transient membrane depolarization in the cells of the Arabidopsis root apex similar to previous reports (Dennison and Spalding, 2000; Sivaguru et al., 2003). After switching the flowing bathing solution to a Glu-free medium, the membrane potential returned to near its initial potential. A second application of Glu produced a smaller depolarization; the large initial component was desensitized. The second depolarization was repeatedly reversible, i.e. it did not desensitize. When the experiment was performed at an external pH of 7.7, the initial depolarization was similar to the response obtained at pH 5.7. However, at the higher pH, responses to subsequent applications of Glu were greatly suppressed. The effect of pH on the nondesensitized component receives a possible explanation below.
Figure 1.
Desensitization of ionic responses to Glu. A, Glu application-washout cycles indicated by the alternating black and white bar show that a large, rapid membrane depolarization is observed only in response to the first exposure to Glu (1,000 μm, black). The initial response was pH independent, but the smaller subsequent responses to Glu were strongly suppressed by raising the pH from 5.7 to 7.7. Both traces are the averages of four independent experiments with SEM shown at arbitrarily selected time points. B, Pretreatment of aequorin-expressing wild-type seedlings with the indicated concentration of Glu caused a reduction in the response to a subsequent application of 1,000 μm Glu made 3 h later. The traces shown are the averages of at least three trials. Inset, A plot of Ca2+ response versus Glu pretreatment made by integrating each of the trials and then averaging the integrals. Data points are the mean ± se. Desensitization of the Ca2+ response occurred over the range of Glu concentrations that roots are expected to experience in soils.
The Ca2+ rise also displayed desensitization. Figure 1B demonstrates that pretreatment with 10 μm Glu caused a 25% reduction in the Ca2+ response to a subsequent application of 1,000 μm Glu, while 100 μm Glu desensitized responsiveness by 56%. The reduced responsiveness was not due to a decrease in the capacity of the aequorin to report Ca2+ because even a 2-h pretreatment with 1,000 μm Glu did not affect the Ca2+ response to cold shock (data not shown). The above data indicate that both the Ca2+ rise and the membrane depolarization display the phenomenon of desensitization, as would be expected if both were the result of Glu activating GLR ion channels.
Genetic Link between Ionic Responses to Amino Acids and GLR3.3
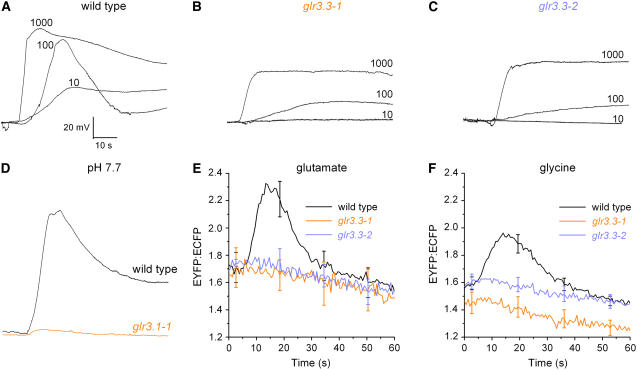
A Glu-gated Ca2+ rise involving influx across the plasma membrane and desensitization is only circumstantial evidence of a GLR-mediated Ca2+-influx mechanism operating in plants. A more formal link between the ionic phenomena and the GLR gene family would be established if a mutation in one or more family member affected the ionic response to Glu. T-DNA insertion (knockout) mutants for 18 members of the family were obtained from the Salk collection (http://signal.salk.edu/cgi-bin/tdnaexpress) and glr homozygotes were screened for aberrant electrophysiological responses to Glu. One of the knockouts, glr3.3, responded very differently than wild type to certain amino acid applications. While 10 μm Glu induced a membrane depolarization of 34 ± 4 mV (n = 3) in wild-type roots (Fig. 2A), the response was completely absent in two independent glr3.3 knockout plants (Fig. 2, B and C). Increasing Glu to 100 μm increased the peak magnitude of the wild-type voltage change to 65 ± 4 mV (n = 9), but the average responses of glr3.3-1 and glr3.3-2 roots were only 20 ± 4 mV and 22 ± 5 mV, respectively (n = 9 for each). The residual depolarization in the glr3.3 mutants increased substantially when the Glu treatment was increased to 1,000 μm (Fig. 2, B and C). The GLR3.3-independent component of the depolarization may be the result of H+-coupled amino acid symport (Fischer et al., 1998) because changing external pH from 5.7 to 7.7 almost completely abolished it (Fig. 2D), as was observed for the nondesensitizing component of the wild-type response (Fig. 1A). The onset of the putative amino acid symport activity at a Glu concentration between 10 and 100 μm agrees well with previous studies of electrogenic Glu uptake by an Arabidopsis amino acid transporter (Boorer et al., 1996).
Figure 2.
Comparison of ionic responses induced by Glu in roots of wild type and glr mutants. A, Depolarizations increased in magnitude and changed shape as Glu concentration was increased from 10 to 1,000 μm in the wild type. The external medium was pH 5.7. B, Responses of glr3.3-1 to 10 μm Glu were not detectable. A sustained depolarization was induced in the mutant by Glu concentrations at or greater than 100 μm. C, The dependence of the depolarization on GLR3.3 is confirmed with the independent glr3.3-2 allele. D, At pH 7.7, the wild-type response to 1,000 μm Glu (black) was large and the mutant response was nearly absent (red). All membrane potential traces shown are representative of between three and nine independent trials. Average peak responses are stated in the text. E, The change in cytoplasmic Ca2+ induced by 1,000 μm Glu in the wild type (n = 12) was abolished in two independent glr3.3 mutant alleles (n = 6, 7). F, The change in cytoplasmic Ca2+ induced by 1,000 μm Gly in the wild type (n = 12) was absent in two independent glr3.3 mutant alleles (n = 7, 8).
To determine if the Ca2+ rise also depended on the GLR3.3 glutamate receptor, a fluorescent Ca2+ reporter (yellow cameleon YC2.1; (Allen et al., 1999) was introduced into the two glr3.3 lines by crossing. Figure 2E shows that even the high concentration of 1,000 μm Glu did not produce a rise in Ca2+ in either mutant line, while the wild-type response was robust. Gly, a co-agonist of some animal glutamate receptors, has been shown to elicit a Glu-like Ca2+ response in Arabidopsis (Dubos et al., 2003; Meyerhoff et al., 2005). The glr3.3 mutations blocked the Ca2+ response to Gly as well (Fig. 2F). The YC2.1 reporter was functional in the glr3.3 lines because cold shock produced a Ca2+ rise in the mutant similar to wild type (data not shown). Photobleaching the yellow fluorescent protein (YFP) acceptor decreased the FRET ratio and almost completely suppressed the response to Gly (data not shown), indicating that the recorded signal was a bona fide measure of cytosolic Ca2+ concentration. These results unequivocally demonstrate that the rise in cytoplasmic Ca2+ concentration and concomitant membrane depolarization triggered by Glu or Gly depend on GLR3.3. These results constitute a formal genetic connection between the GLR gene family and the rapid ionic responses triggered by amino acids.
Regulation of Ca2+ Influx by Micromolar Glu
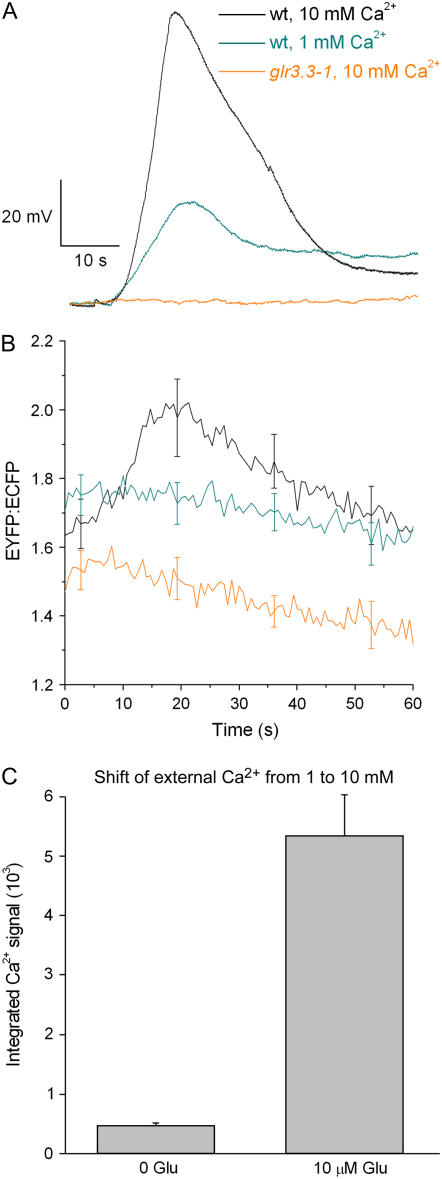
The above results are consistent with GLR3.3 mediating an inward Ca2+ flux across the plasma membrane. If so, then the magnitude of the depolarization and cytosolic Ca2+ responses to Glu should depend on extracellular Ca2+ concentration. Figure 3A shows that increasing external Ca2+ from 1 to 10 mm increased the peak membrane depolarization induced by 25 μm Glu (from 41 ± 6 mV; n = 4 to 86 ± 6 mV; n = 5). The Ca2+ rise triggered by 25 μm Glu was also enhanced by increasing external Ca2+ concentration (Fig. 3B). Both Ca2+-dependent responses were abolished by the glr3.3-1 mutation (Fig. 3, A and B). These results indicate that GLR3.3 is required for Ca2+ transport across the plasma membrane from the apoplast in response to Glu.
Figure 3.
Effects of increasing extracellular Ca2+ on GLR3.3-mediated responses to micromolar Glu. A, Increasing external Ca2+ from 1 mm to 10 mm increased the magnitude of the membrane depolarization induced by 25 μm Glu. Even at the higher Ca2+ concentration, glr3.3-1 root cells did not depolarize in response to this low level of Glu. The glr3.3-2 allele also failed to respond (data not shown). See the text for mean peak values. B, A rise in cytosolic Ca2+ in response to 25 μm Glu was detected in wild-type root cells bathed in 10 mm Ca2+ (n = 7) but not when external Ca2+ was 1 mm (n = 4). Even in the presence of 10 mm external Ca2+, glr3.3-1 mutants did not show a detectable response to 25 μm Glu (n = 6). C, A rise in cytosolic Ca2+ (assayed by aequorin luminescence) in response to shifts of external Ca2+ from 1 mm to 10 mm was greatly enhanced by pretreatment with 10 μm Glu. n = 8 for the 0 Glu trials and n = 13 for the 10 μm Glu trials.
By stimulating GLR activity without causing major desensitization, low concentrations of Glu in the rhizosphere may regulate the Ca2+ permeability of the root plasma membrane. This was tested by determining the effect of changes in extracellular Ca2+ on cytosolic Ca2+ in the continuous presence or absence of 10 μm Glu. Seedlings expressing aequorin were used for this assay of the effect of Glu on Ca2+ entry driven by a change in the Ca2+ electrochemical potential gradient. In the absence of exogenous Glu, a 10-fold increase in extracellular Ca2+ concentration (from 1–10 mm) had little effect on cytoplasmic Ca2+ concentration (Fig. 3C). Either the membrane was not very permeable to Ca2+ or the influx resulting from this change was managed efficiently by a homeostatic efflux mechanism, or both. However, in the continuous presence of 10 μm Glu, the same shift in extracellular Ca2+ caused a substantial rise in cytoplasmic Ca2+ (Fig. 3C). Thus, chronic exposure to 10 μm Glu increased the ease with which Ca2+ entered the cytoplasm.
Broad Agonist Profile of GLR3.3
Although Glu is often the most abundant amino acid found in soils, several others are frequently present in appreciable quantities (Abuarghub and Read, 1988; Kielland, 1994; Jones et al., 2005). To determine if others may trigger GLR3.3-dependent activity in roots, the effectiveness of all 20 l-amino acids as well as d-Glu, d-Ser, d-Ala, NMDA, and γ-aminobutyric acid (GABA) was determined. Of the tested compounds, Ala, Asn, Cys, and Ser triggered large, transient membrane depolarizations that were GLR3.3 dependent and in all respects similar to Glu or Gly responses (Table I; Supplemental Fig. S1A). Each of these effective amino acids also triggered a rise in Ca2+ as assayed by aequorin luminescence (Supplemental Fig. S1A; Supplemental Table S1). This result is surprising because Ala, Asn, and Cys are not known to be agonists of animal glutamate receptors and are structurally dissimilar. Of these, Cys was selected for further study. The large rise in cytosolic Ca2+ triggered by 1,000 μm Cys in the wild type was completely absent in cameleon-expressing glr3.3 mutants but normal in a line heterozygous for the glr3.3 mutation (data not shown). Thus, six amino acids (Glu, Gly, Ser, Ala, Asn, and Cys) may be considered agonists of a GLR3.3-dependent Ca2+-influx mechanism. The ineffective compounds (the remainder of the amino acids, the d-isomers, NMDA, and GABA) produced smaller, persistent depolarizations similar to the responses of glr3.3 mutants to Glu or Gly. Some of these responses are shown in Supplemental Figure S1B and Supplemental Table S1. The small, persistent depolarizations induced by Gln and Asp were further investigated and found to be both pH sensitive and independent of GLR3.3 (data not shown). The small responses evoked by the ineffective amino acids may reflect proton-coupled uptake of the compounds.
Table I.
Peak change in membrane potential induced by 1,000 μM of the six potent amino acids or glutathione in wild type and glr3.3 mutants
Values shown are the mean peak change in mV ± SEM.
| Genotype | Amino Acids
|
Glutathione
|
||||||
|---|---|---|---|---|---|---|---|---|
| Ala | Asn | Cys | Glu | Gly | Ser | GSH | GSSG | |
| Wild type | 117 ± 11 | 87 ± 7 | 91 ± 1 | 83 ± 5 | 98 ± 8 | 90 ± 12 | 104 ± 15 | 43 ± 3 |
| (n = 3) | (n = 3) | (n = 4) | (n = 8) | (n = 7) | (n = 3) | (n = 7) | (n = 10) | |
| glr3.3-1 | 46 ± 13 | 41 ± 3 | 45 ± 3 | 38 ± 2 | 47 ± 4 | 47 ± 4 | 19 ± 6 | 22 ± 3 |
| (n = 4) | (n = 5) | (n = 3) | (n = 7) | (n = 6) | (n = 3) | (n = 4) | (n = 5) | |
| glr3.3-2 | 41 ± 3 | 50 ± 4 | 40 ± 2 | 44 ± 6 | 38 ± 5 | 43 ± 6 | 31 ± 4 | 18 ± 4 |
| (n = 3) | (n = 3) | (n = 3) | (n = 7) | (n = 6) | (n = 3) | (n = 4) | (n = 4) | |
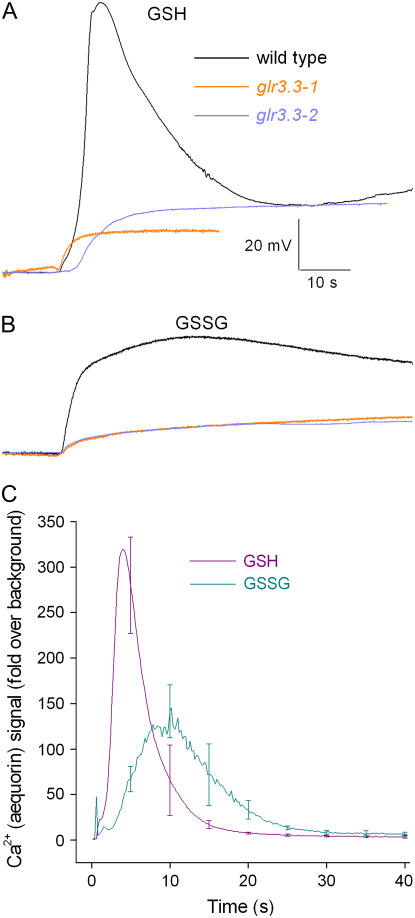
Given that six different amino acids were each found to induce Ca2+ transients and large membrane depolarizations, it was hypothesized that short peptides composed of one or more of these agonists would have similar effects. Glutathione, an abundant tripeptide in plants, is composed of three of the six effective amino acids: Glu, Cys, and Ser. Glutathione plays a number of important roles in plant metabolism and responses to stress, partly because of its ability to be reversibly oxidized and reduced (Noctor et al., 2002; Ogawa, 2005). The reduced form of glutathione (GSH) depolarized root cells by approximately 100 mV in a GLR3.3-dependent manner (Table I; Fig. 4A). GSH also caused a substantial Ca2+ transient (Fig. 4C). The oxidized form of glutathione (GSSG) was much less effective than GSH, though its action was also dependent on GLR3.3 (Table I; Fig. 4, B and C).
Figure 4.
Glutathione activates GLR3.3 activity in roots. A, GSH (1,000 μm) triggered a large, transient membrane depolarization in the wild type but not in glr3.3 mutants. B, GSSG (1,000 μm) triggered a smaller, broader depolarization that was also GLR3.3 dependent. All membrane potential traces shown are representative of three to five independent trials. C, GSH triggered a large, transient change in cytosolic Ca2+ (n = 5). Consistent with the depolarization measurements, GSSG triggered a slower, smaller Ca2+ response than GSH (n = 4). SEM values are shown at arbitrarily selected time points.
DISCUSSION
In soils, Glu originates from decomposing organic matter and exudates produced by living roots (Lynch and Whipps, 1990; Nguyen, 2003; Jones et al., 2004). Its concentration is typically in the low micromolar range (Abuarghub and Read, 1988; Kielland, 1994; Jones et al., 2005). A study of tomato (Lycopersicon esculentum) root exudation estimated rhizosphere Glu concentration to be 9 μm (Simons et al., 1997). The other five effective amino acids are also among the most prevalent in root exudates and in soils. For example, Glu, Ala, and Gly were among the four most abundant amino acids detected in maize (Zea mays) root exudates (Kraffczyk et al., 1984). Metabolic interplay between microbes and roots (Phillips et al., 2004; Singh et al., 2004; Somers et al., 2004) may dynamically change amino acid concentrations in the rhizosphere, thereby producing chemical signals potentially useful to the root. Figure 2 shows that 10 μm Glu activated GLR3.3 and Figure 1B demonstrates that this same low level of agonist partially desensitized the Ca2+-signaling mechanism. Thus, GLR3.3 would naturally encounter regulatory concentrations of effective amino acids. This argues in favor of GLR3.3-dependent Ca2+ influx being physiologically relevant rather than a spurious pharmacological effect. The ability of GLR3.3 to distinguish between oxidized and reduced forms of glutathione raises the possibility that it could also sense the redox poise of the rhizosphere.
Amino acids are not only released to the rhizosphere but also are present in the apoplast throughout the plant. One study of Arabidopsis reported that the GLR3.3 activators Glu, Ser, and Asn are among the four most abundant amino acids in root xylem and leaf exudates (Pilot et al., 2004). In the leaf exudate, Glu accounts for approximately 12% of the total amino acids (Pilot et al., 2004). Therefore, GLR-mediated ionic signaling mediated by different amino acids could be occurring between cells throughout the plant.
The surprisingly broad agonist profile of GLR3.3 may reflect the unusual structure of its extracellular amino terminus, relative to neuronal glutamate receptors (Turano et al., 2001). A recent bioinformatic analysis of amino acid binding motifs in prokaryotic and eukaryotic genomes (Acher and Bertrand, 2005) found that the Arabidopsis GLR genes were unique in containing two distinct amino acid-binding domains homologous to those found in bacterial periplasmic binding proteins. Together these domains may be responsible for the activating effect of six different amino acids and a tripeptide on GLR3.3-dependent Ca2+ influx.
The breadth of the agonist profile found here is difficult to reconcile with the conclusion that Glu binds only to GLR1.1 and that Gly is probably the exclusive ligand of the other 19 GLRs (Dubos et al., 2003). Homology modeling of plant GLR ligand-binding domains using animal glutamate-receptor structures as templates (Dubos et al., 2003) may not be precise enough to predict ligands with accuracy. Alternatively, the logic used here to infer agonists may be overextended. For example, it is formally possible that treatment with any of the six effective amino acids causes the immediate release of the true GLR3.3 ligand, so that in effect what is measured is a secondary response to the initial treatment. The data presented here do not provide good evidence that the effective amino acids actually bind to GLR3.3. They may interact with another protein that acts through GLR3.3 to open a Ca2+ conductance. Nonetheless, it is clear that GLR3.3-dependent ion fluxes are triggered directly or indirectly by multiple amino acids that naturally occur in effective concentrations in the rhizosphere.
GLR3.3 mediates amino acid-gated Ca2+ influx in the root, but it does not necessarily form a channel. The possibility that membrane depolarization due to glutamate-receptor activation triggers Ca2+ influx via separate, voltage-dependent Ca2+ channels (Courtney et al., 1990; White et al., 2002) was investigated by an experiment not presented here. Extracellular K+ concentration was raised from 10 to 1,000 μm to depolarize the membrane, but this did not result in a detectable Ca2+ increase as reported by aequorin (data not shown). Despite minimal sequence similarity in the pore regions of Arabidopsis and mammalian glutamate receptors (Davenport, 2002), the currently preferred hypothesis is that GLR3.3 plays a direct role in the conduction of Ca2+ across the plasma membrane in response to amino acids.
A growth or developmental phenotype that would help connect GLR3.3-mediated ion fluxes to a higher-level biological function was not identified in the knockout mutants reported here. Functional redundancy among members of gene families is a common explanation of phenotype absence but it does not easily explain the present case because glr3.3 mutations essentially eliminated the ionic responses in the cells studied. Another family member does not appear to compensate at the cell physiology level for the glr3.3 mutation in the root apex. In contrast to the lack of obvious outward phenotype in glr3.3, mutation of the OsGLR3.1 gene in rice (Oryza sativa; Li et al., 2006) very obviously slowed root growth by impairing meristematic activity and cell viability at the root apex. Perhaps a similar phenotype will be observed in Arabidopsis glr3.3 mutants when they are cultured in the presence of microorganisms or exudates that naturally activate the GLR3.3-dependent Ca2+-signaling mechanism.
The GLR3.3-mediated Ca2+-signaling system may participate in a number of physiological processes indicated by previous studies. One is the balancing of carbon and nitrogen metabolism, which was affected in certain conditions by antisense suppression of GLR1.1 (Kang and Turano, 2003). Also, the amount of lateral root development in proportion to primary root growth is influenced by micromolar levels of Glu and therefore may be a GLR-mediated process (Walch-Liu et al., 2006). However, of the effective amino acids in Table I, Glu was the only one capable of causing the root architecture changes (Walch-Liu et al., 2006), which argues against GLR3.3 playing a role in this developmental response. Pharmacological data indicated a role for GLRs in light-induced inhibition of hypocotyl growth (Lam et al., 1998). Signal transduction chains linking photoreceptors to some stages of this process are thought to involve fluxes of ions, including Ca2+ (Spalding, 2000). Therefore, detailed studies of photomorphogenesis in glr3.3 seedlings may uncover a phenotype. A role for GLRs in Ca2+ nutrition was suggested by Kim et al. (2001) because overexpression of GLR3.2 led to Ca2+ deficiency symptoms but not lower Ca2+ levels. Perhaps GLR3.3-mediated Ca2+ signaling plays a role in regulating Ca2+ nutrition. Lastly, the responses to harmful elements in the rhizosphere such as Al3+ may also depend on Ca2+ signals mediated by glutamate receptors (Sivaguru et al., 2003). One attractive hypothesis has Al3+ triggering the release of amino acids that activate GLR3.3. The resulting rise in Ca2+ and associated change in microtubules would result in the observed reduction in root growth rate (Sivaguru et al., 2003).
Establishing that GLR3.3 mediates ion fluxes, including a large Ca2+ influx across the Arabidopsis plasma membrane, prompts some intriguing questions. Has a chemosensing mechanism with prokaryotic origins (Kuner et al., 2003) been molded by evolution to serve the chemosensing needs of roots and the communication needs of a central nervous system? Are the signaling components that function downstream of Glu-triggered Ca2+ signals in neurons, such as CREB transcription factors (Ghosh and Greenberg, 1995), conserved in plants? Do plants have a Ca2+-based, cell-to-cell communication system that is molecularly homologous with the mechanism underpinning learning? Do GLRs mediate interspecific and intraspecific signaling between roots? Further work on the Arabidopsis GLR molecules and glr mutants can be expected to provide answers.
MATERIALS AND METHODS
Plant Growth
Arabidopsis (Arabidopsis thaliana) seeds (Columbia ecotype) were surface sterilized with 75% ethanol and sown on vertical 0.7% agar plates containing 1 mm KCl, 1 mm CaCl2, 5 mm MES, pH 5.7, adjusted with Bis-Tris propane. The plates were maintained at 4°C in darkness for 48 to 72 h before transfer to a growth chamber with a 16/8-h light/dark photoperiod cycle and grown vertically for 4 to 6 d.
Membrane Potential Measurement
Seedlings were lifted from the growth plates, mounted horizontally on a thin layer of growth media gelled with 0.6% agar at the bottom of a 3.5-cm diameter recording chamber, then covered with a thin layer of agar-free growth medium and allowed to recover for 3 h. Measurements of membrane potential were made as described previously (Dennison and Spalding, 2000), except that data acquisition in this study was achieved with a PCI-MIO-16XE-10 analog to digital converter (National Instruments) controlled by custom software written in the Labview computer language (National Instruments). The sample rate was 20 Hz. Perfusion of the recording chamber (7 mL min−1) was driven by a peristaltic pump (Dynamax RP-1; Rainin) and controlled by electronic valves. The initial (control) perfusion solution was the same as the growth medium (minus the agar) and could be switched to one supplemented with an amino acid via the data acquisition software. It was important to position the inflow tube as close as possible to the site of impalement because reproducible electrophysiological responses required abrupt (within approximately 2 s) exchange of solutions. Experiments proceeded only if a stable membrane potential more negative than −120 mV was obtained.
Measurement of Intracellular Ca2+ with Aequorin
Seeds of Arabidopsis aequorin-expressing plants described previously (Lewis et al., 1997) were sown and grown on plates as described above. To reconstitute the aequorin with its substrate, 4-d-old seedlings were transferred to a darkroom and under green safelight placed in growth media (minus agar) except for containing 10 μm coelenterazine hcp (Molecular Probes) and allowed to soak overnight. For each trial, five seedlings were loaded into a luminometer cuvette containing 200 μL of the growth medium (minus agar). The seedlings were allowed to recover from the handling in darkness at 25°C for 2 to 4 h. Programmable, rapid delivery of agonist solutions into the cuvette was performed by the onboard injectors of the luminometer used to record the aequorin signal (TD-20/20; Turner Designs). A computer collected data from the luminometer at a rate of 5 Hz. While resting in the dark, the level of aequorin luminescence emitted from five seedlings varied but was generally less than 100 relative light units. The variability was probably due to different degrees of coelenterazine incorporation and different amounts of tissue in the cuvettes. Treatment with 1,000 μm Glu caused a rapid luminescence increase, typically into the thousands of relative light units. A statistical analysis showed that there was a strong, direct correlation between the level of pretreatment aequorin luminescence and the peak response to agonist. Those tubes with higher aequorin levels gave larger responses and the correlation was near perfect (R2 = 0.98 for Glu, 0.99 for Gly). Therefore, for each individual trace, the data were divided by the average background (pretreatment) luminescence, producing a fold increase in aequorin luminescence trace. Recordings normalized in this fashion are shown as traces (e.g. Supplemental Fig. S1), averaged traces (e.g. Figs. 1B and 4C), or were integrated over time and then averaged (e.g. Fig. 1B, inset).
To test the effect of Glu pretreatments on a subsequent response to high levels of Glu, seedlings expressing aequorin reconstituted with coelenterazine were pretreated with the indicated Glu concentration for 3 h and then their responses to 1,000 μm Glu were recorded as described above. Four independent trials were performed for each Glu pretreatment concentration. To test the effects of increasing extracellular Ca2+ on the response to low Glu levels (Fig. 3C), the seedlings were pretreated for 3 to 4 h with either 1 mm Ca2+ (n = 9) or 10 mm Ca2+ (n = 8) before being treated with the same Ca2+ concentration plus 25 μm Glu.
Measurement of Intracellular Ca2+ with YC2.1
Seeds of transgenic plants expressing yellow cameleon YC2.1 (Allen et al., 1999) were kindly provided by Simon Gilroy, Penn State University. Seedlings grown for 4 d were lifted from the growth plates, mounted horizontally on a thin layer of growth medium gelled with 0.6% agar at the bottom of a recording chamber, bathed in agar-free growth medium, and allowed to recover for at least 1 h. Unless stated otherwise, media contained 1 mm CaCl2. The recording chamber was mounted horizontally on the stage of a Zeiss LSM 510 Meta confocal microscope. Root tips were imaged with an Olympus LUMPlanFl×40W objective immersed in the solution bathing the root. Excitation with 458-nm light was provided by a 200-mW argon laser. Enhanced cyan fluorescent protein (ECFP) fluorescence and the FRET-based enhanced YFP (EYFP) signal were collected using the Meta detector using bandwidths of 462.6 to 484.0 nm and 526.8 to 548.2 nm, respectively. Patches of epidermal and cortical cells in the elongating zone were selected as the ROI and scanned at approximately 600-ms intervals. For each time point, four separate scans were averaged to minimize noise. Agonist solution was introduced into and removed from the chamber at a rate of approximately 2 mL min−1 using a syringe pump (SP120p; World Precision Instruments). Mean ECFP and EYFP signals were determined for the ROI for each scan and used to produce the calcium-dependent EYFP to ECFP ratio.
Mutant Genotyping
Seeds of plant lines containing a T-DNA insertion in the gene of interest were obtained from the Salk Institute (http://signal.salk.edu/cgi-bin/tdnaexpress). The lines used here were Salk_040458 (glr3.3-1, second exon insertion) and Salk_066009 (glr3.3-2, first intron insertion). To isolate homozygous mutant individuals, DNA was isolated from leaf samples and evaluated for the presence or absence of allele-specific PCR products. Whole leaves were placed into a 96-well plate containing chloroform and extraction buffer (400 mm Tris, pH 8.0; 480 mm NaCl; 50 mm EDTA, pH 8.0; 1% SDS) and ground using a tissue grinder (GenoGrinder 2000; Spex CertiPrep). Following a 1,200g spin, the DNA-containing fraction was spun twice at 6,100g, first in isopropanol and then in an 80% ethanol wash. The DNA pellet was then resuspended in distilled water after drying. A left-border T-DNA primer (5′-TGGTTCACGTAGTGGGCCATCG-3′) was used in combination with gene-specific primers to test for the presence or absence of the T-DNA insertion alleles in segregating populations using PCR and agarose gel electrophoresis. The following are the gene-specific primers used to confirm the genotype of both of the glr3.3 mutant lines (SALK_040458 and SALK_066009): Forward 5′-GAA ACC AAA AGT TGT GAA AAT CGG T-3′ and Reverse 5′-GAC ACA TTG TCT CTT AGG TGG GCC T-3′.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Survey of changes in membrane potential and cytosolic Ca2+ in response to l-isomers of the 20 common amino acids and GABA.
Supplemental Table S1. Mean peak changes in membrane potential and integrated aequorin signals in response to each of the common amino acids.
Supplementary Material
Acknowledgments
The authors are grateful for the plant materials supplied by the Arabidopsis Biological Resource Center and to Nathan Miller, University of Wisconsin, for constructing the data acquisition software and perfusion control apparatus.
This work was supported by the U.S. Department of Energy (grant no. 04ER15527 to E.P.S.) and by the National Science Foundation (Major Research Instrumentation grant no. DBI−0421266).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Edgar P. Spalding (spalding@wisc.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Abuarghub SM, Read DJ (1988) The biology of mycorrhiza in the Ericaceae. XII. Quantitative analysis of individual ‘free’ amino acids in relation to time and depth in the soil profile. New Phytol 108: 433–441 [Google Scholar]
- Acher FC, Bertrand HO (2005) Amino acid recognition by venus flytrap domains is encoded in an 8-residue motif. Biopolymers 80: 357–366 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI (1999) Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J 19: 735–747 [DOI] [PubMed] [Google Scholar]
- Boorer KJ, Frommer WB, Bush DR, Kreman M, Loo DDF, Wright EM (1996) Kinetics and specificity of a H+/amino acid transporter from Arabidopsis thaliana. J Biol Chem 271: 2213–2220 [DOI] [PubMed] [Google Scholar]
- Chiu J, DeSalle R, Lam H-M, Meisel L, Coruzzi G (1999) Molecular evolution of glutamate receptors: a primitive signaling mechanism that existed before plants and animals diverged. Mol Biol Evol 16: 826–838 [DOI] [PubMed] [Google Scholar]
- Courtney MJ, Lambert JJ, Nicholls DG (1990) The interactions between plasma-membrane depolarization and glutamate receptor activation in the regulation of cytoplasmic free calcium in cultured cerebellar granule cells. J Neurosci 10: 3873–3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport R (2002) Glutamate receptors in plants. Ann Bot (Lond) 90: 549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Essah P, Tester M (2004) Glutamate activates cation currents in the plasma membrane of Arabidopsis root cells. Planta 219: 167–175 [DOI] [PubMed] [Google Scholar]
- Dennison KL, Spalding EP (2000) Glutamate-gated calcium fluxes in Arabidopsis. Plant Physiol 124: 1511–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51: 7–61 [PubMed] [Google Scholar]
- Dubos C, Huggins D, Grant GH, Knight MR, Campbell MM (2003) A role for glycine in the gating of plant NMDA-like receptors. Plant J 35: 800–810 [DOI] [PubMed] [Google Scholar]
- Etherton B, Rubinstein B (1978) Evidence for amino acid-H+ co-transport in oat coleoptiles. Plant Physiol 61: 933–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer WN, Andre B, Rentsch D, Krolkiewicz S, Tegeder M, Breitkreuz K, Frommer WB (1998) Amino acid transport in plants. Trends Plant Sci 3: 188–195 [Google Scholar]
- Ghosh A, Greenberg ME (1995) Calcium signaling in neurons—molecular mechanisms and cellular consequences. Science 268: 239–247 [DOI] [PubMed] [Google Scholar]
- Hepler PK (2005) Calcium: a central regulator of plant growth and development. Plant Cell 17: 2142–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM, Brownlee C (2004) The generation of Ca2+ signals in plants. Annu Rev Plant Biol 55: 401–427 [DOI] [PubMed] [Google Scholar]
- Jones DL, Shannon D, Junvee-Fortune T, Farrarc JF (2005) Plant capture of free amino acids is maximized under high soil amino acid concentrations. Soil Biol Biochem 37: 179–181 [Google Scholar]
- Jones DL, Hodge A, Kuzyakov Y (2004) Plant and mycorrhizal regulation of rhizodeposition. New Phytol 163: 459–480 [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL (1996) The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci 19: 96–101 [DOI] [PubMed] [Google Scholar]
- Kang J, Turano FJ (2003) The putative glutamate receptor 1.1 (AtGLR1.1) functions as a regulator of carbon and nitrogen metabolism in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 6872–6877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielland K (1994) Amino acid absorption by arctic plants: implications for plant nutrition and nitrogen cycling. Ecology 75: 2373–2383 [Google Scholar]
- Kim SA, Kwak JM, Jae S-K, Wang M-H, Nam HG (2001) Overexpression of the AtGluR2 gene encoding an Arabidopsis homolog of mammalian glutamate receptors impairs calcium utilization and sensitivity to ionic stress in transgenic plants. Plant Cell Physiol 42: 74–84 [DOI] [PubMed] [Google Scholar]
- Kinraide TB, Etherton B (1980) Electrical evidence for different mechanisms of uptake for basic, neutral, and acidic amino acids in oat coleoptiles. Plant Physiol 65: 1085–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB, Etherton B (1982) Energy coupling in H+-amino acid cotransport: ATP dependence of the spontaneous electrical repolarization of the cell membranes in oat coleoptiles. Plant Physiol 69: 648–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraffczyk I, Trolldenier G, Beringer H (1984) Soluble root exudates of maize: influence of potassium supply and rhizosphere microorganisms. Soil Biol Biochem 16: 315–322 [Google Scholar]
- Kuner T, Seeburg PH, Guy HR (2003) A common architecture for K+ channels and ionotropic glutamate receptors? Trends Neurosci 26: 27–32 [DOI] [PubMed] [Google Scholar]
- Lacombe B, Becker D, Hedrich R, DeSalle R, Hollman M, Kwak JM, Schroeder JI, Novère NL, Nam HG, Spalding EP, et al (2001) The identity of plant glutamate receptors. Science 292: 1486–1487 [DOI] [PubMed] [Google Scholar]
- Lam H-M, Chiu J, Hsieh M-H, Meisel L, Oliveira IC, Shin M, Coruzzi G (1998) Glutamate-receptor genes in plants. Nature 396: 125–126 [DOI] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, Berkowitz GA (2004) Cyclic adenosine monophosphate regulates calcium channels in the plasma membrane of Arabidopsis leaf guard and mesophyll cells. J Biol Chem 279: 35306–35312 [DOI] [PubMed] [Google Scholar]
- Lewis BD, Karlin-Neumann G, Davis RW, Spalding EP (1997) Ca2+-activated anion channels and membrane depolarizations induced by blue light and cold in Arabidopsis seedlings. Plant Physiol 114: 1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhu SH, Song XW, Shen Y, Chen HM, Yu J, Yi KK, Liu YF, Karplus VJ, Wu P, et al (2006) A rice glutamate receptor-like gene is critical for the division and survival of individual cells in the root apical meristem. Plant Cell 18: 340–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JM, Whipps JM (1990) Substrate flow in the rhizosphere. Plant Soil 129: 1–10 [Google Scholar]
- Madden D (2002) The structure and function of glutamate receptor ion channels. Nature Rev Neurosci 3: 91–101 [DOI] [PubMed] [Google Scholar]
- Meyerhoff O, Muller K, Roelfsema MR, Latz A, Lacombe B, Hedrich R, Dietrich P, Becker D (2005) AtGLR3.4, a glutamate receptor channel-like gene is sensitive to touch and cold. Planta 222: 418–427 [DOI] [PubMed] [Google Scholar]
- Nguyen C (2003) Rhizodeposition of organic C by plants: mechanisms and controls. Agronomie (Paris) 23: 375–396 [Google Scholar]
- Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot 53: 1283–1304 [DOI] [PubMed] [Google Scholar]
- Ogawa K (2005) Glutathione-associated regulation of plant growth and stress responses. Antioxid Redox Signal 7: 973–981 [DOI] [PubMed] [Google Scholar]
- Peiter E, Maathuis FJM, Mills LN, Knight H, Pelloux M, Hetherington AM, Sanders D (2005) The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434: 404–408 [DOI] [PubMed] [Google Scholar]
- Phillips DA, Fox TC, King MD, Bhuvaneswari TV, Teuber LR (2004) Microbial products trigger amino acid exudation from plant roots. Plant Physiol 136: 2887–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot G, Stransky H, Bushey DF, Pratelli R, Ludewig U, Wingate VPM, Frommer WB (2004) Overexpression of glutamine dumper1 leads to hypersecretion of glutamine from hydathodes of Arabidopsis leaves. Plant Cell 16: 1827–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell (Suppl) 14: S401–S417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Permentier HP, de Weger LA, Wijffelman CA, Lugtenberg BJJ (1997) Amino acid synthesis is necessary for tomato root colonization by Pseudomonas fluorescens strain WCS365. Mol Plant Microbe Interact 10: 102–106 [Google Scholar]
- Singh BK, Millard P, Whiteley AS, Murrell JC (2004) Unravelling rhizosphere-microbial interactions: opportunities and limitations. Trends Microbiol 12: 386–393 [DOI] [PubMed] [Google Scholar]
- Sivaguru M, Pike S, Gassmann W, Baskin TI (2003) Aluminum rapidly depolymerizes cortical microtubules and depolarizes the plasma membrane: evidence that these responses are mediated by a glutamate receptor. Plant Cell Physiol 44: 667–675 [DOI] [PubMed] [Google Scholar]
- Somers E, Vanderleyden J, Srinivasan M (2004) Rhizosphere bacterial signalling: a love parade beneath our feet. Crit Rev Microbiol 30: 205–240 [DOI] [PubMed] [Google Scholar]
- Spalding EP (2000) Ion channels and the transduction of light signals. Plant Cell Environ 23: 665–674 [DOI] [PubMed] [Google Scholar]
- Turano FJ, Panta GR, Allard MW, van Berkum P (2001) The putative glutamate receptors from plants are related to two superfamilies of animal neurotransmitter receptors via distinct evolutionary mechanisms. Mol Biol Evol 18: 1417–1420 [DOI] [PubMed] [Google Scholar]
- Véry A-A, Sentenac H (2002) Cation channels in the Arabidopsis plasma membrane. Trends Plant Sci 7: 168–175 [DOI] [PubMed] [Google Scholar]
- Walch-Liu P, Liu LH, Remans T, Tester M, Forde BG (2006) Evidence that L-glutamate can act as an exogenous signal to modulate root growth and branching in Arabidopsis thaliana. Plant Cell Physiol 47: 1045–1057 [DOI] [PubMed] [Google Scholar]
- White PJ, Bowen HC, Demidchik V, Nichols C, Davies JM (2002) Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochim Biophys Acta 1564: 299–309 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.