Abstract
The white petals of chrysanthemum (Chrysanthemum morifolium Ramat.) are believed to contain a factor that inhibits the accumulation of carotenoids. To find this factor, we performed polymerase chain reaction-Select subtraction screening and obtained a clone expressed differentially in white and yellow petals. The deduced amino acid sequence of the protein (designated CmCCD4a) encoded by the clone was highly homologous to the sequence of carotenoid cleavage dioxygenase. All the white-flowered chrysanthemum cultivars tested showed high levels of CmCCD4a transcript in their petals, whereas most of the yellow-flowered cultivars showed extremely low levels. Expression of CmCCD4a was strictly limited to flower petals and was not detected in other organs, such as the root, stem, or leaf. White petals turned yellow after the RNAi construct of CmCCD4a was introduced. These results indicate that in white petals of chrysanthemums, carotenoids are synthesized but are subsequently degraded into colorless compounds, which results in the white color.
Carotenoids are 40-carbon isoprenoids with polyene chains that may contain up to 15 conjugated double bonds. More than 700 naturally occurring carotenoids have been identified (Britton et al., 2004). Carotenoids are essential for photosynthesis, and they furnish flowers and fruits with distinct colors designed to attract insects and other animals. Carotenoids also serve as precursors for the biosynthesis of the plant growth regulator abscisic acid (Schwartz et al., 1997).
The chrysanthemum (Chrysanthemum morifolium Ramat.), which has been bred for more than 2,000 years, is one of the most important ornamental flowers in the world. The petal color of yellow-flowered cultivars originates mainly from carotenoids. Understanding the mechanism that controls carotenoid accumulation in petals will not only contribute greatly to the breeding of chrysanthemums and other flowering plants but also provide important information about the molecular evolutionary mechanisms responsible for different petal colors. Cultivated chrysanthemums are thought to have originated from hybrids between white- and yellow-flowered wild species. On the basis of an experiment in which white- and yellow-flowered chrysanthemums were crossed, Hattori (1991) observed that the white petal color is dominant over yellow and suggested that a single dominant gene that inhibits carotenoid accumulation may exist. The detailed function of such a gene, however, is still unknown. Kishimoto and Ohmiya (2006) demonstrated no significant difference between the expression levels of carotenoid biosynthetic genes in white and yellow petals during the course of development. In addition, the carotenoid content in immature white petals is almost equal to that in yellow petals, and the carotenoid content decreases to undetectable levels as the white petals mature. These results indicate that the formation of white color is caused neither by down-regulation nor by disruption of the carotenoid biosynthetic pathway.
To find a factor that controls carotenoid content in chrysanthemum petals, we performed PCR-Select subtraction screening to search for cDNAs that were differentially expressed in white and yellow petals. In this study, we show that a gene encoding a carotenoid cleavage dioxygenase (CCD; designated as CmCCD4a) is expressed specifically in white petals and that this enzyme contributes to the formation of white color in chrysanthemum petals.
RESULTS
Cloning of CmCCD4a by PCR-Select Subtraction Screening
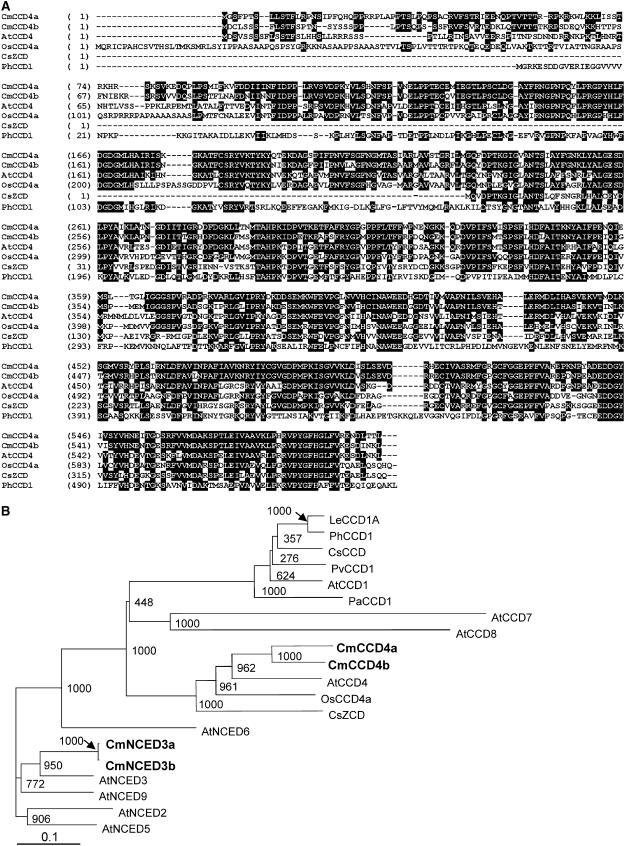
We performed subtraction of messages expressed in the white petals of chrysanthemum cv Paragon from those in the yellow petals of cv Yellow Paragon, a bud sport arising from Paragon. We then screened for cDNAs that were differentially expressed in the white and yellow petals and obtained 31 cDNA clones whose expression was higher in Paragon, and 71 clones whose expression was higher in Yellow Paragon. On the basis of signal-to-noise ratios, we selected 15 clones for further analysis. Quantitative real-time reverse transcription (RT)-PCR analysis showed that only one of the clones was expressed specifically in the petals of white cultivars. We obtained full-length cDNA of the clone by the RACE method, and we determined its nucleotide sequence. The nucleotide sequence of the clone contained an open reading frame of 1,797 bp, predicting a 599-amino acid polypeptide, and had an estimated molecular mass of 67 kD. Comparison of the deduced amino acid sequence of the protein encoded by the clone with available databases revealed the sequence to be highly homologous with that of CCD (Fig. 1A). The protein showed the highest homology with AtCCD4, a CCD homolog found in Arabidopsis (Arabidopsis thaliana; Fig. 1B); we therefore designated the clone as CmCCD4a. The amino acid sequence of CmCCD4a showed 61% homology with the sequence of AtCCD4, and 51% homology with that of OsCCD4a from rice (Oryza sativa).
Figure 1.
Sequence comparison of CCDs of various plant species. A, Alignment of amino acid sequence of CmCCD4a and CmCCD4b with related sequences. Identical amino acids are indicated with black backgrounds. Four conserved His residues are marked with asterisks. B, ClustalW tree analysis of chrysanthemum CCD and NCED homologs in various plant species. AtCCD1 (At3g63520), AtCCD4 (At4g19170), AtCCD7 (At2g44990), and AtCCD8 (At4g32810) are CCDs of Arabidopsis. AtNCED2 (At4g18350), AtNCED3 (At3g14440), AtNCED5 (At1g30100), AtNCED6 (At3g24220), and AtNCED9 (At1g78390) are NCEDs of Arabidopsis. Other CCDs include PhCCD1 of petunia (AY576003), LeCCD1A of tomato (Lycopersicon esculentum; AY576001), OsCCD4a of rice (AP007825), CsCCD (AJ132927) and CsZCD (AJ489276) of crocus, PaCCD1 (AF224670) of avocado (Persea americana), and PvCCD1 (AY029525) of kidney bean (Phaseolus vulgaris). Numbers at branch points indicate bootstrap values (1,000 replicates).
Cloning of CCD and NCED Homologs and Expression in Petals
Homologs of CCD and NCED (9-cis-epoxycarotenoid dioxygenase) were screened from cDNAs of petals and leaves of chrysanthemums using degenerate primers of CCD and NCED, respectively. In addition to CmCCD4a, we obtained one CCD homolog and two NCED homologs from leaf cDNA. The amino acid sequence of the protein encoded by the CCD homolog showed the highest homology to AtCCD4, and the proteins encoded by the NCED homologs showed the highest homology to AtNCED3 among the Arabidopsis CCD family. Therefore, we designated these proteins as CmCCD4b, CmNCED3a, and CmNCED3b, respectively (Fig. 1B). We performed genomic PCR using degenerate primers to search for additional homologs, but we did not find any. On the basis of sequence comparison of these homologs, we designed primers specific to each homolog for genomic PCR and real-time RT-PCR analyses.
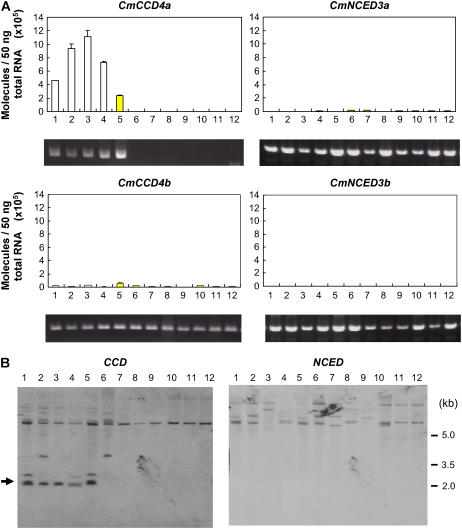
Figure 2A shows the levels of transcripts of CCD and NCED homologs in the white and yellow petals of various chrysanthemum cultivars. All the white petals tested showed high levels of expression of CmCCD4a, whereas transcripts in the yellow petals were not detected, except in Yellow Paragon, which had approximately half the amount of CmCCD4a transcripts as Paragon. The levels of CmCCD4b, CmNCED3a, and CmNCED3b transcripts in petals were extremely low compared to the level of CmCCD4a transcripts. In addition, there was no significant difference between the expression levels of these homologs in white and yellow petals.
Figure 2.
Color-specific expression and genomic PCR analyses. Lanes 1 to 4, white-flowered cultivars; lanes 5 to 12, yellow-flowered cultivars: 1, Paragon; 2, White Marble; 3, Fiducia; 4, Sei-Marine; 5, Yellow Paragon; 6, Florida Marble; 7, Homaro; 8, Susie; 9, Morning Sun; 10, Statesman; 11, Super Yellow; and 12, Sunny Orange. A, Expression of chrysanthemum CCD and NCED homologs in petals of white- and yellow-flowered cultivars of chrysanthemum. Quantitative real-time RT-PCR analysis was performed in triplicate using specific primers for each homolog, and the expression levels were normalized against actin levels; mean values ±se are shown. Genomic PCR with homolog-specific primers of the same chrysanthemum cultivars analyzed in real-time RT-PCR are presented below. B, Genomic Southern-blot analysis of the chrysanthemum cultivars analyzed in A. Genomic DNAs (30 μg) were digested with BamHI and loaded onto each lane. Fragments were hybridized with DIG-labeled CmCCD4a cDNA. The filter was reprobed with DIG-labeled CmNCED3a cDNA. The arrowhead indicates the band that corresponds to CmCCD4a.
Genomic PCR analysis showed that the bands that corresponded to CmCCD4a were not amplified in yellow-flowered cultivars with extremely low expression of CmCCD4a (Fig. 2A). In contrast, the bands corresponding to CmCCD4b, CmNCED3a, and CmNCED3b were amplified in all the cultivars we tested. Genomic CmCCD4a has two BamHI sites: one at −464 bp and another at 1,565 bp from the start codon; BamHI digestion produced a 2.0-kb fragment. In Southern-blot analysis probed with the CCD fragment, the band corresponding to CmCCD4a was not detected in these cultivars (Fig. 2B). The band at 7 kb existed in all the cultivars tested. Because the CmCCD4b gene existed in all the cultivars, the band may correspond to CmCCD4b. The band pattern of Paragon coincided with that of Yellow Paragon, a bud sport of Paragon. On the other hand, Florida Marble, a bud sport of White Marble, had three bands in common with White Marble but lacked a band corresponding to CmCCD4a. When the same filter was reprobed with the NCED fragment, all cultivars showed two to three bands of different sizes. Different band patterns were observed in membranes hybridized with CCD and NCED probes, which indicates that these probes are not cross hybridized.
Organ- and Stage-Specific Expression of CCD and NCED Homologs
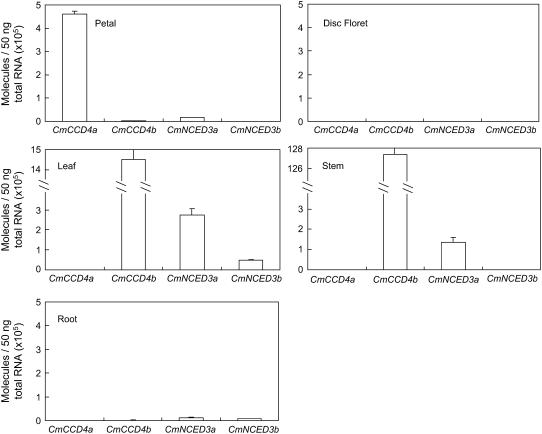
Figure 3 shows the organ-specific expression pattern of CCD and NCED homologs. Expression of CmCCD4a was strictly limited to flower petals and was not detected in other organs, such as the root, stem, or leaf. The chrysanthemum flower is often dimorphic: in the center of the capitulum is the small disc floret; in the marginal ray floret, the corolla is conspicuous as a long petal. White-flowered cultivars usually have yellow disc florets. The level of CmCCD4a transcripts in the yellow disc floret of Paragon was extremely low compared with the level in the white petals of the ray floret. Yellow disc florets of the other white-flowered cultivars, such as Sei-Marine, Fiducia, and White Marble, also showed extremely low levels of CmCCD4a transcripts (data not shown). The abundance of transcripts of CmCCD4b, CmNCED3a, and CmNCED3b showed different organ specificity from that of CmCCD4a. These transcripts were extremely low in petals, disc florets, and roots. The levels of CmCCD4b and CmNCED3a transcripts were high in leaves. The expression of CmCCD4b was extremely high in stems; this high level of expression may have been caused partly by the normalization against actin levels, if stem tissue had a substantially lower level of actin transcripts than other tissues. Expression of CmNCED3b was low in all the tissues we tested.
Figure 3.
Expression of chrysanthemum CCD and NCED homologs in various tissues of the white-flowered cultivar Paragon. Quantitative real-time RT-PCR analysis was performed in triplicate using primers specific to each homolog, and the expression levels were normalized against actin levels; mean values ± se are shown.
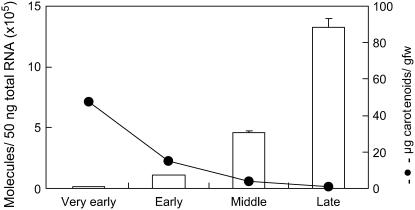
White petals of Paragon contained 47 μg/g fresh weight of carotenoids at the very early stage of flower development (Fig. 4). Carotenoid content decreased as the petals matured, and carotenoids were not detected in the fully opened petals. In contrast, the level of CmCCD4a transcripts increased drastically during the course of petal development.
Figure 4.
Changes in carotenoid concentrations (black circles) and CmCCD4a transcript levels (bars) during flower petal development of Paragon. Quantitative real-time RT-PCR analysis was performed in triplicate using CmCCD4a-specific primers, and the expression levels were normalized against actin levels; mean values ± se are shown.
Suppression of CmCCD4a Expression by RNAi
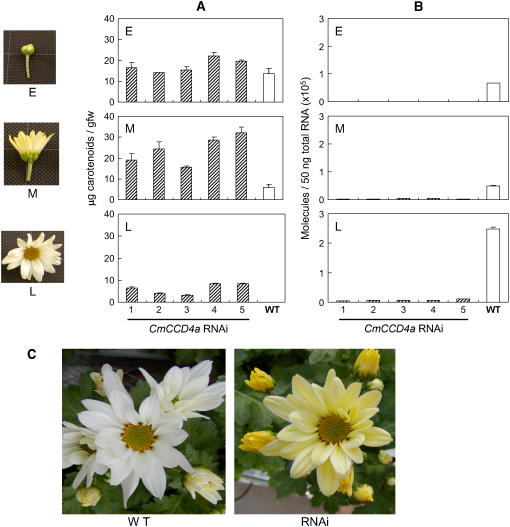
To determine the role of CmCCD4a gene products in the formation of petal color, we produced transgenic chrysanthemum plants with reduced expression of CmCCD4a. We introduced the RNAi construct of CmCCD4a under the control of the tobacco (Nicotiana tabacum) elongation factor 1α promoter into the white-flowered chrysanthemum cultivar Sei-Marine. We analyzed five independently derived transgenic plants. Carotenoid content values in middle-stage petals of these transgenic lines were about 3 to 6 times the values observed in wild-type plants (Fig. 5A). During late-stage petal development, when petals of wild-type plants completely lost their carotenoids, the petals of transgenic lines contained 3 to 8 μg/g fresh weight of carotenoids and looked yellow (Fig. 5B). Quantitative real-time RT-PCR analysis showed that the petals of the transgenic lines expressed the CmCCD4a gene at only 2% to 4% of the wild-type level.
Figure 5.
Suppression of CmCCD4a expression by RNAi. A, Changes in carotenoid concentrations during petal development of wild-type (WT) and five independent CmCCD4a RNAi plants (1–5). Measurements were performed in triplicate, and mean values ± se are shown . E, Early; M, middle; L, late. B, Changes in CmCCD4a expression during petal development of wild-type and CmCCD4a RNAi plants. Quantitative real-time RT-PCR analyses were performed in triplicate using CmCCD4a-specific primers, and the expression levels were normalized against actin levels; mean values ± se are shown. C, Photographs of fully opened flowers of wild-type Sei-Marine and CmCCD4a RNAi plants (line 5).
CmCCD4a in Wild Chrysanthemum Species
Genomic PCR analysis was also performed in white- and yellow-flowered wild species of chrysanthemum (Fig. 6). The bands that corresponded to CmCCD4a were observed in all the white-flowered species but not in the yellow-flowered species. In contrast, the bands that corresponded to CmCCD4b were observed in both white- and yellow-flowered species.
Figure 6.
Genomic PCR of white- and yellow-flowered wild species of chrysanthemum with CmCCD4a and CmCCD4b primers: 1, Chrysanthemum boreale; 2, Chrysanthemum indicum; 3, Chrysanthemum makinoi; 4, Chrysanthemum japonese; 5, Chrysanthemum yezoense; 6, Chrysanthemum zawadskii; and 7, Chrysanthemum yoshinaganthum.
Light Microscope Observation of Transverse Sections of Petals
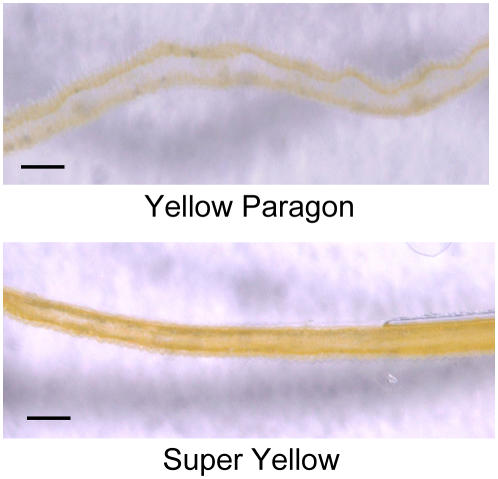
Among yellow-flowered cultivars, only Yellow Paragon expressed CmCCD4a in petals. It is possible that petals of Yellow Paragon are periclinal chimera, and either the L1 or the L2 layer may behave genetically in a manner identical to that of the white progenitor Paragon. Periclinal structures were determined by microscope examination of transverse sections of petals (Fig. 7). In the sections of Yellow Paragon, yellow pigmentation was localized in the adaxial epidermis (L1 layer), and the underlying mesophyll (L2 layer) appeared to be white. In contrast, both the L1 and L2 layers were yellow in petals of Super Yellow.
Figure 7.
Light microscope observation of transverse section through petals of yellow-flowered cultivars Yellow Paragon and Super Yellow. Bars = 100 μm.
DISCUSSION
By means of PCR-Select subtraction screening, we obtained a clone that encoded a CCD and was differentially expressed in white and yellow petals of chrysanthemums. Several types of CCDs were recently reported in various plant species. Arabidopsis contains nine homologs of the CCD family (Tan et al., 2003). Five of the homologs have been designated as 9-cis-epoxycarotenoid dioxygenases (AtNCED2, AtNCED3, AtNCED5, AtNCED6, and AtNCED9), which catalyze cleavage of the 11,12 double bond of 9-cis-neoxanthin and 9-cis-violaxanthin to form xanthoxin, an abscisic acid precursor. The rest of these CCDs (designated AtCCD1, AtCCD4, AtCCD7, and AtCCD8) cleave carotenoids into apocarotenoids at different double-bond positions. AtCCD1 symmetrically cleaves the 9,10 (9′,10′) double bonds of multiple carotenoid substrates in vitro (Schwartz et al., 2001). Homologs of this enzyme have recently been identified in various plant species; some of the homologs contribute to the formation of flavor volatiles, such as β-ionone, pseudoionone, and geranylacetone (Simkin et al., 2004a, 2004b). AtCCD7 and AtCCD8 sequentially cleave β-carotene into 13-apo-β-carotenone (C18), a signaling molecule that is necessary for the regulation of lateral branching (Schwartz et al., 2004). The biochemical and enzymatic activities of AtCCD4 have not been characterized. The deduced amino acid sequence of CmCCD4a showed the highest homology with that of AtCCD4 among members of the Arabidopsis CCD family, showing 61% homology. CmCCD4a also shares a common feature with AtCCD4 in that both contain a plastid-targeting transit peptide at the N terminus and four highly conserved His residues that may be involved in coordinating a nonheme iron that is required for enzymatic activity (Schwartz et al., 1997; Tan et al., 1997). Sequencing of a genomic clone encoding CmCCD4a revealed the presence of a 105-bp intron at 1,442 bp from the start codon (data not shown); this intron was not present in AtCCD4.
Quantitative real-time RT-PCR analysis showed a clear relationship between CmCCD4a mRNA abundance and carotenoid content in various chrysanthemum tissues: when the level of CmCCD4a transcripts was high, the carotenoid content was low. Kishimoto and Ohmiya (2006) compared expression of genes encoding isoprenoid and carotenoid biosynthetic enzymes in both white- and yellow-flowered cultivars. The difference they observed in the levels of transcripts, however, could not account for the differences between the carotenoid content values for yellow and white petals. In addition, white petals of chrysanthemums accumulate carotenoids during the early stage of petal development at levels similar to the levels in the yellow petals, which shows that the carotenoid biosynthetic pathway is not blocked in white petals. It is possible that white petals can synthesize carotenoids even when the carotenoid content decreases to undetectable levels. We therefore assumed that in white petals, carotenoid degradation by CmCCD4a causes the white color, even though carotenoid biosynthesis continues to take place.
We therefore performed an RNAi experiment to see whether the suppression of CmCCD4a expression affected the petal color of chrysanthemums. In RNAi lines of white-flowered cultivars, in which CmCCD4a expression was reduced to 2% to 4%, petal color became yellow. This result clearly indicates that CmCCD4a contributed to the white color formation in chrysanthemum petals by cleaving carotenoids into colorless compounds. During petal development of yellow-flowered chrysanthemum cultivars, carotenoid concentration increases approximately 10-fold, owing to the accumulation of lutein and its derivatives (Kishimoto and Ohmiya, 2006). In transgenic lines introduced in the RNAi construct of CmCCD4a, carotenoids decreased as petals matured, and carotenoid levels in late-stage petals were lower than those in yellow-flowered cultivars. This phenomenon is probably because of the enzymatic activity of CmCCD4a that existed in transgenic lines, although the level was low. It may be possible to increase the carotenoid content in petals to make a vivid yellow petal color by completely knocking out the expression of CmCCD4a.
We obtained one CCD homolog (CmCCD4b) and two NCED homologs (CmNCED3a and CmNCED3b). The expression of these homologs in petals was lower than the expression of CmCCD4a. In addition, significant differences between the expression levels of these homologs in white and yellow petals were not observed. We therefore assumed that among the currently available CCD and NCED homologs of chrysanthemums, only CmCCD4a was involved in white color formation in petals. Expression of CmCCD4a was detected only in white petals, which indicates that the function of CmCCD4a was limited to white color formation in petals. Kishimoto and Ohmiya (2006) reported that at the very early stage of petal development, Paragon contains carotenoids such as lutein and β-carotene but that the levels of these compounds are drastically decreased during petal development. Their results suggest that CmCCD4a cleaves these carotenoids into colorless compounds.
Some CCDs are present in flowers and contribute to the formation of aroma and pigment compounds. Simkin et al. (2004b) demonstrated that the petunia (Petunia hybrida) PhCCD1, a homolog of Arabidopsis AtCCD1, contributes to the formation of β-ionone and geranylacetone, important constituents of petunia flavor. Significant expression of PhCCD1 also occurs in roots and leaves. What role expression of these genes would play in vegetative tissues is not clear. Bouvier et al. (2003) reported a unique CCD (designated CsZCD) whose expression is restricted to the style branch tissues of the crocus (Crocus sativus). CsZCD catalyzes the cleavage of zeaxanthin at the 7,8 and 7′,8′ positions, initiating the synthesis of the saffron pigment and aroma. Among CCD homologs reported so far, there is no CCD whose expression is limited to petals. Very few data are available concerning the relationship between pigment degradation and petal color. Vaknin et al. (2005) suggested that active anthocyanin degradation occurs in the petals of Brunfelsia calycina, resulting in white color. However, the precise mechanism remains to be elucidated.
Sporting, a well-known phenomenon in chrysanthemums, is a process by which new cultivars arise vegetatively from the parental cultivar. Generally, variants that arise from radiation breeding or bud sports are involved in genomic deletions. Dowrick and Bayoumi (1966) showed that changes in chromosome number and chromosome fragmentation are usually responsible for color changes. About one-third of commercial chrysanthemum cultivars are thought to have arisen in this way (Wasscher, 1956). In the case of chrysanthemums, yellow-flowered bud sports arise from white-flowered cultivars, but to our knowledge the mutation for the reverse orientation has never been observed. A single gene was postulated by Hattori (1991): the dominant allele gives white cultivars, and the recessive gives yellow cultivars. He speculated that such a gene might act as a suppressor of carotenoid formation, although the precise mechanism remains to be elucidated. We propose herein that CmCCD4a is this so-called suppressor of carotenoid formation; however, our results suggest that rather than suppressing carotenoid formation it catalyzes carotenoid breakdown. It is assumed that yellow-flowered bud sports may lose the CmCCD4a gene during somatic mutation, and loss of the gene results in accumulation of carotenoids. In fact, Florida Marble, a bud sport of White Marble, lacks the band corresponding to CmCCD4a in Southern-blot analysis, while the other bands existed in common in both White Marble and Florida Marble.
Most yellow-flowered cultivars do not have the CmCCD4a gene in their genome. Among the yellow-flowered cultivars we tested, only Yellow Paragon, a bud sport that arose from Paragon, had the CmCCD4a gene, and this gene was expressed in the petals. Cultivars that arise as sports are frequently periclinal chimera and have cell layers that are genetically different. Langton (1980) demonstrated that some yellow-flowered sports of chrysanthemum have carotenoids in only one layer of their petals. In this study, light microscope observation revealed that petals of Yellow Paragon are periclinal chimera, consisting of a yellow L1 layer and a white L2 layer. Because CmCCD4a expressed in petals of Yellow Paragon has the same nucleotide sequence as Paragon (data not shown), the L2 layer of the petals of Yellow Paragon may behave genetically in a manner identical to that of its white progenitor Paragon, and the L1 layer of petals may lose its enzymatic activity not by nucleotide change but by genomic deletion. CmCCD4a was not expressed in the L1 layer, which resulted in lower level of expression in petals than that observed in Paragon.
Cultivated chrysanthemums are thought to originate from hybrids of white- and yellow-flowered wild species, although their exact origin is uncertain. Genomic PCR analysis showed that yellow-flowered wild species of chrysanthemum did not have the CmCCD4a gene, whereas all white-flowered wild species that we tested did have the CmCCD4a gene in their genome. We believe that the CmCCD4a gene is a dominant factor that determines the petal color—yellow or white—of chrysanthemums. Our findings thus shed light on the molecular evolutionary mechanisms resulting in different petal colors. It is of particular interest to determine whether the mechanism of white color formation found in chrysanthemum petals is applicable to other plant species.
MATERIALS AND METHODS
Plant Material
Plant tissues were obtained from chrysanthemums (Chrysanthemum morifolium Ramat.) grown in greenhouses at the National Institute of Floricultural Science (Tsukuba, Ibaraki, Japan). Petal development was divided into four stages on the basis of petal length: very early (2–3 mm), early (8–10 mm), middle (15–18 mm), and late (30–35 mm). RNAs and carotenoids were extracted from petals in all four stages.
PCR-Select Subtraction Screening
Total RNA was extracted from petals of Paragon (a white-flowered cultivar) and Yellow Paragon (a yellow-flowered sport that arose from Paragon) by means of a cetyltrimethylammonium bromide method (Chang et al., 1993); and poly(A)+ RNA was purified using an mRNA purification kit (Amersham Bioscience). A PCR-based cDNA subtraction method was used to selectively amplify differentially expressed cDNA fragments in white and yellow petals using the PCR-Select cDNA subtraction kit (BD Biosciences CLONTECH). mRNAs derived from petals of Paragon and Yellow Paragon were converted into double-stranded cDNAs. Tester (Paragon) and driver (Yellow Paragon) cDNAs were hybridized, and hybrid sequences were removed. The remaining molecules were then subjected to PCR to enrich the differentially expressed sequences. These cDNAs were inserted into the pUC18 vector and transferred into Escherichia coli DH5α. Individual clones were amplified by PCR with M13-20 and M13-reverse primers, dotted onto membranes, and tested for differential expression using the PCR-Select differential screening kit (BD Biosciences CLONTECH).
Phylogenetic Method
Multiple alignments of amino acid sequences were produced with a Web-based version of ClustalW (http://crick.genes.nig.ac.jp/homology/clustalw-e.shtml). The phylogenic tree was calculated using the neighbor-joining method and bootstrap analysis (1,000 replicates) using PHYLIP via the same Web site and visualized with Treeviewer version 1.6.6 (http://taxonomy.zoology.gla.ac.uk/rod/rod.html).
Real-Time RT-PCR Analysis
cDNAs were synthesized using the Superscript first-strand synthesis system (Invitrogen) from poly(A)+ RNA treated with DNase I. Levels of CmCCD4a transcripts were analyzed by real-time RT-PCR with the QuantiTect SYBR Green PCR kit (QIAGEN). Reactions were carried out using a LightCycler system (Roche Diagnostics). A chrysanthemum actin cDNA (GenBank accession no. AB205087) was identified by RT-PCR and used as a constitutive control. Primer sequences for chrysanthemum actin were as follows: forward, 5′-ACATGCTATCTTGCGTTTGG-3′; reverse, 5′-CTCTCACAATTTCCCGTTCA-3′. A plasmid containing CmCCD4a was used in a standard curve assay, and levels of CmCCD4a transcripts are given as copy number per 50 ng of total RNA.
Degenerate primers corresponding to the conserved regions of either NCED or CCD were designed to isolate their homologs from chrysanthemums: NCED forward, 5′-TTYGAYGGNGAYGGNATGGTNCAY-3′; NCED reverse, 5′-YTCCCANGCRTTCCANARRTGRAA-3′; CCD forward, 5′-TAYCAYYMRTTYGAYGGNGAYGGNATG-3′; CCD reverse, 5′-TANCGNGGNAARNACNCCNAAYCT-3′. We screened cDNAs of petals and leaves of chrysanthemums and obtained one CCD homolog (designated as CmCCD4b) and two NCED homologs (designated as CmNCED3a and CmNCED3b) from leaf cDNAs. Full-length cDNAs for these homologs were obtained by RACE. By avoiding homologous parts among these homologs, we designed specific primers for real-time RT-PCR and genomic PCR analyses for each homolog as follows: CmCCD4a forward, 5′-CCATCCCATTTCAACATCAACCA-3′; CmCCD4a reverse, 5′-ATTAGCTTTTTCAGCCATTTTCTTT-3′; CmCCD4b forward, 5′-CACCAACCAACTCTTACTCTTC-3′; CmCCD4b reverse, 5′-ATGTTTTTTCACTTGTTCATCAC-3′; CmNCED3a forward, 5′-TCTACCTAGAGACAATGCTAGTGA-3′; CmNCED3a reverse, 5′-CCTCGATGGTAACATAACTGTC-3′; CmNCED3b forward, 5′-GCATTCGATCACAAGTTTTC-3′; CmNCED3b reverse, 5′-TTTTCATTATCTTTTATTCGGTC-3′.
Southern-Blot Analysis
Total genomic DNA was isolated from approximately 1 g of chrysanthemum leaves by a cetyltrimethylammonium bromide method as described by Doyle and Dickson (1987), completely digested by BamHI, separated on a 0.8% agarose gel, and blotted onto Hybond N+ membrane (Amersham Biosciences). Coding regions of CmCCD4a and CmNCED3a were labeled with digoxigenin (DIG) using PCR DIG probe synthesis kit (Roche Diagnostics), and used as probes. Primer sequences used for amplification of the probes were as follows: CmCCD4a forward, 5′-CAGACAGAGAAAGATGCGGAATCA-3′ and reverse, 5′-AGATTCCGGATTGAAAGAGGGTAC-3′; CmNCED3a forward, 5′-CAGTAAAATTCGATCAAGGTGAAG-3′ and reverse, 5′-AAATCATTACAAACAAGCTGCTACTAT-3′. The filter was hybridized with DIG-labeled CmCCD4a probe and washed twice with 2× SSC, 0.1% SDS at RT for 15 min, and then twice with 0.1× SSC, 0.1% SDS at 68°C for 15 min. We removed the CmCCD4a probe by dipping the filter in 0.2 n NaOH, 1% SDS for 30 min; the filter was then washed with 2× SSC and rehybridized with the DIG-labeled CmNCED3a probe.
RNAi Construct and Agrobacterium-Mediated Transformation
A 442-bp sense fragment of CmCCD4a containing BamHI and SacI sites was amplified by PCR using primers 5′-GGAGTCGGGGATCCAATGCCTAAA-3′ and 5′-TATCTCATAAATGAGCTCTCTAGTAGGAG-3′. A 293-bp antisense fragment of CmCCD4a containing BamHI and SalI sites was amplified by PCR using primers 5′-GGAACCCAAGGATCCATATGCGGAC-3′ and 5′-CATAAATGATGTGTCGACTAGGAGTCGT-3′. A 150-bp fragment from the 5′ end of the sense fragment was used as a linker between sense and antisense orientations. The sense construct was prepared by excising the β-glucuronidase fragment from pBIEF1α (Aida et al., 2005) with BamHI/SacI and inserting the sense fragment of CmCCD4a at the same sites. The inverted repeat construct (RNAi construct) was then made by inserting the antisense fragment into the BamHI/SalI sites.
Chrysanthemum cultivar Sei-Marine was transformed as described by Aida et al. (2005), using Agrobacterium strain EHA105 carrying the RNAi construct. Transformed chrysanthemum lines were selected on Murashige and Skoog medium (Murashige and Skoog, 1962) with half-strength minerals (1/2 Murashige and Skoog), solidified with 0.8% (w/v) agar containing 6-benzyladenine (1 μg/mL), naphthylacetic acid (2 μg/mL), carbenicillin (300 μg/mL), and paromomycin (50 μg/mL), at 20°C under a 16-h light/8-h dark photoperiod with fluorescent light (photon flux density 7 μmol s−1 m−2). Paromomycin-resistant shoots were then transferred to soil.
Measurement of Carotenoid Concentration
Tissues (0.5 g) were ground in acetone, then were partitioned between diethyl ether and aqueous NaCl. Carotenoid content was determined by measuring the A445 of the diethyl ether layer and is expressed as microgram of lutein equivalent per gram fresh weight of the tissue.
Light Microscope Observation
Petals were cut into 5 × 5 mm pieces and embedded in 4% agarose. Thin sections (thickness, 100 μm) were cut with a Micro Slicer DTK-1000 (D.S.K.). Sections were examined using a VH-Z75 microscope (Keyence).
The GenBank accession numbers for the cDNAs mentioned in this article are as follows: CmCCD4a, AB247158; CmCCD4b, AB247160; CmNCED3a, AB247159; and CmNCED3b, AB247161.
Acknowledgments
We thank S. Sugaya, University of Tsukuba, for providing degenerate primers for NCED amplification; M. Shibata, National Institute of Floricultural Science, for helpful discussions; and M. Mori, National Institute of Floricultural Science, for technical assistance.
This work was supported by a Grant-in-Aid (Development of innovative crops through the molecular analysis of useful genes) from the Ministry of Agriculture, Forestry, and Fisheries of Japan.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Akemi Ohmiya (ohmiya@affrc.go.jp).
Open Access articles can be viewed online without a subscription.
References
- Aida R, Nagaya S, Yoshida K, Kishimoto S, Shibata M, Ohmiya A (2005) Efficient transgene expression in Chrysanthemum morifolium Ramat., with the promoter of a gene for tobacco elongation factor 1 α protein. JARQ 39: 269–274 [Google Scholar]
- Bouvier F, Suire C, Mutterer J, Camara B (2003) Oxidative remodeling of chromoplast carotenoids: identification of the carotenoid dioxygenase CsCCD and CsZCD genes involved in crocus secondary metabolite biogenesis. Plant Cell 15: 47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton G, Liaaen-Jensen S, Pfander H, editors (2004) Carotenoids Handbook. Birkhauser, Basel
- Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolation of RNA from pine trees. Plant Mol Biol Rep 11: 113–116 [Google Scholar]
- Dowrick GD, Bayoumi A (1966) The induction of mutations in chrysanthemum using X- and gamma radiation. Euphytica 15: 204–210 [Google Scholar]
- Doyle JJ, Dickson EE (1987) Preservation of plant samples for DNA restriction endonuclease analysis. Taxon 36: 715–722 [Google Scholar]
- Hattori K (1991) Inheritance of carotenoid pigmentation in flower color of chrysanthemum. Jpn J Breed 41: 1–9 [Google Scholar]
- Kishimoto S, Ohmiya A (2006) Regulation of carotenoid biosynthesis in petals and leaves of chrysanthemum (Chrysanthemum morifolium Ramat.). Physiol Plant (in press)
- Langton FA (1980) Chimerical structure and carotenoid inheritance in Chrysanthemum morifolium (Ramat.). Euphytica 29: 807–812 [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Schwartz SH, Qin X, Loewen M (2004) The biochemical characterization of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. J Biol Chem 279: 46940–46945 [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Qin X, Zeevaart JAD (2001) Characterization of a novel carotenoid cleavage dioxygenase from plants. J Biol Chem 276: 25208–25211 [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JAD, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276: 1872–1874 [DOI] [PubMed] [Google Scholar]
- Simkin AJ, Schwartz SH, Auldridge M, Taylor MG, Klee HJ (2004. a) The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles β-ionone, pseudoionone, and geranylacetone. Plant J 40: 882–892 [DOI] [PubMed] [Google Scholar]
- Simkin AJ, Underwood BA, Auldridge M, Loucas HM, Shibuya K, Schmelz E, Clark DG, Klee HJ (2004. b) Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of B-ionone, a fragrance volatile of petunia flowers. Plant Physiol 136: 3504–3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR (2003) Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35: 44–56 [DOI] [PubMed] [Google Scholar]
- Tan BC, Schwartz SH, Zeevaart JAD, McCarty DR (1997) Genetic control of abscisic acid biosynthesis in maize. Proc Natl Acad Sci USA 94: 12235–12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaknin H, Bar-Akiva A, Ovadia R, Nissim-Levi A, Forer I, Weiss D, Oren-Shamir M (2005) Active anthocyanin degradation in Brunfelsia calycina (yesterday-today-tomorrow) flowers. Planta 222: 19–26 [DOI] [PubMed] [Google Scholar]
- Wasscher J (1956) The importance of sports in some florist's flowers. Euphytica 5: 163–170 [Google Scholar]