Abstract
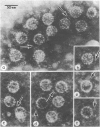
Haemagglutination-inhibition (HAI) antibodies to BK virus, including BK-virus-specific IgM, were determined before and after renal transplantation in 20 patients, in 57 patients with malignant disease, and in 66 healthy controls, Before transplantation 11 of the renal transplant recipients were seronegative, but eight later serocconverted, two before and six after transplantation. Twenty of the patients with malignant disease and 22 controls were also seronegative. The geometric mean titre of BK HAI antibodies was significantly higher among transplanted patients (1/180) than among controls (1/90). BK-virus-specific IgM antibody was detected in seven renal transplant recipients, six patients with malignant disease, and 13 healthy controls. In transplant recipients BK-virus-specific IgM antibody usually persisted throughout the duration of the study, and studies on controls from whom second serum samples were available suggested that they too had persistent BK-virus-specific IgM responses. The geometric mean titre of BK-virus-specific IgM HAI antibody was significantly greater in post-transplantation sera (1/223) than in control sera (1/28). The specificity of the detection of BK-virus-specific IgM HAI antibody was confirmed by direct visualisation of antibody by immune electron microscopy. The persistence of BK-virus-specific IgM suggested that BK virus continued to provide an antigenic stimulus. Nevertheless, there was no obvious association between the serological findings and any clinical features, and prospective studies will be needed to elucidate any such association.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Nakib W., Best J. M., Banatvala J. E. Rubella-specific serum and nasopharygeal immunoglobulin responses following naturally acquired and vaccine-induced infection. Prolonged persistence of virus-specific IgM. Lancet. 1975 Jan 25;1(7900):182–185. doi: 10.1016/s0140-6736(75)91356-2. [DOI] [PubMed] [Google Scholar]
- Alford C. A., Jr Studies on antibody in congenital rubella infections. I. Physicochemical and immunologic investigations of rubella neutralizing antibody. Am J Dis Child. 1965 Oct;110(4):455–463. doi: 10.1001/archpedi.1965.02090030475019. [DOI] [PubMed] [Google Scholar]
- Almeida J. D., Brown F., Waterson A. P. The morphologic characteristics of 19S antibody. J Immunol. 1967 Jan;98(1):186–193. [PubMed] [Google Scholar]
- Banatvala J. E., Best J. M., Waller D. K. Epstein-Barr virus-specific IgM in infectious mononucleosis, Burkitt lymphoma, nasopharyngeal carcinoma. Lancet. 1972 Jun 3;1(7762):1205–1208. doi: 10.1016/s0140-6736(72)90925-7. [DOI] [PubMed] [Google Scholar]
- Bellanti J. A., Artenstein M. S., Olson L. C., Buescher E. L., Luhrs C. E., Milstead K. L. Congenital rubella. Clinicopathologic, virologic, and immunologic studies. Am J Dis Child. 1965 Oct;110(4):464–472. doi: 10.1001/archpedi.1965.02090030484020. [DOI] [PubMed] [Google Scholar]
- Best J. M., Banatvala J. E., Watson D. Serum IgM and IgG responses in postnatally acquired rubella. Lancet. 1969 Jul 12;2(7611):65–68. doi: 10.1016/s0140-6736(69)92386-1. [DOI] [PubMed] [Google Scholar]
- Brady M. I., Furminger I. G. Purification of influenza virus with Arcton 113. J Clin Microbiol. 1976 May;3(5):524–527. doi: 10.1128/jcm.3.5.524-527.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. C., Baublis J. V., O'Leary T. P. Development and duration of mumps fluorescent antibodies in various immunoglobulin fractions of human serum. J Immunol. 1970 Jan;104(1):86–94. [PubMed] [Google Scholar]
- Caul E. O., Smyth G. W., Clarke S. K. A simplified method for the detection of rubella-specific IgM employing sucrose density fractionation and 2-mercaptoethanol. J Hyg (Lond) 1974 Dec;73(3):329–340. doi: 10.1017/s0022172400042674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. V., Gardner S. D., Field A. M. Human polyomavirus infection in renal allograft recipients. Br Med J. 1973 Aug 18;3(5876):371–375. doi: 10.1136/bmj.3.5876.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J. H., Haire M., Hadden D. S. Measles immunoglobulins in subacute sclerosing panencephalitis. Br Med J. 1971 Jan 2;1(5739):23–25. doi: 10.1136/bmj.1.5739.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead J. E., Hanshaw J. B., Carpenter C. B. Cytomegalovirus infection after renal allotransplantation. JAMA. 1967 Sep 4;201(10):725–728. [PubMed] [Google Scholar]
- Edelman R., Schneider R. J., Vejjajiva A., Pornpibul R., Voodhikul P. Persistence of virus-specific IgM and clinical recovery after Japanese encephalitis. Am J Trop Med Hyg. 1976 Sep;25(5):733–738. doi: 10.4269/ajtmh.1976.25.733. [DOI] [PubMed] [Google Scholar]
- Fiala M., Payne J. E., Berne T. V., Moore T. C., Henle W., Montgomerie J. Z., Chatterjee S. N., Guze L. B. Epidemiology of cytomegalovirus infection after transplantation and immunosuppression. J Infect Dis. 1975 Oct;132(4):421–433. doi: 10.1093/infdis/132.4.421. [DOI] [PubMed] [Google Scholar]
- Gardner S. D., Field A. M., Coleman D. V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971 Jun 19;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Gardner S. D. Prevalence in England of antibody to human polyomavirus (B.k.). Br Med J. 1973 Jan 13;1(5845):77–78. doi: 10.1136/bmj.1.5845.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMPARIAN V. V., MULLER F., HUMMELER K. Elimination of nonspecific components from viral antigens by fluorocarbon. J Immunol. 1958 Jun;80(6):468–475. [PubMed] [Google Scholar]
- Hanshaw J. B., Steinfeld H. J., White C. J. Fluorescent-antibody test for cytomegalovirus macroglobulin. N Engl J Med. 1968 Sep 12;279(11):566–570. doi: 10.1056/NEJM196809122791102. [DOI] [PubMed] [Google Scholar]
- Henry K., Bird R., Watson G., Hugh-Jones K. Aplastic anaemia, bone-marrow transplantation, and polyoma and other virus infections. Lancet. 1977 Mar 26;1(8013):695–696. doi: 10.1016/s0140-6736(77)92130-4. [DOI] [PubMed] [Google Scholar]
- Ishii K., Matsunaga Y., Kono R. Immunoglobulins produced in response to Japanese encephalitis virus infections of man. J Immunol. 1968 Oct;101(4):770–775. [PubMed] [Google Scholar]
- Jung M., Krech U., Price P. C., Pyndiah M. N. Evidence of chronic persistent infections with polyomaviruses (BK type) in renal transplant recipients. Arch Virol. 1975;47(1):39–46. doi: 10.1007/BF01315591. [DOI] [PubMed] [Google Scholar]
- Kiessling W. R., Hall W. W., Yung L. L., ter Meulen V. Measles-virus-specific immunoglobulin-M response in subacute sclerosing panencephalitis. Lancet. 1977 Feb 12;1(8007):324–327. doi: 10.1016/s0140-6736(77)91132-1. [DOI] [PubMed] [Google Scholar]
- Lecatsas G., Prozesky O. W., van Wyk J., Els H. J. Papova virus in urine after renal transplantation. Nature. 1973 Feb 2;241(5388):343–344. doi: 10.1038/241343a0. [DOI] [PubMed] [Google Scholar]
- Mäntyjärvi R. A., Meurman O. H., Vihma L., Berglund B. A human papovavirus (B.K.), biological properties and seroepidemiology. Ann Clin Res. 1973 Oct;5(5):283–287. [PubMed] [Google Scholar]
- Nagington J. Cytomegalovirus antibody production in renal transplant patients. J Hyg (Lond) 1971 Dec;69(4):645–660. doi: 10.1017/s0022172400021926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagington J. Reactivation of hepatitis b after transplantation operations. Lancet. 1977 Mar 12;1(8011):558–560. doi: 10.1016/s0140-6736(77)91995-x. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Roizman B. Infection with herpes-simplex viruses 1 and 2. II. N Engl J Med. 1973 Oct 4;289(14):719–725. doi: 10.1056/NEJM197310042891404. [DOI] [PubMed] [Google Scholar]
- Narayan O., Weiner L. P. Biological properties of two strains of Simian virus 40 isolated from patients with progressive multifocal leukoencephalopathy. Infect Immun. 1974 Jul;10(1):173–179. doi: 10.1128/iai.10.1.173-179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973 Apr;127(4):467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Eckroade R. J., Dessel B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971 Jun 19;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Pattison J. R., Mace J. E., Dane D. S. The detection and avoidance of false-positive reactions in tests for rubella-specific IgM. J Med Microbiol. 1976 Aug;9(3):355–357. doi: 10.1099/00222615-9-3-355. [DOI] [PubMed] [Google Scholar]
- Pattison J. R., Mace J. E. The detection of specific IgM antibodies following infection with rubella virus. J Clin Pathol. 1975 May;28(5):377–382. doi: 10.1136/jcp.28.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison J. R. Persistence of specific IgM after natural infection with rubella virus. Lancet. 1975 Jan 25;1(7900):185–187. doi: 10.1016/s0140-6736(75)91357-4. [DOI] [PubMed] [Google Scholar]
- Penney J. B., Jr, Narayan O. Studies of the antigenic relationships of the new human papovaviruses by electron microscopy agglutination. Infect Immun. 1973 Aug;8(2):299–300. doi: 10.1128/iai.8.2.299-300.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portolani M., Marzocchi A., Barbanti-Brodano G., La Placa M. Prevalence in Italy of antibodies to a new human papovavirus (BK virus). J Med Microbiol. 1974 Nov;7(4):543–546. doi: 10.1099/00222615-7-4-543. [DOI] [PubMed] [Google Scholar]
- Reese J. M., Reissing M., Daniel R. W., Shah K. V. Occurrence of BK virus and BK virus-specific antibodies in the urine of patients receiving chemotherapy for malignancy. Infect Immun. 1975 Jun;11(6):1375–1381. doi: 10.1128/iai.11.6.1375-1381.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds D. W., Stagno S., Stubbs K. G., Dahle A. J., Livingston M. M., Saxon S. S., Alford C. A. Inapparent congenital cytomegalovirus infection with elevated cord IgM levels. Casual relation with auditory and mental deficiency. N Engl J Med. 1974 Feb 7;290(6):291–296. doi: 10.1056/NEJM197402072900601. [DOI] [PubMed] [Google Scholar]
- Ross C. A., McDaid R. Specific IgM antibody in serum of patients with herpes zoster infections. Br Med J. 1972 Dec 2;4(5839):522–523. doi: 10.1136/bmj.4.5839.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVEHAG S. E., MANDEL B. THE FORMATION AND PROPERTIES OF POLIOVIRUS-NEUTRALIZING ANTIBODY. I. 19S AND 7S ANTIBODY FORMATION: DIFFERENCES IN KINETICS AND ANTIGEN DOSE REQUIREMENT FOR INDUCTION. J Exp Med. 1964 Jan 1;119:1–19. doi: 10.1084/jem.119.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVEHAG S. E., MANDEL B. THE FORMATION AND PROPERTIES OF POLIOVIRUS-NEUTRALIZING ANTIBODY. II. 19S AND 7S ANTIBODY FORMATION: DIFFERENCES IN ANTIGEN DOSE REQUIREMENT FOR SUSTAINED SYNTHESIS, ANAMNESIS, AND SENSITIVITY TO X-IRRADIATION. J Exp Med. 1964 Jan 1;119:21–39. doi: 10.1084/jem.119.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H., Dennis J. Characterization of antibodies produced in natural and experimental coxsackievirus infections. J Immunol. 1968 Jan;100(1):99–106. [PubMed] [Google Scholar]
- Shah K. V., Daniel R. W., Warszawski R. M. High prevalence of antibodies to BK virus, an SV40-related papovavirus, in residents of Maryland. J Infect Dis. 1973 Dec;128(6):784–787. doi: 10.1093/infdis/128.6.784. [DOI] [PubMed] [Google Scholar]
- Simmons R. L., Lopez C., Balfour H., Jr, Kalis J., Rattazzi L. C., Najarian J. S. Cytomegalovirus: Clinical virological correlations in renal transplant recipients. Ann Surg. 1974 Oct;180(4):623–634. doi: 10.1097/00000658-197410000-00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer E. S., Andersen H. K. Antibodies to the Epstein-Barr virus in kidney transplant recipients. Acta Med Scand. 1972 Jan-Feb;191(1-2):107–110. [PubMed] [Google Scholar]
- Strauch B., Andrews L. L., Siegel N., Miller G. Oropharyngeal excretion of Epstein-Barr virus by renal transplant recipients and other patients treated with immunosuppressive drugs. Lancet. 1974 Feb 16;1(7851):234–237. doi: 10.1016/s0140-6736(74)92546-x. [DOI] [PubMed] [Google Scholar]