Abstract
Continuous rotation of an arena in a cue-rich room dissociates the stationary room-bound information from the rotating arena-bound information. This disrupted spatial discharge in the majority of place cells from rats trained to collect randomly scattered food. In contrast, most place cell firing patterns recorded from rats trained to solve a navigation task on the rotating arena were preserved during the rotation. Spatial discharge was preserved in both the task-relevant stationary and the task-irrelevant rotating reference frames, but firing was more organized in the task-relevant frame. It is concluded that, (i) the effects of environmental manipulations can be understood with confidence only when the rat's purposeful behavior is used to formulate interpretations of the data, and (ii) hippocampal place cell activity is organized in multiple overlapping spatial reference frames.
Hippocampal pyramidal cells called “place cells” selectively discharge in “firing fields,” which are cell-specific parts of an environment. Soon after their discovery (1), place cell discharge was shown to be controlled by multiple cues in the environment, none of which was essential (2). The idea that “multiple replaceable stimuli” control place cell firing is consistent with the cognitive mapping theory that the cells function to represent the overall environment rather than cell-specific subsets of environmental stimuli (3). This view has received considerable support from experiments in geometrically simple recording chambers, which demonstrated that environmental changes, for example from a cylinder to a box, caused place cell firing to change in unpredictable ways even if the physical appearance of the chamber remained similar (4). This phenomenon, called “remapping” (5), indicates that a different hippocampal representation was instantiated, and within the cognitive mapping view implies that the animal understands the environment to be different. It is remarkable that this assumption has not been verified (6, 7). In fact, the evidence that place cell discharge controls spatial behavior comes from one early experiment where place cell firing was predicted by the rat's choice of a goal arm on a plus maze when the rat had to make its choice after the defining cues were removed (8).
Although many studies have confirmed that hippocampal lesions disrupt place learning, it does not follow that place cells control spatial behavior. Indeed, with special training, rats with hippocampal lesions can solve navigation tasks (9, 10), constituting proof that place cells do not necessarily control spatial behavior.
Other work has led to a “combinatorial” view that place cells do not encode the collection of stimulus relations called an environment; rather they encode cell-specific subsets of the relations amongst stimuli (11). These authors argue that the hippocampus is charged with the computations of an “episodic memory space,” that is, a neural construct within which arbitrary features of temporally contiguous experience are encoded and organized into a type of event matrix that allows the animal to organize and cross reference the recollections of the temporally discontinuous events it has experienced. Although the concept of an episodic memory space appears to us rather vague and thus difficult to address experimentally, we recognize that the existing data on hippocampal phenomena make it also difficult to accept that this structure is exclusively involved in encoding global spatial information.
The operations of the hippocampus are undoubtedly complex and governed by internal cognitive (12) and perceptual (13) features. Therefore, in our opinion, a comprehensive understanding of hippocampal function from single unit recording will be most readily achieved when the recordings are made, like in ref. 8, during behavioral conditions that allow the experimenter to understand what the rat is thinking. To understand what the rat is thinking is obviously overly ambitious, but in line with this goal one can arrange single unit recording experiments so that the organization of the rat's behavior with respect to the experimental conditions can be assessed and thus understood in terms of how both the rat's decisions and hippocampal discharge were changed by experimental alterations.
We here report the first results from ongoing place cell experiments in which the rat's purposeful behavior was such that we could use it to formulate our interpretations of place cell recordings. These experiments are motivated by a recent finding that rats trained on a stable arena to avoid footshock in a part of the arena learned to avoid a particular part of the room and at the same time acquired a separate memory for avoiding the corresponding part of the arena floor (14). This was found by first training the rat on the stable arena then during extinction dissociating the reference frames of the room and floor by continuous rotation of the arena. During the rotation, the rats avoided both the part of the room and the part of the floor.
In the present study, place cells were recorded while rats collected randomly scattered food on an arena when it was stable or rotating. On the basis of the place avoidance study, we expected firing fields established on the stable arena to be maintained during the rotation. Instead we found that most fields were disrupted (15). To directly test the assumption that stable place cell activity is necessary for navigation, we trained rats to solve a room-defined “place preference” navigation task (16) on the stable and rotating arena. In contrast, most place cell activity in these animals was preserved during the rotation. Thus the hippocampus encodes environmental information in a way that depends not only on the animal's overt behavior but also on what problem the animal is solving. In addition, firing fields in the place preference-trained animals were preserved not only in the task-relevant stationary reference frame of the room but also in the task-irrelevant reference frame of the rotating arena. Thus the hippocampus encodes what the rat needs to know as well as information that is potentially useful but unnecessary. We argue that recognizing the coexistence of several reference frames and applying the concept of a reference frame to place cell studies may provide a framework within which to resolve the appar- ently contradictory facts about place cells and hippocampal encodings.
Methods
Subjects.
Male Long Evans rats from the Institute breeding colony weighed 300–350 g and were housed at 22°C under natural lighting conditions. They had free access to water and were food deprived to about 85% of their free feeding weight. Their treatment complied with both Czech and United States guidelines.
Apparatus.
Experiments were conducted in a cylindrical arena with black featureless walls (80 cm diameter, 40 cm high). The arena was in an evenly lit 4 m × 6 m recording room with many polarizing cues.
Position Tracking.
The rat's position was recorded by using an overhead television camera connected to a hardware tracker in a computer. Every 100 ms, the position (0.7 cm spatial resolution) of an infrared light-emitting diode (LED) on the recording headstage was recorded along with the position of a second LED on the outside of the arena. The second LED was used to calculate the position of the rat in the reference frame of the rotating arena. The computer controlled both the arena rotation and the release of food from a feeder that was 2 m above the arena.
Behavioral Training.
Foragers.
All rats were trained for a week in a food-retrieval task (17). Every 10 s the feeder dropped a 20-mg pasta morsel to an undetermined location in the arena. After 3–7 days, the rats foraged continuously, searching for pellets as they fell. The arena was stable during this training. The rats that had only this experience were called “foragers.”
Navigators.
Rats in the “navigator” group were trained in the place-preference task (16), which preserves the undirected foraging behavior while also requiring the rat to make target-directed movements to an unmarked goal. Food was dispensed only when the rat entered a circular unmarked target 20 cm in diameter. The preoperative training lasted 7–10 days, for which the target was in the same quadrant of the arena. The food fell to a random location requiring the rat to search the arena. The rat had to stay outside the target for at least 3 sec before the next visit was rewarded. Eventually the rats released about 90 pellets in a 30-min session. Thus before surgery, for over 2 wk, the navigators were trained only on the stable arena. After surgery, the target could be located in any of the quadrants of the arena, and the rats learned to find this place by exploring at the start of a session.
Electrophysiology.
After initial training, under thiopental anesthesia (50 mg/kg), a driveable bundle of 8 formvar-insulated 25-μm nichrome electrodes was implanted above CA1, 3.5 mm posterior, and 2–2.5 mm lateral to Bregma (18). After recordings were completed, the final position of the electrode array was marked by passing anodal current (18 μA for 15 s) through one of the wires. Under deep anesthesia, the animals were perfused transcardially with saline followed by 10% formalin. Their brains were removed, marked by the Prussian blue reaction, sectioned at 30-μm intervals, then stained with cresyl violet to locate the electrode tracks. The electrodes passed through CA1 in each rat.
At least 3 days after surgery, the rats were placed in the stable arena where they retrieved pellets for 15 min each day for 2–5 days. On subsequent days, the rats either retrieved pellets or performed the place-preference task during rotation while the experimenter checked the electrodes for place cell activity.
Recordings were made in 3 consecutive 10-min sessions without manipulating the rat between the sessions. First the arena was stable for 10 min, then it rotated (1 rpm) for 10 min, then it was stable again for 10 min. The rat was either doing undirected foraging or place preference navigation for the full 30 min. Useful unit recordings from the navigators were made only after their place preference performance during rotation was asymptotic.
An integrated circuit headstage amplifier (gain 10) was attached to the electrode connector, and the signals were carried to a ceiling-mounted commutator by a weight counterbalanced cable. After further amplification (total gain 5,000) and filtering (300 Hz − 5 kHz), the signals were digitized (32 kHz, 12 bits), and 1 ms action potential waveforms were time stamped and stored by using a Brainwave Discovery system (Boulder, Co).
The electrodes were advanced in 50-μm steps (typically 100 μm per day) until unitary action potentials from place cells were detected. If an isolated cell did not emit a few complex spikes and did not have a firing field, then it was not analyzed.
The digitized waveforms were categorized into unitary waveform classes by using a two-step template-matching algorithm. The first step selects only spikes from discharge bursts to identify a set of representative waveforms for each unit. The mean waveform for each unit is calculated along with the mean and variance of the deviations of the individual waveforms from this mean. These are then used in the second step to calculate the standard normal deviate that measures how much a waveform deviates from the unit represented by each template. The discrimination on the basis of this algorithm was supplemented by cluster analysis by using additional parameters from the spike waveforms.
Spatial Firing Analysis.
The arena surface was divided into 5 cm × 5 cm pixels, and a spatial firing rate distribution was calculated for each cell. The mean firing rate in each pixel was calculated as the total number of spikes observed in the pixel divided by the total time the rat was in the pixel. When the arena was rotated, separate firing rate distributions were calculated for two separate reference frames. From the point of view of the experimenter, there was a “stationary” reference frame defined by the room and a “rotating” reference frame defined by the arena surface. Note that all analyses were performed on the entire firing rate distribution rather than on an arbitrarily defined notion of a firing field. Autoscaled color-coded firing rate maps were created to visualize firing rate distributions (17). White represents undefined firing rate in places the rat did not visit. Yellow is for pixels that were visited but no spikes occurred. The other colors in ascending order, orange, red, green, blue, and purple, represent active pixels such that each category contains 0.8 of the number of pixels in the next lower category. The median value for each category is given. Because a firing rate pattern recorded by a single electrode may appear to change if the electrode moves and another unit with similar waveform appears, we took the conservative measure of considering only those cells with firing rate patterns that returned after the rotation.
Whether a cell had location-specific discharge was determined by calculating the spatial information (19) signaled by each spike as
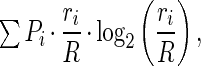 |
where R is the session average firing rate, ri is the rate in pixel i, and Pi is the probability that the rat was detected in the pixel. A Monte Carlo method was used to determine whether the observed spatial information could be expected by chance. The spike and position time series were offset by a random number of 100-ms units, and the firing rate distribution and the spatial information were recalculated. This was done 100 times to estimate the distribution of spatial information that could be expected by chance given a particular spike time series. The mean and variance was used to calculate a standard score (zinfo) measuring the probability that the observed spatial information occurred by chance. Only firing rate distributions with zinfo > 1.65 (less than 5% likely to come from the chance distribution) were considered to be location specific.
Patchiness measures the global smoothness of a firing rate pattern (20). It is calculated as the number of contiguous regions (patches) in the highest firing rate category (purple) of the firing rate map. Spatial coherence measures the local smoothness of a firing rate pattern (20) and is calculated as the z-transform of the correlation between the rate in a pixel and the mean rate in the immediately neighboring pixels.
The similarity between two firing rate distributions was calculated by superimposing the two patterns and calculating the pixel-to-pixel correlation (rsim) (21). t-tests between groups were performed on the z-transformed correlation (zsim).
Results
Effect of Continuous Rotation on Place Cells from Rats Trained Only in Undirected Foraging.
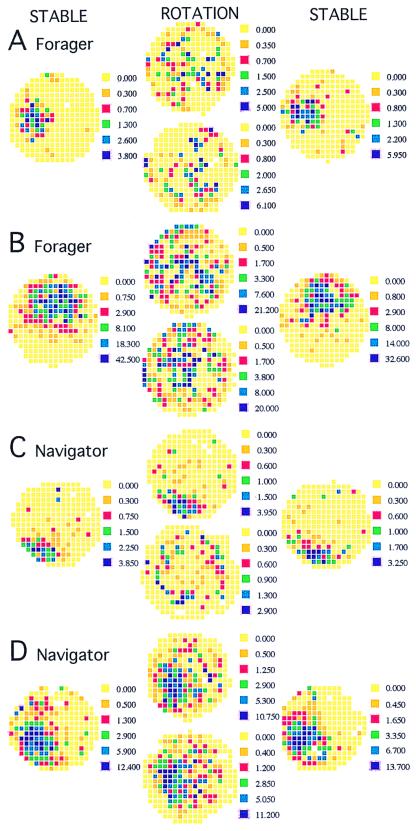
The effect of rotation was studied in 14 place cells from 2 foragers. Rotating the arena typically dispersed both the stationary and rotating firing rate patterns (Fig. 1 A and B). Overall, the rotation degraded spatial firing patterns, and there was a trend for firing rates to increase (Table 1). The proportion of the arena in which the cells fired and the patchiness increased. The coherence and the spatial information decreased. These changes were equal for discharge in the stationary and rotating reference frames.
Figure 1.
The different effects of rotation are demonstrated in these examples from foragers (A and B) and navigators (C and D). Recordings on the stable arena before and after rotation are in columns 1 and 3, respectively. Recordings during rotation are in column 2. The stationary frame map is above the rotating frame map. Rotation characteristically disrupted firing patterns in the foragers. (A) The cell with a field at 9:00 on the stable arena continued to discharge during the rotation, but the activity was dispersed when viewed from both the stationary (rsim = 0.12) and rotating (rsim = −0.09) reference frames. When the rotation was stopped after 10 revolutions, the field was again prominent at 9:00 (rsim = 0.79). Qualitatively, rotation had the same disruptive effect on the cell in B; however, according to the numerical criterion, the rotating pattern had a marginally significant resemblance to the stable (rsim = 0.26) but not the stationary pattern (rsim = 0.17). When the rotation stopped, the pattern returned (rsim = 0.80). Place cell discharge from the navigators was more often preserved during rotation. (C). In this example, only stationary frame firing was preserved. The stable field at 7:00 was maintained in the stationary frame (rsim = 0.76), but not in the rotating frame (rsim = 0.14) and persisted when the rotation stopped (rsim = 0.70). (D) In this example, the field at 7:30 was stable in both the stationary (rsim = 0.62) and rotating (rsim = 0.72) reference frames, as well as after the rotation stopped (rsim = 0.71). The field in the rotating reference frame has shifted away from the arena wall. The only way that the same spikes could produce stable firing fields in both the stationary and rotating frames is that the cell mostly fired after each full revolution, when the two frames were in register.
Table 1.
Discharge properties of place cells from the foragers when the arena was stable and in the stationary and rotating frames during rotation (mean ± SEM)
| Stable | Stationary (paired t-test vs. stable) | Rotating (paired t-test vs. stable, paired t-test vs. stationary) | |
|---|---|---|---|
| Mean rate (AP/s) | 1.82 ± 0.33 | 3.21 ± 0.71 (t13 = 2.08, P = 0.06) | 3.21 ± 0.71 (t13 = 2.08, P = 0.06) |
| (t13 = 0, P = 1.0) | |||
| Proportion of arena | 0.52 ± 0.08 | 0.67 ± 0.06 (t13 = 2.76, P = 0.02) | 0.65 ± 0.07 (t13 = 2.30, P = 0.04) |
| (t13 = 1.67, P = 0.12) | |||
| Patchiness | 3.7 ± 0.89 | 10.2 ± 1.24 (t13 = 4.95, P = 3 × 10−4) | 9.2 ± 1.32 (t13 = 4.64, P = 5 × 10−4) |
| (t13 = 1.36, P = 0.2) | |||
| Coherence | 0.78 ± 0.15 | 0.39 ± 0.08 (t13 = 2.91, P = 0.01) | 0.33 ± 0.08 (t13 = 4.46, P = 6 × 10−4) |
| (t13 = 0.93, P = 0.4) | |||
| Information (bits/AP) | 2.10 ± 0.39 | 1.36 ± 0.22 (t13 = 2.26, P = 0.04) | 1.35 ± 0.20 (t13 = 2.24, P = 0.04) |
| (t13 = 0.16, P = 0.88) |
Comparison of place cells during undirected foraging and navigation.
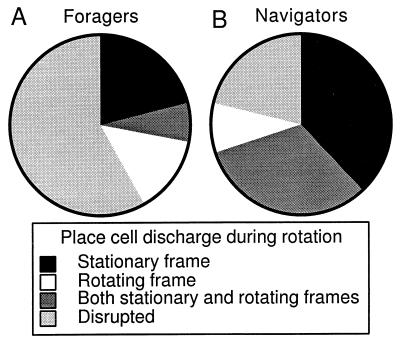
The disruptive effect of rotation was quantified by using rsim to judge whether the spatial firing rate patterns during rotation were related to the patterns on the stable arena. First, the correlation of the firing rate patterns was calculated between pairs of stable sessions from 65 units in 7 rats trained in either or both undirected foraging and navigation. Assuming that this distribution represents the similarity that can be expected between a pair of stable recordings (mean = 0.53; SD = 0.21), we took correlations that would fall in the lower 5% of this distribution (<1.65 SD below the mean; i.e., < 0.19) to be from units with a disrupted firing pattern. This criterion tends to accept firing patterns as similar unless they are really very different. On this basis, the effects of rotation were classified into four categories (Fig. 2A): (i) Stationary frame preserved in which only the stationary firing pattern resembled the stable pattern (3/14; stationary rsim = 0.48 ± 0.25; rotating rsim = 0.06 ± 0.06); (ii) rotating frame preserved in which only the rotating pattern resembled the stable pattern (2/14; stationary rsim= 0.17 ± 0.01; rotating rsim= 0.29 ± 0.03); (iii) stationary and rotating frame preserved in which both the stationary and rotating patterns resembled the stable pattern (1/14; stationary rsim= 0.52; rotating rsim= 0.48); and (iv) disrupted, in which neither the stationary nor rotating patterns resembled the stable pattern (8/14; stationary rsim= 0.07 ± 0.03; rotating rsim= 0.05 ± 0.04). Note that the cells classified to be stable in the rotating frame had low rsim values that were just above the threshold of significance. In fact, by inspection it was difficult to see a similarity between the rotating and stable patterns (Fig. 1B).
Figure 2.
During rotation, place cell discharge in the rats trained only to forage for scattered food was different than the discharge from cells in rats trained to navigate. Firing rate patterns during rotation were judged to be similar to the stable pattern if rsim > 0.19. If rsim ≤ 0.19, they were judged to be disrupted. Firing rate patterns were classified as either preserved in the stationary, rotating, or both reference frames. The majority of firing rate patterns was disrupted in the forager group (A), whereas the majority was preserved in the navigator group (B). The place cells in the navigator group were more likely to be preserved in the stationary frame that defined the goal.
Comparison of Place Cells During Undirected Foraging and Navigation.
We first looked for a difference between firing fields in the navigators recorded during undirected foraging and place preference navigation. Thirty-nine cells in four rats with navigator training were recorded in separate undirected foraging and navigation sessions on the stable arena. To test for changes caused by doing the navigation task, the similarity of discharge patterns during 32 pairs of undirected foraging sessions (UF-UF) were compared with the similarity of the 39 firing rate patterns during an undirected foraging session and a place preference session (UF-PP). The mean similarity (zsim ± SEM) for the UF-UF pairs was 0.14 ± 0.027; it was 0.16 ± 0.02 for UF-PP pairs, which is no different (t70 = 0.72; P = 0.48). Neither could we find a difference in measures of field size, firing rate, coherence, or spatial information. Thus for analysis of the effects of rotation in the navigators, the recordings during undirected foraging and navigation were pooled.
Effect of Continuous Rotation on Place Cells from Rats Trained to Navigate.
If place cells encode the environment and this encoding is used to navigate, then the rats that can do stationary frame-place preference navigation during rotation should have better preserved firing patterns in the stationary reference frame compared with cells from rats trained only for undirected foraging. Of 32 place cells from 2 rats trained to navigate, a minority had firing fields that were dissipated by rotation. Some fields were preserved in the stationary reference frame that defined the place-preference goal (Fig. 1C), some fields were preserved in the rotating reference frame, and some fields were preserved in both frames (Fig. 1D). The overall effects of rotation are summarized in Table 2. Rotation did not change firing rates, but it increased both the proportion of the arena where spikes were emitted and the patchiness; these increases were more pronounced in the rotating frame. The spatial information decreased by about 25% in both the stationary and rotating frames, whereas in the rotating frame alone, the coherence decreased by 55%. These changes were generally milder than the changes induced by rotation in the foragers. No parameter was different between the two groups when the arena was stable, but during rotation, firing rates in the foragers were higher (t45 = 2.92; P = 0.01) as was the proportion of active pixels in both the stationary (t45 = 2.33; P = 0.02) and rotating frames (t45 = 2.21; P = 0.03). Patchiness was higher in the foragers but only in the stationary frame (t45 = 3.31; P = 0.002). Stationary frame coherence was lower in the foragers (t45 = 3.82; P = 4 × 10−4).
Table 2.
Discharge properties of place cells from navigation-trained rats when the arena was stable and in the stationary and rotating frames during rotation (mean ± SEM)
| Stable | Stationary (paired t-test vs. stable) | Rotating (paired t-test vs. stable, paired t-test vs. stationary) | |
|---|---|---|---|
| Mean rate (AP/s) | 1.23 ± 0.23 | 1.28 ± 0.21 (t31 = 1.28, P = 0.21) | 1.28 ± 0.21 (t31 = 1.28, P = 0.21) |
| (t31 = 0, P = 1.0) | |||
| Proportion of arena | 0.46 ± 0.04 | 0.53 ± 0.03 (t31 = 3.2, P = 0.003) | 0.51 ± 0.03 (t31 = 1.97, P = 0.06) |
| (t31 = 2.76, P = 0.01) | |||
| Patchiness | 3.7 ± 0.53 | 5.9 ± 0.69 (t31 = 3.8, P = 6 × 10−4) | 7.6 ± 0.73 (t31 = 4.84, P = 3 × 10−5) |
| (t31 = 2.24, P = 0.03) | |||
| Coherence | 0.85 ± 0.10 | 0.93 ± 0.09 (t31 = 0.78, P = 0.43) | 0.38 ± 0.04 (t31 = 3.98, P = 4 × 10−4) |
| (t31 = 13.6, P = 2 × 10−7) | |||
| Information (bits/AP) | 1.82 ± 0.16 | 1.35 ± 0.10 (t31 = 4.09, P = 3 × 10−4) | 1.39 ± 0.11 (t31 = 3.96, P = 4 × 10−4) |
| (t31 = 0.98, P = 0.34) |
The effect of rotation on place cells of the navigators was classified into four categories according to the criterion used for the foragers (Fig. 2B): (i) Stationary frame preserved (12/32; stationary rsim = 0.38 ± 0.05; rotating rsim= 0.04 ± 0.03); (ii) Rotating frame preserved (3/32; stationary rsim = 0.02 ± 0.11; rotating rsim = 0.37 ± 0.06); (iii) stationary and rotating frame preserved (10/32; stationary rsim = 0.38 ± 0.08; rotating rsim = 0.40 ± 0.04); and (iv) disrupted (7/32; stationary rsim = 0.09 ± 0.03; rotating rsim = 0.01 ± 0.03). Thus the effect of rotation on the foragers and navigators was different (X32 = 38.3; P = 4 × 10−8). The majority of firing patterns from the foragers was disrupted (58%), and in contrast the majority of firing rate patterns from the navigators was preserved (78%). In addition, in the navigators there were more task-relevant (stationary) than task-irrelevant (rotating) frame firing patterns preserved than expected on the assumption that they were equally likely to be preserved (X12 = 9.8; P = 0.02).
Comparison of Activity in the Task-Relevant and Task-Irrelevant Reference Frames.
Discharge in the task-relevant and task-irrelevant frames was directly compared in the 10 cells from the navigation-trained rats, with discharge patterns that were preserved in both the stationary and rotating frames. The proportion of the stationary frame area in which spikes were emitted (0.60 ± 0.09) was the same as in the rotating frame (0.59 ± 0.09; t9 = 1.68; P = 0.13). Neither did the stationary frame spatial information (1.34 ± 0.31) differ from the rotating frame information (1.28 ± 0.31; t9 = 0.89; P = 0.4). In contrast, stationary frame discharge was more smooth, both globally (patchiness: 3.7 ± 1.23) and locally (coherence: 0.89 ± 0.21), compared with the rotating frame discharge (patchiness: 7.1 ± 1.58; t9 = 2.68; P = 0.03; coherence: 0.36 ± 0.09; t9 = 2.44; P = 0.04). These differences, however, did not cause there to be a difference in the overall similarity of the discharge patterns to those in the stable arena (stationary r = 0.46 ± 0.07; rotating r = 0.40 ± 0.05; t9 = 0.71; P = 0.50).
Discussion
Although place cell discharge remaps in different environments (4, 22) and when the rat performs a different task in the same space (6), the effect of rotation on the foragers did not resemble a remapping. Only the spatial organization of the action potentials was disrupted and all the cells continued to discharge. Firing rates even increased by over 70%.
The present results indicate two rather different answers to the question: how does dissociating information from distal and local sources affect place cell discharge? This question was recently addressed by the “double-rotation” experiments of Eichenbaum and colleagues (23, 24). They concluded that place cell activity is controlled by arbitrary cell-specific subsets of cues. On the basis of the data from the forager group, they would presumably come to the same conclusion, because some firing patterns were preserved in the stationary frame, some in the rotating frame, others in both, and most were disturbed. However, the changes in firing rate properties were the same for cells with disrupted or preserved spatial firing. Thus, in contradiction to the combinatorial view, the dissociation of distal and local information by continuous rotation disturbs the spatial discharge of nearly all place cells, indicating that in support of the multiple replaceable cue view, place cells were controlled by information from both the distal (stationary) and local (rotating) sets of cues. It is likely that the dissociation of distal and local information itself was disrupting, because in the foragers, place cell firing in the rotating frame was preserved when the rotation occurred in darkness (15).
The data from the navigation-trained animals support a different interpretation. Regardless of whether these rats were doing the navigation task or collecting randomly scattered food, the majority of their place cells continued to fire predictably during rotation. Some cells' discharge remained fixed to the stationary frame of reference, and other cells' discharge remained fixed to the rotating frame of reference. This contradicts the multiple replaceable cue view but is not surprising within the combinatorial view. Some cells are driven by the subset of cues in the stationary frame, some by those in the rotating frame, others by cues in both frames. This latter set of cells can have two subclasses. In one, the cells require inputs from either reference frame, and thus the firing of these cells will appear scattered across both frames. Our short-duration recordings of single cells are not sufficient to determine whether the dissipated cells in either the foragers or navigators match this description. The other subclass of cell requires the conjunction of inputs from both reference frames. These are the 31% of cells in the navigators that had preserved fields in both the stationary and rotating frames. These cells were most likely to discharge when the initial correspondence between the stationary and rotating frames was restored with each full revolution. This appears as a 1-min periodicity in the spike autocorrellogram (not shown). This class of cell indicates that at least a subpopulation of the hippocampus operates in two reference frames at once (but see ref. 25), signaling that the stationary and rotating coordinate systems are in register.
The results show that how environmental stimuli control firing fields depends on what problem the animal has been trained to solve in the environment. We stress that the difference between place cells from the foragers and navigators is that the latter learned to solve a navigation problem in the environment. The two groups had approximately the same amount of exposure to rotation, and there was no effect of time order on the behavior of the cells.
The place cell literature also supports apparently incompatible views of what these cells signal (compare ref. 26 with ref. 27). In support of cognitive mapping, complex-spike cells are controlled by multiple replaceable stimuli and seem to encode the geometric features of the environment (refs. 28–31; reviewed by ref. 32). Knowing the discharge of many cells integrated over a second or so may allow one to predict the rat's position (refs. 33 and 34, but see ref. 35). On the other hand, these same cells arbitrarily change their firing in the same physical environment when the rat's task or pattern of movement changes (6) and when the rat is constrained from moving (36). Some of these cells remap in one half of a environment that is visually identical to the other half, whereas simultaneously, other cells have symmetric firing fields in the two visually identical halves (7). This “partial” remapping contradicts any simple model that the hippocampus as a whole acts as a neural entity encoding the map of the current environment (37). Finally, the discharge of these cells seems to be organized into reference frames anchored to distal and local environmental features such that in simultaneous recordings of many cells, some will discharge in places defined by the reference frame of the experimental room, others in the reference frame of a local landmark that indicates a goal, still others in the frame determined by entry to a box, others to the exit from the box, and so on (25).
The concept of a reference frame may have general utility for understanding the results of place cell experiments. Rats clearly understand where they are within coordinate systems defined by different sets or classes of information (14). Without recognizing the existence of separate spatial reference frames and testing whether changes in place cell activity correspond to shifts in the use of a particular reference frame, many place cell results may remain baffling.
The notion that place cells encode a holistic cognitive map may be compatible with the idea that place cells encode arbitrary subsets of stimulus relations if these ideas are put in the context of there being multiple reference frames, an old idea (3) but really only ever applied by ref. 25. A reference frame is a global coordinate system used for defining a space. This is the essence of any map. A reference frame is also defined only by subsets of information specific to the frame. Thus we argue that when place cell firing segregates into discordant subpopulations (7, 24, 25), it might be understood in terms of there being some limited number of subpopulations of cells, each subgroup signaling position in a particular fixed coordinate frame. Because the hippocampus itself seems to be involved in most spatial computations, one would expect that either specific intrahippocampal circuits and/or specific parts of the rat navigation system are specialized for processing information in idiothetic, local (substratal) cue-, or distal cue-defined reference frames, and that there is a mechanism for indicating when they are in or out of register.
The relationship of place cells to a goal can be understood easily from the perspective of spatial reference frames. In the current work, changing the goal location did not alter place cell discharge. This has already been demonstrated (38) but contrasts with ref. 25, where a subset of cells discharged in relation to a moving landmark/goal constellation. We interpret this to be that the cells were principally bound to the spatial reference frame set up by the landmarks and only incidentally associated with the goal. In the current work, the room-defined goal could be different at the start of a 30-min recording session. Because place cell discharge was unaffected by changing the goal (data not shown), it seems that a goal itself does not establish a reference frame within which place cells discharge. This conclusion is confirmed in a new study correlating place cell discharge and goal choice in a Y-maze alternation task (39). Rather than goals and other abstract environmental features, we expect that the physical attributes of a space, like walls, surfaces, and prominent landmarks, establish reference frames. Testing whether particular kinds of information, like local objects, or distal visual cues are “naturally” coordinated into one reference frame but not another would be important for determining whether this view is useful.
Although in the navigation-trained rats the spatial discharge in the task-irrelevant (rotating) frame was less preserved and less organized, it is not clear whether this was because the rotating frame was irrelevant to the navigation task or, alternatively, the spatial activity within this frame is inherently less organized because it depends on idiothetic and local cues, which may not supply spatial information as precise as distal landmarks. Arguably, the rotating frame may have been important for finding the food once its release was triggered. The rat may have used idiothesis and local landmarks in the rotating frame to chart the places it had searched. Nonetheless, it is interesting that organized hippocampal activity in both knowable frames of reference is the sort of thing that would be expected of a system that encodes experience in general, irrespective of its current relevance.
The experimental approach to understanding place cell activity we advocate is one that asks the rat to organize its behavior according to definable coordinate systems. Our initial work has raised several questions, and we suggest that navigation on a rotating arena provides a useful paradigm to answer them, especially in combination with ensemble recording. One will be able to learn whether different hippocampal representations are simultaneously active. Cells with firing fields preserved in the stationary frame will periodically overlap with cells with fields preserved in the rotating frame, and with sufficiently long sampling and sufficiently many cells recorded simultaneously, it will be possible to determine whether cells discharge simultaneously if they are affiliated with separate reference frames. One will be able to understand whether head direction cell (40) activity is organized in separate reference frames. One will be able to learn whether and how cells that discharge in separate reference frames influence each other if their two firing fields are made to overlap for long periods of time.
Acknowledgments
We are grateful to Bruno Poucet and Robert Muller for their animated and valuable commentary. This work was supported by Granting Agency of the Czech Republic Grant 309/97/0555 and McDonnell Foundation Grant 98–38 CNS-QUA.05.
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050576397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050576397
References
- 1.O'Keefe J, Dostrovsky J. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 2.O'Keefe J, Conway D H. Exp Brain Res. 1978;31:573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- 3.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon; 1978. [Google Scholar]
- 4.Muller R U, Kubie J L. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bostock E, Muller R U, Kubie J L. Hippocampus. 1991;1:193–206. doi: 10.1002/hipo.450010207. [DOI] [PubMed] [Google Scholar]
- 6.Markus E J, Qin Y, Leonard B, Skaggs W E, McNaughton B L, Barnes C A. J Neurosci. 1995;15:7079–7094. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skaggs W E, McNaughton B L. J Neurosci. 1998;18:8455–8466. doi: 10.1523/JNEUROSCI.18-20-08455.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Keefe J, Speakman A. Exp Br Res. 1987;68:1–27. doi: 10.1007/BF00255230. [DOI] [PubMed] [Google Scholar]
- 9.Eichenbaum H, Stewart C, Morris R G M. J Neurosci. 1990;10:3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whishaw I Q, Cassel J C, Jarrard L E. J Neurosci. 1995;15:5779–5788. doi: 10.1523/JNEUROSCI.15-08-05779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 12.Quirk G J, Muller R U, Kubie J L. J Neurosci. 1990;10:2008–2017. doi: 10.1523/JNEUROSCI.10-06-02008.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rotenberg A, Muller R U. Philos Trans R Soc London B. 1997;352:1505–1513. doi: 10.1098/rstb.1997.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenton A A, Wesierska M, Kaminsky Yu, Bures J. Proc Natl Acad Sci USA. 1998;95:11493–11498. doi: 10.1073/pnas.95.19.11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bures J, Fenton A A, Kaminsky Yu, Rossier J, Sacchetti B, Zinyuk L. Philos Trans R Soc London B. 1997;352:1515–1524. doi: 10.1098/rstb.1997.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossier, J., Kaminsky, Yu., Schenk, F. & Bures, J. (2000) Behav. Neurosci., in press. [PubMed]
- 17.Muller R U, Kubie J L, Ranck J B., Jr J Neurosci. 1987;7:1935–1950. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paxinos G, Watson M. The Rat Brain in Stereotaxic Coordinates. New York: Academic; 1986. [Google Scholar]
- 19.Skaggs W E, McNaughton B L, Gothard K M, Markus E J. In: Advances in Neural Information Processing. Hanson S J, Cowan J D, Giles C L, editors. Vol. 5. San Mateo, CA: Morgan Kaufmann; 1993. pp. 1030–1037. [Google Scholar]
- 20.Muller R U, Kubie J L. J Neurosci. 1989;9:4101–4110. doi: 10.1523/JNEUROSCI.09-12-04101.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharp P, Muller R U, Kubie J L. J Neurosci. 1990;10:3093–3105. doi: 10.1523/JNEUROSCI.10-09-03093.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson L T, Best P J. J Neurosci. 1989;9:2382–2390. doi: 10.1523/JNEUROSCI.09-07-02382.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shapiro M L, Tanila H, Eichenbaum H. Hippocampus. 1997;7:624–642. doi: 10.1002/(SICI)1098-1063(1997)7:6<624::AID-HIPO5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 24.Tanila H, Shapiro M L, Eichenbaum H. Hippocampus. 1997;7:613–623. doi: 10.1002/(SICI)1098-1063(1997)7:6<613::AID-HIPO4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 25.Gothard K M, Skaggs W E, Moore K M, McNaughton B L. J Neurosci. 1996;16:823–835. doi: 10.1523/JNEUROSCI.16-02-00823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Keefe J. Hippocampus. 1999;9:352–364. doi: 10.1002/(SICI)1098-1063(1999)9:4<352::AID-HIPO3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro M L, Eichenbaum H. Hippocampus. 1999;9:365–384. doi: 10.1002/(SICI)1098-1063(1999)9:4<365::AID-HIPO4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 28.O'Keefe J, Burgess N. Nature (London) 1996;381:425–428. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- 29.Cressant A, Muller R U, Poucet B. J Neurosci. 1997;17:2531–2542. doi: 10.1523/JNEUROSCI.17-07-02531.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fenton A A. Ph.D. thesis. Brooklyn: State University of New York; 1998. [Google Scholar]
- 31.Fenton, A. A. & Muller, R. U. (2000) J. Gen. Physiol., in press.
- 32.Muller R U, Poucet B, Fenton A A, Cressant A. Hippocampus. 1999;9:413–422. doi: 10.1002/(SICI)1098-1063(1999)9:4<413::AID-HIPO7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Wilson M A, McNaughton B L. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 34.Brown E N, Frank L M, Tang D, Quirk M C, Wilson M A. J Neurosci. 1998;18:7411–7425. doi: 10.1523/JNEUROSCI.18-18-07411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fenton A A, Muller R U. Proc Natl Acad Sci USA. 1998;9:3182–3187. doi: 10.1073/pnas.95.6.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foster T C, Castro C A, McNaughton B L. Science. 1989;244:1580–1582. doi: 10.1126/science.2740902. [DOI] [PubMed] [Google Scholar]
- 37.Samsonovich A, McNaughton B L. J Neurosci. 1997;17:5900–5920. doi: 10.1523/JNEUROSCI.17-15-05900.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Speakman A, O'Keefe J. Eur J Neurosci. 1990;2:544–555. doi: 10.1111/j.1460-9568.1990.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 39.Lenck-Santini, P. P., Save, E. & Poucet, B. (2000) J. Neurosci., in press. [DOI] [PubMed]
- 40.Taube J S, Goodridge J P, Golob E J, Dudchenko P A, Stackman R W. Brain Res Bull. 1996;40:477–486. doi: 10.1016/0361-9230(96)00145-1. [DOI] [PubMed] [Google Scholar]