Abstract
Taxol (paclitaxel), a complex diterpene obtained from the Pacific yew, Taxus brevifolia, is arguably the most important new drug in cancer chemotherapy. The mechanism of cytotoxic action for paclitaxel—i.e., the stabilization of microtubules leading to mitotic arrest—is now shared by four recently identified natural products, eleutherobin, epothilones A and B, and discodermolide. Their ability to competitively inhibit [3H]paclitaxel binding to microtubules strongly suggests the existence of a common binding site. Recently, we have developed nonaromatic analogues of paclitaxel that maintain high cytotoxicity and tubulin binding (e.g., nonataxel). We now propose a common pharmacophore that unites paclitaxel, nonataxel, the epothilones, eleutherobin, and discodermolide, and rationalizes the extensive structure–activity relationship data pertinent to these compounds. Insights from the common pharmacophore have enabled the development of a hybrid construct with demonstrated cytotoxic and tubulin-binding activity.
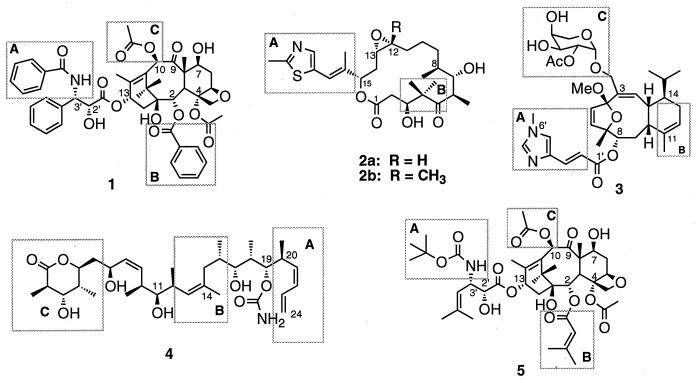
Taxol (paclitaxel, 1; see Fig. 1) is a powerful resource in cancer chemotherapy (1–7). It was approved by the Food and Drug Administration in the United States for the treatment of advanced ovarian cancer (1992) and metastatic breast cancer (1994). Clinical studies on the treatment of other cancers with paclitaxel and closely related synthetic analogues, as well as combination protocols, are being pursued (1–7).
Figure 1.
Structures of paclitaxel (1), epothilones A and B (2a and 2b), eleutherobin (3), discodermolide (4), and nonataxel (5). Labeled boxed regions are areas of common overlap as represented in Fig. 4.
Recently, four natural products, structurally dissimilar to paclitaxel, have been found to share its mechanism of action (8, 9). Epothilones A (2a: R = H) and B (2b: R = Me) (10, 11), eleutherobin (3) (12), and discodermolide (4) (13) (Fig. 1) show activities comparable to those of paclitaxel in various assays (11–15). These compounds competitively inhibit [3H]paclitaxel binding to microtubules (11, 16, 17), strongly suggesting the existence of a common binding site.¶ Clearly, the identification of a three-dimensional pharmacophore common to these structurally diverse agents could guide the selection of the next generation of paclitaxel-like cytotoxic agents. Herein we propose a common pharmacophore for these agents based on their structural correlation to the NMR-defined conformation of the nonaromatic paclitaxel mimic nonataxel (5).‖ The pharmacophore succeeds in explaining the substantial structure–activity relationships (SAR) of these agents and is corroborated by the synthesis of a hybrid construct with demonstrated cytotoxic and tubulin-binding ability.
MATERIALS AND METHODS
Conformational Analysis of Nonataxel and Molecular Modeling.
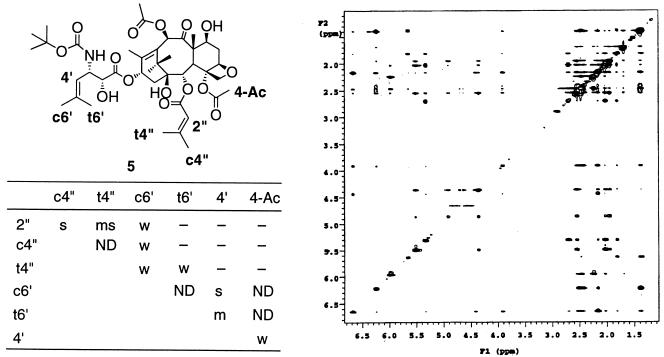
Nonataxel (5) was subjected to conformational analysis using distance constraints based on the cross-peak intensity obtained from the two-dimensional nuclear Overhauser enhancement (NOE) spectroscopy experiment at 4°C in DMSO-d6/D2O (3:1; D2O = 2H2O) with solvent peak suppression (Fig. 2), carried out on a Varian INOVA 500-MHz spectrometer. Simulated annealing of several starting structures of 5 (10 cycles each of the following sequence per conformer: equilibration at 1000 K for 2 ps, followed by exponential cooling from 1000 K to 300 K over 10 ps) including the key NOE-based constraints for the C-2 and C-3′ side chains was carried out (sybyl 6.4), and the lowest-energy conformer consistent with these NOEs was chosen as the template conformation for the superimposition study.
Figure 2.
(Right) Two-dimensional NOE spectrum in DMSO/D2O (3:1) for nonataxel (5) at 4°C (500-MHz Varian INOVA.) In table on Left, NOE intensities and corresponding distance constraints: s = strong (1.8–2.5 Å); ms = medium-strong (1.8–3.0 Å); m = medium (1.8–3.5 Å); mw = medium-weak (1.8–4.0 Å); w = weak (1.8–5.0 Å); —- = no observed NOE; ND = not quantifiable.
Several low-energy conformations of epothilone B (2b), eleutherobin (3), and discodermolide (4) obtained from the high-temperature molecular dynamics study (sybyl 6.4, Tripos force field, charges calculated by the Gasteiger–Hückel method) were compared with the template conformation of 5. It should be noted that the purpose of this study was not to obtain a statistical distribution of the various possible overlays, but to arrive at the best fit that generally satisfied the vast existing SAR and structural data for these compounds.** Numerous overlay modes were generated, starting from these conformers, that were optimized with template forcing studies [discover 95.0, consistent valence force field (CVFF)].‡‡ To refine the structures resulting from the template forcing procedure, the models were subjected to restrained and unrestrained molecular dynamics (sybyl 6.4, Tripos force field using Gasteiger–Hückel charges), typically for 100 ps for each conformer at 1000 K with sampling at every 1 ps, followed by dynamics at 300 K for 5 ps for each sampled conformer. Each of these conformers was then minimized and the resultant low-energy conformers within the range of 10 kcal/mol were reevaluated for overlay with the template nonataxel conformation. Similar analysis was performed for the overlay mode proposed by Winkler and Axelsen (18) for paclitaxel and epothilone B (see below).
Synthesis of Hybrid Construct 6.
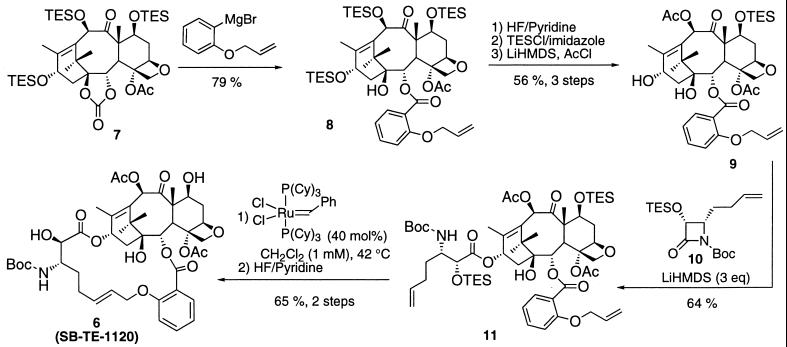
Fig. 3 outlines the synthesis of hybrid construct 6 starting from 7,10,13-tri-TES-1,2-carbonate baccatin 7. Nucleophilic ring-opening of the carbonate moiety of 7 by using the Grignard reagent from 2-allyloxyphenyl bromide yields baccatin 8 in 79% yield. Deprotection of the TES groups with HF/pyridine, followed by reprotection at the 7-position with TES and acetylation at the 10-position, gave baccatin 8 in 56% yield over three steps. Coupling of baccatin 9 with β-lactam 10, obtained in good yields and high enantiomeric excess by the previously published procedure (6, 19), provided coupling product 11 in 64% yield. The ring-closing metathesis of 11 with Ru–benzylidene complex in CH2Cl2 (1 mM) proceeded smoothly to afford the protected 18-membered macrocycle as the pure E isomer, which afforded hybrid construct 6 on deprotection with HF/pyridine in 65% yield for the two steps. All intermediates were characterized by satisfactory NMR (1H and 13C) and high-resolution MS data.
Figure 3.
Synthetic scheme for the hybrid construct 6 (SB-TE-1120). TES, triethylsilyl; LiHMDS, lithium hexamethyldisilazide; Cy, cyclohexyl.
Characterization Data for Hybrid Construct 6.
mp 190–192°C; [α]D22 −109° (c 0.11, CHCl3); 1H NMR (250 MHz, CDCl3) δ 1.14 (s, 3 H), 1.26 (s, 3 H), 1.38 (s, 9 H), 1.55 (m, 1 H), 1.70 (s, 3 H), 1.89 (m, 1 H), 1.91 (s, 3 H), 2.14 (s, 3 H), 2.23 (s, 3 H), 2.25–2.55 (m, 4 H), 2.77 (m, 1 H), 2.99 (d, J = 8.0 Hz, 1 H), 3.03 (s, 1 H), 3.79 (d, J = 7.1 Hz, 1 H), 4.00 (d, J = 7.7 Hz, 1 H), 4.07 (m, 1 H), 4.24 (d, J = 8.4 Hz, 1 H), 4.42 (m, 1 H), 4.42 (d, J = 8.3 Hz, 1 H), 4.55 (d, J = 9.7 Hz, 1 H), 4.67 (d, J = 15.0 Hz, 1 H), 4.72 (d, J = 15.0 Hz, 1 H), 4.90 (d, J = 8.1 Hz, 1 H), 5.69 (m, 3 H), 5.69 (t, J = 6.7 Hz, 1 H), 6.26 (s, 1 H), 6.98 (d, J = 8.5 Hz, 1 H), 7.03 (t, J = 7.6 Hz, 1 H), 7.47 (t, J = 7.9 Hz, 1 H), 7.68 (d, J = 7.4 Hz, 1 H); 13C NMR (62.9 MHz, CDCl3) δ 9.8, 15.3, 20.9, 22.0, 22.9, 26.6, 28.2, 28.4, 29.7, 32.2, 35.5, 37.7, 42.7, 45.7, 51.7, 58.6, 70.4, 71.9, 73.1, 75.5, 75.8, 76.4, 77.8, 80.0, 80.9, 84.8, 113.6, 121.3, 128.7, 129.0, 132.1, 133.0, 133.7, 142.8, 155.6, 168.6, 169.7, 171.4, 203.9. High-resolution MS (fast atom bombardment) m/z calculated for C44H57NO16⋅H+: 856.3757; found: 856.3756 (Δ = 0.1 ppm).
RESULTS AND DISCUSSION
Nonataxel (5) is a nonaromatic paclitaxel mimic lacking the conventional N-benzoylphenylisoserine side chain at the C-13α hydroxyl of baccatin III and the benzoyl ester at the C-2α hydroxyl group. Despite its structural difference, nonataxel exhibits 2- to 8-fold higher activity than paclitaxel in various cytotoxicity assays (7) and at 10 μM enhances tubulin polymerization to the same extent as paclitaxel at that concentration. Nonataxel, in contrast to paclitaxel, is amenable to a conformationally defining NMR analysis. High-temperature restrained molecular dynamics (RMD) study (sybyl 6.4, Tripos) on nonataxel, maintaining distance restraints obtained from the two-dimensional NOE spectroscopy experiments in DMSO-d6/D2O (3:1) revealed a very specific relative orientation of the C-3′ and C-2 alkenyl side chains for the major conformation of nonataxel. The solution conformation of nonataxel differs only slightly from the previously described conformation of paclitaxel (20–23) (see Fig. 4a) in terms of a greater compactness of the hydrophobic clustering of the side chains. It was expected that the nonaromatic groups in the nonataxel template would allow for better mapping to the ring systems of 2 and 3.
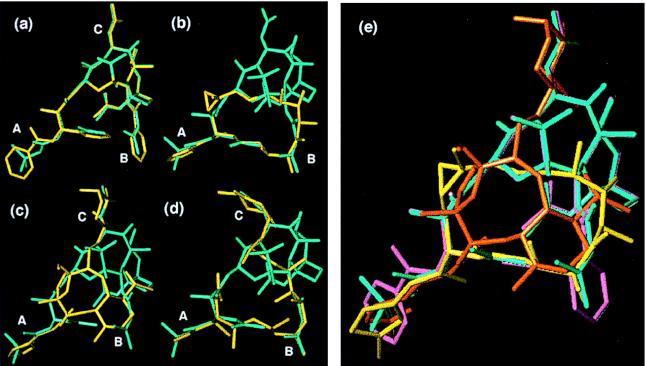
Figure 4.
(a–d) Overlay of nonataxel (4, cyan) with (a) paclitaxel (1), (b) epothilone B (2b), (c) eleutherobin (3), and (d) discodermolide (4) (all in yellow). Designators A, B, and C correspond to regions of common overlap (also see Fig. 1). (e) Overlay of paclitaxel (1, magenta), nonataxel (4, cyan), epothilone B (2b, yellow), and eleutherobin (3, orange) demonstrating the common pharmacophore (discodermolide omitted for clarity).
First, we modeled relationships of the epothilones with nonataxel. The high temperature molecular dynamics (MD) study of epothilone B (sybyl 6.4) revealed several low-energy conformers that were evaluated with regards to superimposition with the template conformation of nonataxel. A combination of template forcing (discover 95.0, Molecular Simulations), restrained molecular dynamics, and unrestrained energy minimizations (sybyl 6.4) on these conformations were used to iteratively refine a large number of overlays to obtain the best fit.** Fig. 4b illustrates the excellent topological homology between epothilone B (2b) and nonataxel (5). The C-1 to C-6 segment of 2b corresponds to the “southern” hydrophobic surface that interacts at the tubulin-binding site, and the C-15 side chain is seen to correspond with the t-Boc group at C-3′N of 5. The essential feature of the proposal is the excellent overlap of (i) the C-2 3-methyl-2-butenoate and C-3′ 2-methyl-1-propenyl substituents of 5 with the epothilone macrolide core, and (ii) the C13-N-t-Boc moiety with the thiazole ring side chain.
The observed structural homology between epothilone B and nonataxel in fact nicely accommodates the vast SAR data that have been accumulated for the former. For example, inversion at C-3, reduction at C-5, or removal of functionality at any of the positions from C-3 to C-8 in epothilone results in abrogation of both cytotoxicity and tubulin-binding ability (24). The sensitivity of this region to structural alteration accords well with its overlay with the highly sensitive C-3′ and C-2 alkyl substituents of nonataxel. The ring conformation of epothilone B may potentially be stabilized by hydrogen bonding between the C-1 carbonyl oxygen and C-3 hydroxyl hydrogen, which could logically explain the loss of activity observed on epimerization at the C-3 position (24). Similarly, the deleterious consequence of excision of the C-9 methylene (producing a 15-membered macrolide) is consistent with the anticipated change in conformation. The previously demonstrated need for the thiazole sector is well accommodated by the proposed common pharmacophore, since the aryl sector of 2 overlays with the critical C-13 acyl side chain. Also, deletion of the olefin spacer, between the aryl and the macrolide sectors, results in a predictable loss of activity. The C-12 methyl group in epothilone B is not defined in a clear way as part of the common pharmacophore. In practice, it can be deleted (compare epothilone A) or further extended to a propyl group while retaining good cytotoxicity (24).
An important triumph of the proposed common pharmacophore is that it accommodates the nonessential nature of the C12–C13 epoxide in the epothilones. Thus, this oxido oxygen points away from the overlapping structural terrain and is therefore not central to the tubulin-binding cytotoxicity phenomena. In fact, the 12,13-desoxy version of epothilone B is showing a far more promising profile of in vivo activity than either 2b or paclitaxel (25, 26). Similarly, the more severely distorted 12,13-(E) analogue of 12,13-desoxyepothilone B also retains excellent tubulin-binding activity.
Next, we turned our attention to the structural homology between eleutherobin and nonataxel. As Fig. 4c shows, the tricyclic 4,7-oxaeunicellane core of eleutherobin fits neatly into the cavity between the baccatin core and the southern hydrophobic surface of nonataxel. The largely hydrophobic C-8 to C-14 segment of eleutherobin traces the southern hydrophobic surface, whereas the C-8 N-(6′)-methylurocanic acid ester side chain closely corresponds to the t-Boc group at C-3′N of the C-13 side chain of nonataxel. Two essential points are immediately recognized: (i) the overlap for the C13-N t-Boc with the C-8 N-(6′)-methylurocanic acid ester side chain, and (ii) the potentially nonessential role played by the C-3 sugar side chain, which overlays with the baccatin core rather than the essential pharmacophore of nonataxel.
Again, the pharmacophore proposal accommodates the preliminary SAR data available for eleutherobin from these laboratories (27). For example, replacement of the l-arabinose moiety with a simple acetyl group or the d-arabinose moiety (neo-eleutherobin) results in compounds that retain tubulin polymerization ability as well as significant, albeit reduced, cytotoxicity (27). Nicolaou and co-workers have also recently reported sarcodictyings—i.e., sugar-free eleutherobin analogues—with tubulin polymerization abilities comparable to those of paclitaxel, the epothilones, and elutherobin (28). In sharp contrast to the C-3 modifications, the deletion of the C-8 side chain results in abrogation of tubulin polymerization ability and diminution of cytotoxic potency by nearly three orders of magnitude (27). These results clearly indicate the critical role of the C-8 side chain, and the expendability of the C-3 sugar moiety, in complete agreement with the common pharmacophore.
Modeling studies of discodermolide (4) have shown it to be a very flexible structure. Given the flexibility of the discodermolide system, the selection process required to interface with our model is somewhat arbitrary. However, we can readily identify a conformation that adheres to our common pharmacophore. The overlay of this conformation with nonataxel (5) (Fig. 4d) shows the efficient overlay in the southern hydrophobic surface traced by the C-11 to C-19 fragment of 4. The C-20 to C-24 diene-containing moiety of 4 overlays nicely with the C3′-N-t-Boc group of 5. Although not conclusive, this overlay suggests that discodermolide generally conforms to the common pharmacophore proposal.
Fig. 4e summarizes the overlays of paclitaxel, nonataxel, epothilone B, and eleutherobin, which facilitates visualization of our proposed pharmacophore. One other pharmacophore hypothesis for epothilone B and paclitaxel had previously been reported (18). Our pharmacophore proposal uniquely succeeds in explaining the key structural requirements for the biological activity of paclitaxel—i.e., the C-2 benzoate and the two C-13 side-chain elements, (i) the C-3′-N-benzoyl that crucially overlaps with the necessary thiazole (or similar aromatic moiety) of epothilone, and (ii) the C-3′ phenyl group. In this regard, the previously reported model (18) does not explain the critical nature of the C-3′ phenyl group toward the tubulin binding and cytotoxicity of paclitaxel, and it places undue importance on the nonessential C-10 acetyl group. At the same time, our proposal also accounts for the activity of sugar-deletion analogues or C-8 side-chain analogues of eleutherobin. Most importantly, our proposal logically suggests the linkage between the C-3′ phenyl group and the C-2α benzoyl group that has allowed us to validate our model through manufacture of an active hybrid construct described below.
Our model paves the way for designing the third-generation taxoids that could be essentially baccatin-free, or “hybrids” integrating the structures of paclitaxel and the new antitumor agents. The ring systems of eleutherobin and epothilone B clearly suggested that the nonataxel C-2 and C-3′ substituents can be linked to create a conformational constraint (Fig. 5). On the basis of the model, we have designed and synthesized cytotoxic hybrid constructs containing 16-, 17-, or 18-membered macrocycles.
Figure 5.
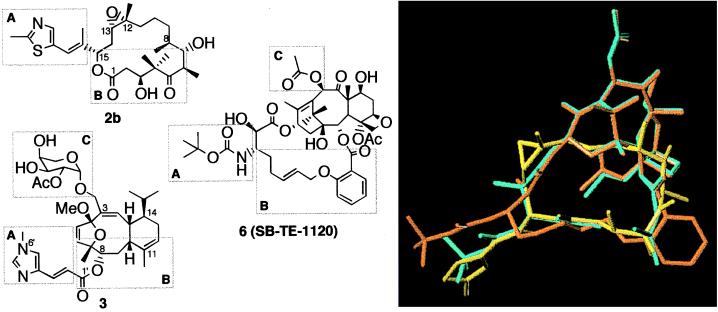
Structural correlation of the hybrid construct SB-TE-1120 (6) with epothilone B (2b) and eleutherobin (3). Designators A, B, and C correspond to regions of common overlap. The overlay of construct 6 (orange) with epothilone B (2b, yellow) and nonataxel (5, cyan) is shown on the right.
Indeed, by this thinking we were led to synthesizing compound 6 (SB-TE-1120). This construct exhibited submicromolar level IC50 values (0.39 μM against the human breast cancer cell line MDA-435/LCC6-WT) and 37% activity as compared with paclitaxel in the tubulin polymerization assay. Fig. 5 shows the overlay of construct 6 with nonataxel, demonstrating good overlay in the region corresponding to the C-2α substituent although the overlay near the C-3′ position, including the C-3′-N-t-Boc group has not reached a desirable level. Although details of the synthesis and SAR of these compounds will be published elsewhere, it is relevant to this discussion to note that the cytotoxicity and tubulin-binding ability of these compounds show high sensitivity to steric bulk of the substituent at the C-2α position (as observed in the nonaromatic taxoid series (7)), size and flexibility of the macrocycle, and presence of heteroatom(s) in the lower surface of the macrocycle (corresponding to the electron density on the lower surface of epothilone B). The preliminary SAR data for this series of compounds are thus highly consistent with the common pharmacophore model, thereby supporting the predictive power of our proposal.†† Further improvement in design will facilitate development of the next generation of tubulin-directed anticancer agents based on this approach.
Acknowledgments
This research was supported by grants from the National Institutes of Health (I.O., S.B.H., and S.J.D.). I.O. and S.C. also acknowledge the support from the National Science Foundation for the NMR Facilities at Stony Brook.
ABBREVIATIONS
- SAR
structure–activity relationship(s)
- NOE
nuclear Overhauser enhancement
Footnotes
We note that, recently, the structure of the αβ-tubulin dimer incorporating a paclitaxel molecule by electron crystallography of zinc-induced tubulin sheets was reported with 3.7-Å resolution [see Nogales et al. (29)]. A proposal for the tubulin-bound structure of paclitaxel, however, was not conclusive because of insufficient resolution. If a highly reliable refined crystal structure of tubulin-bound paclitaxel becomes available, docking experiments of these microtubule-stabilizing drugs with the protein binding pocket can be performed to define their common pharmacophore. In the absence of such reliable information, our approach is directed toward identifying common structural features among these drug molecules that correlate for their tubulin-binding ability.
Prior preliminary studies on the common pharmacophores for nonataxel, paclitaxel, epothilones, and discodermolide have been presented at (i) the 214th American Chemical Society National Meeting, Las Vegas, NV, Sept. 7–11, 1997, by S. Chakravarty and I. Ojima, abstr. MEDI 75; and (ii) the 215th American Chemical Society National Meeting, Dallas, TX, March 29-April 2, 1998, by S. F. Victory, G. L. Grunewald, and G. I. Georg, abstr. MEDI 187.
The proposed bioactive conformation of epothilone B was obtained through the template forcing protocol (discover 95.0), using the whole southern region of the nonataxel structure derived from the NOE-constrained molecular dynamics as the template. This structure is among the lowest-energy conformers generated through this operation, albeit not the global energy minimum structure. It is worth mentioning that the hypothetical bioactive conformation of epothilone B thus obtained is found to be very close to its x-ray crystallographic structure [see Höfle et al. (30)], and only slight deviation is observed at the orientation of the lactone carbonyl. For eleutherobin, the molecular dynamics calculations were carried out without any constraint because of its highly rigid structure except for its side chains. The positions of the two side chains at C-3 and C-8 were defined by using the t-Boc group at C-3′-N and the acetyl group at C-10, respectively, as key positions in the template forcing operations.
While we used two different force fields—i.e., Tripos (sybyl 6.4) and CVFF (discover 95.0)—all energy optimizations were performed with the Tripos force field. CVFF was used only for template forcing operations.
Ojima, I., Lin, S., Inoue, T., Walsh, J. J., Borella, C. P. & Chakravarty, S. (1998) 216th American Chemical Society National Meeting, Boston, August 23–27, 1998; abstr. ORGN 380 and 464, and MEDI 313.
References
- 1.Rowinsky E K, Cazenave L C, Donehower R C. J Natl Cancer Inst. 1990;82:1247–1259. doi: 10.1093/jnci/82.15.1247. [DOI] [PubMed] [Google Scholar]
- 2.Suffness M. Taxol: Science and Applications. Boca Raton, FL: CRC; 1995. [Google Scholar]
- 3.Georg G I, Chen T T, Ojima I, Vyas D M. Taxane Anticancer Agents: Basic Science and Current Status. Washington, DC: Am. Chem. Soc.; 1995. [Google Scholar]
- 4.Ojima I, Kuduk S D, Chakravarty S. Adv Med Chem. 1998;4:69–124. [Google Scholar]
- 5.Ojima I, Slater J C, Michaud E, Kuduk S D, Bounaud P-Y, Vrignaud P, Bissery M-C, Veith J, Pera P, Bernacki R J. J Med Chem. 1996;39:3889–3896. doi: 10.1021/jm9604080. [DOI] [PubMed] [Google Scholar]
- 6.Ojima I, Slater J S, Kuduk S D, Takeuchi C S, Gimi R H, Sun C-M, Park Y H, Pera P, Veith J M, Bernacki R J. J Med Chem. 1997;40:267–278. doi: 10.1021/jm960563e. [DOI] [PubMed] [Google Scholar]
- 7.Ojima I, Kuduk S D, Pera P, Veith J M, Bernacki R J. J Med Chem. 1997;40:279–285. doi: 10.1021/jm9606711. [DOI] [PubMed] [Google Scholar]
- 8.Shiff P B, Fant J, Horwitz S B. Nature (London) 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 9.Jordan M A, Toso R J, Wilson L. Proc Natl Acad Sci USA. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bollag D M, McQueney P A, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods C M. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 11.Kowalski R J, Giannakakou P, Hamel E. J Biol Chem. 1997;272:2534–2541. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- 12.Lindel T, Jensen P R, Fenical W, Long B H, Casazza A M, Carboni J, Fairchild C R. J Am Chem Soc. 1997;119:8744–8745. [Google Scholar]
- 13.ter Haar E, Kowalski R J, Hamel E, Lin C M, Longley R E, Gunasekera S P, Rosenkranz H S, Day B W. Biochemistry. 1996;35:243–250. doi: 10.1021/bi9515127. [DOI] [PubMed] [Google Scholar]
- 14.Kowalski R J, Giannakakou P, Gunasekera S P, Longley R E, Day B W, Hamel E. Mol Pharmacol. 1997;52:613–622. [PubMed] [Google Scholar]
- 15.Giannakakou P, Sackett D L, Kang Y, Zhan Z, Buters J T M, Fojo T, Poruchynsky M S. J Biol Chem. 1997;272:17118–17125. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]
- 16.Kowalski R J, ter Haar E, Longley R E, Gunasekera S P, Lin C M, Day B W, Hamel E. Mol Biol Cell. 1995;6:368a. [Google Scholar]
- 17.Hung D T, Chen J, Schreiber S L. Chem Biol. 1996;3:287–293. doi: 10.1016/s1074-5521(96)90108-8. [DOI] [PubMed] [Google Scholar]
- 18.Winkler J D, Axelsen P H. Bioorg Med Chem Lett. 1996;6:2963–2966. [Google Scholar]
- 19.Ojima I, Lin S, Chakravarty S, Fenoglio I, Park Y H, Sun C, Appendino G, Pera P, Veith J M, Bernacki R J. J Org Chem. 1998;63:1637–1645. [Google Scholar]
- 20.Vander Velde D G, Georg G I, Grunewald G L, Gunn C W, Mitscher L A. J Am Chem Soc. 1993;115:11650–11651. [Google Scholar]
- 21.Williams, H. J., Scott, A. I., Dieden, R. A., Swindell, C. S., Chirlian, L. E., Francl, M. M., Heerding, J. M. & Krauss, N. E. (1994) Can. J. Chem. 252–260.
- 22.Mastropaolo D, Camerman A, Luo Y, Brayer G D, Camerman N. Proc Natl Acad Sci USA. 1995;92:6920–6924. doi: 10.1073/pnas.92.15.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ojima I, Kuduk S D, Chakravarty S, Ourevitch M, Bégué J-P. J Am Chem Soc. 1997;119:5519–5527. [Google Scholar]
- 24.Su D, Balog A, Meng D, Bertinato P, Danishefsky S J, Zheng Y, Chou T, He L, Horwitz S B. Angew Chem Int Ed Engl. 1997;36:2093–2096. [Google Scholar]
- 25.Chou T, Zhang X, Balog A, Su D, Meng D, Savin K, Bertino J R, Danishefsky S J. Proc Natl Acad Sci USA. 1998;95:9642–9647. doi: 10.1073/pnas.95.16.9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou T C, Zhang X G, Harris C R, Kuduk S D, Balog A, Savin K, Danishefsky S J. Proc Natl Acad Sci USA. 1998;95:15798–15802. doi: 10.1073/pnas.95.26.15798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDaid, H. M., Bhattacharya, S. K., Chen, S., He, L., Shen, H., Gutteridge, C. E., Horwitz, S. B. & Danishefsky, S. J. (1999) Cancer Chemother. Pharmacol., in press. [DOI] [PubMed]
- 28.Nicolaou K C, Kim S, Pfefferkorn J, Xu J, Ohshima T, Hosokawa S, Vourloumis D, Li T. Angew Chem Int Ed Engl. 1998;37:1418–1421. doi: 10.1002/(SICI)1521-3773(19980605)37:10<1418::AID-ANIE1418>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Nogales E, Wolf S G, Downing K H. Nature (London) 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 30.Höfle G, Bedorf N, Steinmetz H, Schomburg D, Gerth K, Reichenbach H. Angew Chem Int Ed Engl. 1996;35:1567–1569. [Google Scholar]