Abstract
It has been reported that substitution of Arg258, a residue within the GTPase domain of the heterotrimeric guanine nucleotide binding protein (G protein) α-subunit (αs), to alanine (αs-R258A) results in decreased activation by receptor or aluminum fluoride (AlF4−) and increased basal GDP release. Arg258 interacts with Gln170 in the helical domain, and, presumably, loss of this interaction between the GTPase and helical domain leads to more rapid GDP release, resulting in decreased activation by AlF4− and increased thermolability. In this study, we mutate Gln170 to alanine (αs-Q170A) and demonstrate that this mutant, like αs-R258A, has decreased activation by AlF4−, increased thermolability (both reversed in the presence of excess guanine nucleotide), and an increased rate of GDP release. However, unlike αs-R258A, αs-Q170A does not have impaired receptor-mediated activation. Therefore, this interdomain interaction is critical to maintain normal guanine nucleotide binding (and hence normal activation by AlF4−) but is not important for receptor-mediated activation. In single turnover GTPase assays, the catalytic rate for GTP hydrolysis of αs-R258A was 14-fold higher than normal whereas that of αs-Q170A was unaffected. Examination of the αs crystal structure suggests that Arg258, through interactions with Glu50, might constrain the position of Arg201, a residue critical for catalyzing the GTPase reaction. This is an example of a mutation in a heterotrimeric G protein that results in an increased intrinsic GTPase activity and provides another mechanism by which G protein mutations can impair signal transduction.
Heterotrimeric guanine nucleotide binding proteins (G proteins) mediate the activation of intracellular effectors by heptahelical receptors (reviewed in refs. 1–3). Each G protein has a distinct α-subunit that binds guanine nucleotide and modulates the activity of specific downstream effectors. For the stimulatory G protein α-subunit (αs), these include the stimulation of adenylyl cyclase and modulation of ion channels (4, 5). In the basal (inactive) state, GDP-bound α-subunit is associated with a βγ-dimer. Activation by receptor results in a conformational change in the α-subunit, leading to the exchange of GTP for GDP and dissociation from βγ. The GTP-bound α-subunit interacts with and regulates specific effectors. Hydrolysis of bound GTP to GDP by an intrinsic GTPase activity within the α-subunit returns the G protein to the inactive state. Mutations that slow this intrinsic GTPase activity lead to constitutive activation, and such activating mutations are found in sporadic endocrine tumors (6) and in patients with the McCune-Albright syndrome (7). G proteins also can be activated by guanosine-5′-O-3-thiotriphosphate (GTPγS), a nonhydrolyzable analogue of GTP, or aluminum fluoride (AlF4−), which binds to GDP-bound α-subunits and mimics the γ-phosphate of GTP.
G protein α-subunits have two domains, a ras-like GTPase domain, which includes the sites for guanine nucleotide binding and effector interaction, and a helical domain (8–14). Because the guanine nucleotide resides in a cleft between the two domains, the helical domain may be important in slowing GDP release in the inactive state. Comparison of the structures of inactive (GDP-bound) and activated (GTPγS- or AlF4−-bound) α-subunits reveals three regions within the GTPase domain (named switches 1, 2, and 3) that undergo conformation changes on activation. On activation, switches 2 and 3 move toward each other and form multiple interactions that stabilize the active state (15–17). Residues in switch 3 also make contact with residues in the helical domain, and these interactions have been implicated in the maintenance of guanine nucleotide binding (12, 18) and in receptor-mediated activation (18–20).
An αs mutation in a patient with Albright hereditary osteodystrophy in which the switch 3 residue Arg258 is substituted with tryptophan§ has been identified, and it has been demonstrated that this substitution (as well as the Arg258 to alanine substitution, αs-R258A) leads to increased GDP release (resulting in decreased activation by AlF4− and increased thermolability) and impaired receptor-mediated activation (18). Based on the αs crystal structure (14, 18), Arg258 interacts with residue Gln170 within the helical domain. Loss of this interaction would be predicted to open the cleft between the GTPase and helical domain, resulting in more rapid GDP release, as observed for the Arg258 substitution mutants. It also has been suggested that interactions between Arg258 and the helical domain are important for receptor-mediated activation (18–20). In this study, we show that mutating Gln170 to alanine (αs-Q170A) also leads to increased GDP release (along with decreased activation by AlF4−) but does not affect receptor-mediated activation. Therefore, interactions between Arg258 and Gln170 are important for maintaining guanine nucleotide binding but are not important for activation by receptor. We further show that αs-R258A (but not αs-Q170A) has a markedly elevated intrinsic GTPase rate, which will lead to more rapid inactivation. Arg258, through mutual interactions with Glu50, may constrain Arg201, a residue critical for catalyzing GTP hydrolysis. Disruption of the interaction between Arg258 and Glu50 may relieve this constraint and allow Arg201 to more efficiently interact with the γ-phosphate of GTP in the transition state. This is an example of a mutation in a heterotrimeric G protein that increases the intrinsic GTPase activity and provides another mechanism by which receptor signaling can be impaired by G protein mutations.
MATERIALS AND METHODS
Generation of αs in Vitro Transcription/Translation Products.
To generate αs-Q170A, PCR was performed by using wild-type αs (αs-WT) cDNA as template (18), upstream primer 5′CCCTCCCGAATTCTATGAGCATGCCAAGGCTCTGTGGGAGGATGAAGGAGTGCGTGCCTG3′, and downstream mutagenic primer 5′CTGCTTGATCACGTCGATCTTGTCCAGGAAGTACTGGGCACAGTCAATCAGCGCGTACTCG3′. The PCR product was digested with HincII and Sse8387I and was ligated into pBluescript II SK(+) (Stratagene) containing αs-WT cDNA (splice variant αs-1; ref. 21) in which the same HincII-Sse8387I restriction fragment had been removed. In vitro transcription/translation was performed on αs plasmids as described (18, 22). Synthesis of full-length αs from each construct was confirmed by immune precipitation of in vitro translated products with RM antibody, directed against the αs C-terminal decapeptide (23).
Adenylyl Cyclase and Trypsin Protection Assays.
In vitro transcription/translation products (10 μl of translation medium) were reconstituted into 25-μg purified S49 cyc-plasma membranes and were tested for stimulation of adenylyl cyclase in the presence of various agents at 30°C as described (18, 22, 24). Trypsin protection assays were performed as described (18, 22). In brief, [35S]methionine-labeled in vitro-translated αs was incubated at 30 or 37°C for 1 h with either no activators, 100 μM GTPγS, 10 mM NaF/10 μM AlCl3 (AlF4−), or AlF4− plus 2 mM GDP, and then was digested with 200 μg/ml tosyl-l-phenylyalanine chloromethyl ketone-trypsin for 5 min at 20°C (18, 22). Digestion products were separated on 10% SDS polyacrylamide gels.
GTPγS Binding Assays.
Plasmid pQE60 containing the long form of bovine αs cDNA with a hexa-histidine extension at the C terminus was mutated to αs-Q170A by site-directed mutagenesis by using the Quickchange kit (Stratagene). αs proteins were purified from Escherichia coli and rates of GTPγS binding were measured and calculated as described (18, 25, 26).
Single Turnover GTPase Assays.
Purified αs (100 pmol, 40 nM) was incubated in 50 mM Hepes (pH 8.0), 1 mM DTT, 5 mM EDTA, 0.1% Lubrol-PX, and 5 μM [32P-γ]GTP (3,000 cpm/pmol) at 30°C for 30 min (αs-WT) or 20°C for 20 min (αs-R258A and -Q170A). Reactions were placed on ice for 5 min, and the GTPase reaction was initiated by the addition of MgCl2 and GTP to final concentrations of 20 mM and 200 μM, respectively. At various time points, 50-μl aliquots (corresponding to 10 pmol of αs) were removed and immediately added to 750 μl of ice-cold 5% (wt/vol) Norit-SA3 (Aldrich) in 50 mM NaH2PO4 (pH 2.0). After microcentrifugation, 32P was measured in the supernatant by liquid scintillation. kcat was determined by fitting the data into the equation P = Pmax(1 − e−kt) (where P represents phosphate released at time t and Pmax represents maximal phosphate release) by using prism 2.01 (GraphPad, San Diego).
RESULTS
Substitution of αs Gln170 Leads to Impaired Activation by AlF4− but Normal Receptor-Mediated Activation.
A mutation in a patient with Albright hereditary osteodystrophy encoding a substitution of residue Arg258 within the switch 3 region of αs has been identified, and it has been demonstrated that substitution of this residue impairs AlF4−- and receptor-mediated activation whereas activation by GTPγS is unaffected (18). The mutant has an increased rate of GDP release, and excess GDP restores AlF4−-induced activation. Because GDP binding is a prerequisite for AlF4− binding and activation, the impaired activation by AlF4− is a function of the decreased ability of this mutant to maintain the GDP-bound state. Arg258 interacts with the conserved residue Gln170 within the helical domain (18), and disruption of this interaction would be predicted to open the cleft between the GTPase and helical domains, leading to increased GDP release.
To further characterize the role of this interdomain interaction in maintaining the GDP-bound basal state and in receptor-mediated activation, we examined the effect of substituting Gln170 on G protein function. A Gln170 to alanine substitution mutant (αs-Q170A) was cloned into the transcription vector pBluescript, and the in vitro transcription/translation products were compared with those of αs-WT in various biochemical assays. After reconstitution of translation products into purified S49 cyc-membranes (which lack endogenous αs), αs-Q170A was efficient at stimulating adenylyl cyclase in the presence of GTPγS or activated receptor (the β-adrenergic agonist isoproterenol plus GTP), with a response similar to or slightly greater than that of αs-WT (Table 1). In contrast, the ability of αs-Q170A to stimulate adenylyl cyclase in the presence of AlF4− was decreased significantly. For comparison, previously published results for αs-R258A (expressed as percent of αs-WT) also are shown (18). Although the two mutants respond similarly to GTPγS and AlF4−, receptor-mediated activation is markedly reduced in αs-R258A but is normally maintained in αs-Q170A. Sucrose density gradient experiments demonstrated that both mutants are stable for 1 h at the same temperature that the adenylyl cyclase assays were performed (30°C; ref. 18 and data not shown). Therefore, thermolability is unlikely to explain the decreased function of these mutants in the adenylyl cyclase assay.
Table 1.
Adenylyl cyclase stimulation by αS-Q170A [pmol cAMP/ml translation product/15 min (percent of WT)]
| αS | GTP, 100 μM | Isoproterenol, 10 μM, + GTP, 100 μM | GTPγS, 100 μM | AlF4−b* |
|---|---|---|---|---|
| WT | 25 ± 10 | 233 ± 5 | 259 ± 21 | 225 ± 12 |
| Q170A | 29 ± 6 | 246 ± 24 (106 ± 11) | 305 ± 4 (118 ± 10) | 113 ± 9 (50 ± 5) |
| R258A† | (24 ± 3) | (132 ± 6) | (62 ± 9) |
In vitro transcription/translation products were mixed with purified cyc- membranes and were assayed for adenylyl cyclase stimulation at 30°C. Results are expressed as the mean ± SD, (σn−1) of triplicate determinations and are representative of three independent experiments. The levels of expression of αS-WT and -Q170A were similar as determined by in vitro translation with [35S]methionine, SDS/PAGE, and phosphorimaging. Background values determined from mock transcription/translation reactions (in pmol cAMP/ml translation medium/15 min; GTP, 39 ± 4; isoproterenol/GTP, 46 ± 1; GTPγS, 58 ± 2; and AlF4−; 68 ± 8) were subtracted from each determination.
10 mM NaF, 10 μM AlCl3, and 100 μM GDP.
The results for αS-R258A (shown here as percent of αS-WT) have been published (18) and are shown here for comparison with αS-Q170A.
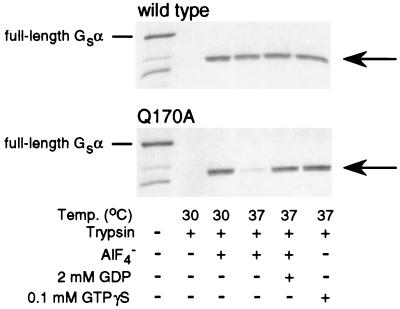
We next examined the ability of AlF4− or GTPγS to protect αs-Q170A from trypsin digestion, which measures the ability of each agent to bind to αs and to induce the active conformation (27). When αs attains the active conformation, specific arginine residues within the switch 2 region become inaccessible to trypsin digestion (9), leading to the generation of a partially protected, 38-kDa digestion product. αs-WT was well protected by AlF4− or GTPγS at temperatures up to 37°C (Fig. 1). αs-Q170A also was well protected by GTPγS at 37°C. Consistent with the results of the adenylyl cyclase assays, AlF4− was less effective than GTPγS in protecting αs-Q170A from trypsin digestion, particularly at higher temperature (37 vs. 30°C; Fig. 1). Moreover, the ability of AlF4− to protect αs-Q170A was restored by addition of excess GDP in the incubation. These results are similar to those obtained previously for αs-R258A (18) and suggest that both mutants have a decreased ability to bind GDP, particularly at higher temperatures.
Figure 1.
Trypsin protection of [35S]methionine-labeled in vitro translates of αs-WT (Upper) and αs-Q170A (Lower). In each gel, the full-length undigested αs (52 kDa) is shown in the first lane, and complete digestion in the absence of activators is shown in the second lane. The next four lanes show the results after preincubation at the temperatures and in the presence of various agents indicated below. The smaller products in the left lane are attributable to initiation of protein synthesis at downstream methionine codons. Gs, stimulatory G protein.
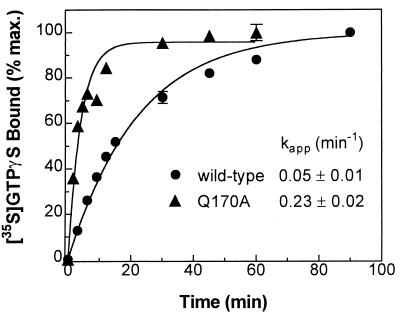
To directly determine the rate of GDP release in the basal state, bovine αs-WT and -Q170A, each with a C-terminal hexahistidine tag, were purified from E. coli, and the time course of GTPγS binding to each was examined. The rate of GTPγS binding has been shown to be limited by the rate of GDP dissociation, and the experimentally determined values of these two rates are essentially identical (18, 28, 29). The kapp for GTPγS binding at 20°C was 0.05 min−1 for αs-WT versus 0.23 min−1 for αs-Q170A (Fig. 2), indicating that the Q170A substitution leads to a significantly increased rate of GDP release (as reflected in the rate of GTPγS binding). Similar results were reported for αs-R258A, with a kapp of 0.36 min−1 (18). αs-Q170A would be predicted to be more thermolabile because a greater proportion will have no bound guanine nucleotide, as has been shown for αs-R258A (18) and for other mutants with decreased affinity for guanine nucleotide (22, 30). Sucrose density gradient experiments of in vitro translates as previously performed for αs-R258A (18) demonstrated that, in fact, αs-Q170A is more thermolabile but can be stabilized in the presence of excess guanine nucleotide and is capable of interacting with βγ dimers (data not shown).
Figure 2.
Time course of GTPγS binding to purified αs. Bovine αs-WT and -Q170A, each with a C-terminal hexahistidine extension, were purified from E. coli, and the rate of GTPγS binding for each was determined. αs-WT (●) and -Q170A (▴) were incubated with 1 μM [35S]GTPγS (≈10,000 cpm/pmol) at 20°C for varying times, and the amount of bound GTPγS was determined (18). Each data point is the mean ± SD of triplicate determinations. The apparent on rates for GTPγS (kapp) are shown (mean ± SE of six experiments).
Impaired Receptor-Mediated Activation of αs-R258A is Associated with Increased Intrinsic GTPase Activity.
The Arg258-Gln170 interdomain interaction is critical for maintaining the GDP-bound basal state because disruption of this interaction by substituting either residue increases GDP release, which leads to decreased activation by AlF4− and increased thermolability. However, the fact that activation by isoproterenol plus GTP is unaffected by the Gln170 substitution suggests that this interdomain interaction is not important for receptor-mediated activation and that substitution of Arg258 impairs receptor-mediated activation by another mechanism.
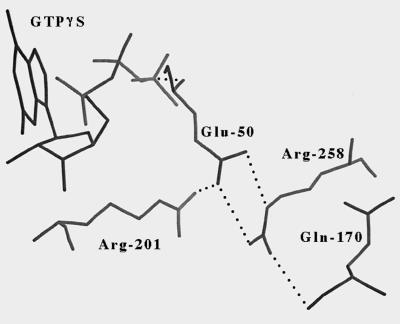
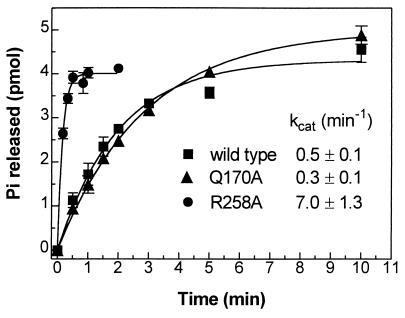
One possible mechanism for defective receptor-mediated activation is an increased rate of GTP hydrolysis leading to more rapid inactivation. Based on the fact that Arg258 interacts indirectly (through Glu50) with Arg201, a residue critical for catalyzing the hydrolysis of bound GTP to GDP (see Fig. 4 and Discussion), we hypothesized that impaired receptor-mediated activation of αs-R258A might be caused by an increase in its intrinsic GTPase activity. To test this directly, the catalytic rates of GTP hydrolysis for purified αs-WT, -R258A, and -Q170A were determined in a single turnover assay (Fig. 3). After loading each with [32P-γ]GTP in the absence of magnesium, the GTPase reaction was initiated by the addition of magnesium and unlabeled GTP, and the rate of phosphate release was measured. At 0°C, the intrinsic catalytic rate of GTP hydrolysis by αs-R258A (kcat 7.0 ± 1.3 min−1) was 14-fold higher than that by αs-WT (kcat 0.5 ± 0.1 min−1). In contrast, the intrinsic GTPase activity of αs-Q170A (kcat 0.3 ± 0.1 min−1) was similar to that of αs-WT. This is consistent with the results of the adenylyl cyclase stimulation assays because, for αs-R258A, increased GTPase activity is associated with reduced stimulation of adenylyl cyclase by receptor agonist (isoproterenol plus GTP) whereas for αs-Q170A, both receptor-mediated activation and the GTPase activity are normal.
Figure 4.
Detailed view of interactions between the side chains of Arg258 in switch 3, Glu50, and Arg201 based on the crystal structure of GTPγS-αs. The interaction between the side chain of Arg258 and the backbone oxygen of Gln170 also is shown. Hydrogen bonds are shown as dotted lines. The figure was generated with look 3.0 (Molecular Applications Group, Palo Alto, CA) by using coordinates for the short form of bovine GTPγS-αs [Protein Data Bank accession code 1AZT (14)], although the numbering in the figure corresponds to the long form of αs.
Figure 3.
Single turnover GTP hydrolysis rate of purified αs isoforms. GTP hydrolysis of αs-WT (■), αs-Q170A (▴), and αs-R258A (●) at 0°C is shown as the amount of phosphate (Pi) released as a function of time. The calculated catalytic rates (kcat) also are shown. Each data point represents the mean ± SE of three experiments. Baseline counts at time zero ranged from 30 to 55% of maximum counts released. The majority of baseline counts is attributable to free inorganic phosphate contamination of the stock [32P-γ]GTP.
DISCUSSION
It has been suggested that interactions between Arg258 in the GTPase domain of αs and residues within the helical domain are important for both guanine nucleotide binding and receptor-mediated activation based on the observations that both are impaired in αs-R258A (18) and that replacing the αs helical domain with the helical domain of αi2 corrects the receptor-mediated activation defect of αs-R258A (20). The GTPγS-αs crystal structure reveals that, amongst the helical domain residues, Arg258 most closely interacts with the conserved residue Gln170 (18). To further examine the importance of interdomain interactions in G protein function, we decided to disrupt the Arg258–Gln170 interaction from the opposite side by mutating the Gln170 to alanine. This mutant, like αs-R258A, had an increased rate of GDP release associated with increased thermolability and decreased activation by AlF4−. This provides further evidence that the interdomain interaction acts as a “lid” over the cleft that contains the guanine nucleotide binding pocket and that disruption of this interaction from either side opens the cleft, leading to increased GDP release in the basal state.
Although the substitution of Gln170 appears to disrupt the interdomain interaction, based on its effects on guanine nucleotide binding, this substitution has no effect on receptor-mediated activation because αs-Q170A was able to normally stimulate adenylyl cyclase in the presence of isoproterenol plus GTP. This suggests that the interdomain interactions (or at least the ones involving Gln170) are not critical for receptor-mediated activation and that the receptor activation defect in αs-R258A is caused by the disruption of interactions between Arg258 and other αs residues.
Examination of the GTPγS-αs crystal structure reveals that Arg258 also interacts with Glu50 within the GTPase domain and that Glu50 interacts with Arg201 (Fig. 4). Arg201 is critical for catalyzing the hydrolysis of bound GTP to GDP, and mutations of this residue lead to constitutive activation because of a markedly reduced intrinsic GTPase activity (31). It is conceivable that disruption of the interaction between Arg258 and Glu50 might relieve constraints on Arg201, which would allow Arg201 to adopt a more favorable position for catalyzing the hydrolysis of GTP. The resulting increased GTPase activity should lead to impaired receptor-mediated activation because the GTP-bound active state would be short-lived. To test this hypothesis, the intrinsic GTPase activity of αs-R258A was measured directly in single turnover GTPase assays and was shown to be 14-fold higher than that of αs-WT. The fact that the GTPase activity of αs-Q170A measured in the same assay was normal demonstrates that the altered GTPase activity of αs-R258A was specific for the Arg258 substitution and was not caused by disruption of the interaction between Arg258 and the helical domain.
Defects in receptor-mediated activation may result from decreased receptor-catalyzed nucleotide exchange, inability to attain the active conformation or interact with effector, or an increased rate of GTP hydrolysis leading to more rapid inactivation. The ability of αs-R258A to attain the active conformation and interact with effector is demonstrated by the fact that the mutant can normally activate adenylyl cyclase in the presence of GTPγS (18) or when the Arg201 residue also is mutated to block hydrolysis of bound GTP (20). Impaired receptor-catalyzed nucleotide exchange may result from inability of the α-subunit to interact with receptor or βγ or inability of the receptor-G protein interaction to stimulate guanine nucleotide exchange. We have demonstrated that αs-R258A is capable of binding to βγ (18). An αs mutant created by Grishina and Berlot (20) with substitution of four switch 3 residues, including the R258A substitution, has somewhat increased apparent affinity for the β-adrenergic receptor, and it is therefore unlikely that the single R258A substitution has a major effect on receptor–G protein interactions. In their study (20), the above mutant (which includes the R258A substitution) has increased guanine nucleotide exchange in the absence of isoproterenol [consistent with our findings for αs-R258A (18)] but has only minor changes in the rate of guanine nucleotide exchange in the presence of isoproterenol. It is therefore unlikely that the R258A substitution has a major effect on receptor-catalyzed guanine nucleotide exchange. In addition, deletion of the entire switch 3 region of transducin has no effect on βγ binding or receptor-catalyzed guanine nucleotide exchange (15). Although it is possible that the αs-R258A mutant has minor defects in receptor-catalyzed guanine nucleotide exchange, given our results and those described above, we feel that the receptor-mediated activation defect of αs-R258A is caused primarily by a markedly increased GTPase activity. Evidence that increased GTPase rates can lead to decreased receptor-mediated activation is provided by the effects of regulators of G protein signaling proteins (which increase the GTPase rate of α-subunits) on receptor signaling (32).
Grishina and Berlot (20) showed that the receptor activation defect of αs-R258A can be corrected by replacing the αs helical domain with that of αi2. Because, in αi2, alanine is the residue in the position that corresponds to Arg258 in αs, they concluded (20) that replacing the αs with the αi2 helical domain restores interdomain interactions in αs-R258A and that this corrects the receptor activation defect. Although this is possible, the fact that we can interrupt an important interdomain interaction in αs-Q170A (confirmed by its effects on GDP binding) without affecting receptor-mediated activation makes this explanation less likely. Although interactions between Arg258 and other helical domain residues might be important for receptor-mediated activation, the only helical domain residues that make direct contacts with Arg258 are identical in αs and αi2. It is also possible that substitution of the helical domain corrects the increased GTPase activity of αs-R258A. It is interesting to note that substituting the αi2 helical domain into αs-WT leads to constitutive activation (19, 20), which could result from decreased GTPase activity.
This is an example in which receptor signaling is impaired by a mutation that activates the intrinsic GTPase activity of a heterotrimeric G protein; mutations that lead to increased GTPase activity have been identified in the small guanine nucleotide binding proteins EF-Tu (33) and ras (34). This underscores the need to examine the GTPase activity when assessing the function of mutant G proteins with impaired receptor-mediated activation. Finally, these studies demonstrate that identification and analysis of naturally occurring G protein mutations in patients can lead to significant advances in our understanding of how G proteins function.
Acknowledgments
We thank J. Nagle for performing DNA sequencing analysis, A. G. Gilman and R. K. Sunahara for providing plasmid pQE60-αs-H6, and P. Fishman and A. Spiegel for helpful comments.
ABBREVIATIONS
- G protein
guanine nucleotide binding protein
- αs
stimulatory G protein α-subunit
- GTPγS
guanosine-5′-O-(3-thiotriphosphate)
- WT
wild-type
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
All numbering is based on the αs-1 sequence reported by Kozasa et al. (21).
References
- 1.Spiegel A M, Shenker A, Weinstein L S. Endocr Rev. 1992;13:536–565. doi: 10.1210/edrv-13-3-536. [DOI] [PubMed] [Google Scholar]
- 2.Neer E J. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 3.Birnbaumer L, Abramowitz J, Brown A M. Biochim Biophys Acta. 1990;1031:163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- 4.Yatani A, Codina J, Imoto Y, Reeves J P, Birnbaumer L, Brown A M. Science. 1987;238:1288–1292. doi: 10.1126/science.2446390. [DOI] [PubMed] [Google Scholar]
- 5.Schreibmayer W, Dessauer W, Vorobiev D, Gilman A G, Lester H A, Davidson N, Dascal N. Nature (London) 1996;380:624–627. doi: 10.1038/380624a0. [DOI] [PubMed] [Google Scholar]
- 6.Lyons J, Landis C A, Harsh G, Vallar L, Grünewald K, Feichtinger H, Duh Q-Y, Clark O H, Kawasaki E, Bourne H R, et al. Science. 1990;249:655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein L S, Shenker A, Gejman P V, Merino M J, Friedman E, Spiegel A M. N Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 8.Noel J P, Hamm H E, Sigler P B. Nature (London) 1993;366:654–663. doi: 10.1038/366654a0. [DOI] [PubMed] [Google Scholar]
- 9.Lambright D G, Noel J P, Hamm H E, Sigler P B. Nature (London) 1994;369:621–628. doi: 10.1038/369621a0. [DOI] [PubMed] [Google Scholar]
- 10.Coleman D E, Berghuis A M, Lee E, Linder M E, Gilman A G, Sprang S R. Science. 1994;265:1405–1412. doi: 10.1126/science.8073283. [DOI] [PubMed] [Google Scholar]
- 11.Wall M A, Coleman D E, Lee E, Iñiguez-Lluhi J A, Posner B A, Gilman A G, Sprang S R. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 12.Mixon M B, Lee E, Coleman D E, Berghuis A M, Gilman A G, Sprang S R. Science. 1995;270:954–960. doi: 10.1126/science.270.5238.954. [DOI] [PubMed] [Google Scholar]
- 13.Lambright D G, Sondek J, Bohm A, Skiba N P, Hamm H E, Sigler P B. Nature (London) 1996;379:297–299. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 14.Sunahara R K, Tesmer J J G, Gilman A G, Sprang S R. Science. 1997;278:1943–1947. doi: 10.1126/science.278.5345.1943. [DOI] [PubMed] [Google Scholar]
- 15.Li Q B, Cerione R A. J Biol Chem. 1997;272:21673–21676. doi: 10.1074/jbc.272.35.21673. [DOI] [PubMed] [Google Scholar]
- 16.Iiri T, Farfel Z, Bourne H R. Nature (London) 1998;394:35–38. doi: 10.1038/27831. [DOI] [PubMed] [Google Scholar]
- 17.Warner D R, Romanowski R, Yu S, Weinstein L S. J Biol Chem. 1999;274:4977–4984. doi: 10.1074/jbc.274.8.4977. [DOI] [PubMed] [Google Scholar]
- 18.Warner D R, Weng G, Yu S, Matalon R, Weinstein L S. J Biol Chem. 1998;273:23976–23983. doi: 10.1074/jbc.273.37.23976. [DOI] [PubMed] [Google Scholar]
- 19.Marsh S R, Grishina G, Wilson P T, Berlot C H. Mol Pharmacol. 1998;53:981–990. [PubMed] [Google Scholar]
- 20.Grishina G, Berlot C H. J Biol Chem. 1998;273:15053–15060. doi: 10.1074/jbc.273.24.15053. [DOI] [PubMed] [Google Scholar]
- 21.Kozasa T, Itoh H, Tsukamoto T, Kaziro Y. Proc Natl Acad Sci USA. 1988;85:2081–2085. doi: 10.1073/pnas.85.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warner D R, Gejman P V, Collins R M, Weinstein L S. Mol Endocrinol. 1997;11:1718–1727. doi: 10.1210/mend.11.11.0013. [DOI] [PubMed] [Google Scholar]
- 23.Simonds W F, Goldsmith P K, Woodard C J, Unson C G, Spiegel A M. FEBS Lett. 1989;249:189–194. doi: 10.1016/0014-5793(89)80622-2. [DOI] [PubMed] [Google Scholar]
- 24.Sternweis P C, Northup J K, Smigel M D, Gilman A G. J Biol Chem. 1981;256:11517–11526. [PubMed] [Google Scholar]
- 25.Lee E, Linder M E, Gilman A G. Methods Enzymol. 1994;237:146–163. doi: 10.1016/s0076-6879(94)37059-1. [DOI] [PubMed] [Google Scholar]
- 26.Carty D J, Iyengar R. Methods Enzymol. 1994;237:38–44. doi: 10.1016/s0076-6879(94)37051-6. [DOI] [PubMed] [Google Scholar]
- 27.Miller R T, Masters S B, Sullivan K A, Beiderman B, Bourne H R. Nature (London) 1988;334:712–715. doi: 10.1038/334712a0. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson K M, Higashijima T, Smigel M D, Gilman A G. J Biol Chem. 1986;261:7393–7399. [PubMed] [Google Scholar]
- 29.Graziano M P, Gilman A G. J Biol Chem. 1989;264:15475–15482. [PubMed] [Google Scholar]
- 30.Iiri T, Herzmark P, Nakamoto J M, Van Dop C, Bourne H R. Nature (London) 1994;371:164–167. doi: 10.1038/371164a0. [DOI] [PubMed] [Google Scholar]
- 31.Landis C A, Masters S B, Spada A, Pace A M, Bourne H R, Vallar L. Nature (London) 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- 32.Berman D M, Gilman A G. J Biol Chem. 1998;273:1269–1272. doi: 10.1074/jbc.273.3.1269. [DOI] [PubMed] [Google Scholar]
- 33.Harmark K, Anborgh P H, Merola M, Clark B F C, Parmeggiani A. Biochemistry. 1992;31:7367–7372. doi: 10.1021/bi00147a022. [DOI] [PubMed] [Google Scholar]
- 34.Frech M, Darden T A, Pedersen G, Foley C K, Charisson P F, Anderson M W, Wittinghofer A. Biochemistry. 1994;33:3237–3244. doi: 10.1021/bi00177a014. [DOI] [PubMed] [Google Scholar]