Abstract
Caspases play an essential role in the execution of programmed cell death in metazoans. Although 14 caspases are known in mammals, only a few have been described in other organisms. Here we describe the identification and characterization of a Drosophila caspase, DRONC, that contains an amino terminal caspase recruitment domain. Ectopic expression of DRONC in cultured cells resulted in apoptosis, which was inhibited by the caspase inhibitors p35 and MIHA. DRONC exhibited a substrate specificity similar to mammalian caspase-2. DRONC is ubiquitously expressed in Drosophila embryos during early stages of development. In late third instar larvae, dronc mRNA is dramatically up-regulated in salivary glands and midgut before histolysis of these tissues. Exposure of salivary glands and midgut isolated from second instar larvae to ecdysone resulted in a massive increase in dronc mRNA levels. These results suggest that DRONC is an effector of steroid-mediated apoptosis during insect metamorphosis.
Keywords: apoptosis, caspase activation, caspase recruitment domain, development, metamorphosis
The caspase family of cysteine proteases are integral components of the metazoan cell death apparatus. In mammals 14 caspases have been described so far, some of which play a direct role in apoptosis, whereas others seem primarily involved in the processing and activation of proinflammatory cytokines (1–6). The function of individual caspases in different cell death pathways is just beginning to be understood. In the nematode Caenorhabditis elegans, a single caspase, CED-3, appears to be essential for all developmental cell death as loss-of-function mutants of the ced-3 gene exhibit a complete inhibition of the death of all 131 cells that are programmed to die during development (7). In mice, targeted disruption of caspase-3 and caspase-9 results in profound developmental abnormalities in the brain, largely because of the suppression of developmental neuronal cell death (8–10), whereas caspase-2-deficient mice show an increased number of female germ cells (11). These recent results suggest that some caspases regulate specific pathways of developmental cell death.
Developmentally programmed cell death in Drosophila begins at stage 11–12 of embryogenesis and becomes widespread in many developing tissues soon after (12). Apoptosis also plays a major role in the deletion of obsolete larval tissues, such as midgut and salivary glands, during insect morphogenesis (13). Three caspases, named DCP-1, DREDD/DCP-2, and drICE, have been reported in Drosophila to date (14–17). Loss of function of dcp-1 gene results in larval lethality and melanotic tumors (14). Additionally, DCP-1 is required for Drosophila oogenesis, as dcp-1 mutants show a defect in transfer of nurse cell cytoplasmic contents to developing oocytes (18). dredd mRNA accumulates in embryonic cells undergoing programmed cell death and in nurse cells at a time that coincides with nurse cell death (15). DREDD also has been shown to act downstream of the apoptotic mediators REAPER, GRIM, and HID (15). The precise role of drICE in programmed cell death in Drosophila has not been established, although in vitro antibody depletion experiments suggest that drICE is required for apoptotic activity in the S2 Drosophila cell line (19). These recent studies suggest that specific caspases may mediate tissue- and stage-specific programmed cell death during Drosophila development.
In this paper, we describe the characterization of DRONC, the fourth Drosophila caspase. DRONC is the first Drosophila caspase that carries a caspase recruitment domain (CARD) in its prodomain region. We show that dronc gene expression is induced by ecdysone, the insect hormone that controls metamorphosis. Thus, DRONC is the first steroid-regulated caspase likely to be involved in programmed cell death during insect metamorphosis.
MATERIALS AND METHODS
Identification and Sequencing of dronc cDNA.
DRONC was identified in a tblastn search as a GenBank expressed sequence tag (EST) (accession no. AA949406) containing a short ORF that showed homology to the prodomain of caspase-2. The EST clone was obtained from the Berkeley Drosophila Genome Project and sequenced in full. An additional EST (accession no. AA950690) from the same cluster also was sequenced.
Expression of Recombinant DRONC and Enzyme Assays.
For expression in Escherichia coli a truncated form of DRONC lacking the putative prodomain (residues 1–113) was amplified by PCR and cloned into the NdeI and BamHI restriction sites of plasmid pET32b. Cleared cell lysates from E. coli BL21 cells harboring the pET32b-dronc were prepared and assayed for caspase activity as described (20, 21). Caspase substrates DEVD-7-amino-4-trifluomethyl coumarin (-afc) and YVAD-afc were purchased from Enzyme Systems Products, Livermore, CA. VDVAD-amino-methylcoumaride (-amc) was from California Peptide Research, Napa, CA.
Expression Constructs.
The 1.35-kb dronc ORF was amplified by PCR using Pfu polymerase from the original pOT2 vector and subcloned into pBluescript (SK+). dronc coding sequence was released by HindIII/XbaI digestion and directionally cloned into pCDNA3 expression vector (Invitrogen). Mutation of the catalytic Cys-318 to Gly was accomplished by using the Quickchange method (Stratagene). To generate constructs in which Aequorea victoria green fluorescent protein (GFP) was fused to DRONC, both wild-type and the catalytic Cys mutant of DRONC were amplified by PCR with appropriate primers and cloned into the EcoRI and BamHI sites of pEGFP-N1 (CLONTECH) to generate a construct in which GFP is fused to the C terminus of DRONC. Expression constructs for CrmA, P35, OpIAP, and MIHA have been described (22). The Bcl-2 expression vector was kindly provided by David Vaux (Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia).
Cell Culture and Transient Transfection.
NIH 3T3 cells were maintained in DMEM with 10% FCS. For cell death assays, 2 × 105 cells were plated per 35-mm dish the day before transfection. Expression vectors (2 μg total DNA) were transfected into cells by using the Fugene reagent (Boeringer Mannheim) according to the manufacturer’s protocol. We used 1.5 μg of pCDNA3-dronc mixed with 0.5 μg of pEF-β-gal, a β-galactosidase expression vector (23), or 2 μg of pEGFP-N1-dronc construct. Where necessary, cells were stained for β-galactosidase and scored for apoptotic morphology as described (23). Cells transfected with GFP expression vectors were observed by using a fluorescence microscope (Olympus BH2-RFCA). In some cases DRONC expression vectors were cotransfected with either CrmA, P35, OpIAP, MIHA, or Bcl-2 expression constructs at a ratio of 1:3.
Northern and in Situ mRNA Analysis.
Total RNA from various developmental stages of Drosophila was prepared by using RNAzol B according to the manufacturer’s (Tel-Test, Friendswood, TX) protocol. Approximately 20 μg of total RNA was electrophoresed onto a 2.2 M formaldehyde gel and transferred to Biodyne A nylon membrane (Pall). The blot was probed with 32P-labeled full-length dronc cDNA and exposed to Kodak XAR-5 film. For in situ RNA analysis, an antisense digoxygenin-labeled riboprobe was prepared by using SP6 RNA polymerase from the original pOT2-dronc cDNA clone linearized by EcoRI digestion. The sense riboprobe was prepared by using T7 RNA polymerase from pOT2-dronc linearized with SspI. Digoxygenin labeling was according to the manufacturer’s instructions (Boeringer Mannheim). In situ hybridization to Drosophila embryos and larval tissues were essentially as described (24), with some modifications. Embryos and dissected larval tissues were fixed in 0.1 M Hepes, 50 mM EGTA, 0.01% NP40, 4% formaldehyde, pH 6.9 for 20 min. The proteinase step was omitted. Dissected ovaries from 3-day-old adult females were fixed as described for embryos and treated with 50% ethanol/50% xylene for 30 min, washed in ethanol, then in methanol and finally in PBS with 0.01% Triton X-100 (PBT). Ovaries then were refixed for 25 min in 4% paraformaldehyde and then treated with proteinase K (5 μg/ml) for 8 min at room temperature. After hybridization, nonspecifically bound probe was removed by digestion with RNase A (125 μg/ml in PBT) for 1 hr at 37°C. Hybridization was detected by using the alkaline phosphatase-coupled secondary antibody detection system according to the manufacturer’s instructions (Boeringer Mannheim).
Ecdysone Treatment.
Larval tissues were dissected in Schneider cell medium (GIBCO/BRL) and incubated with or without 1 mM ecdysone (Sigma) in Schneider cell medium for 1 hr at 25°C. Samples then were fixed as described above and in situ hybridization was carried out by using an antisense dronc riboprobe.
RESULTS
Identification of DRONC as a Unique Caspase.
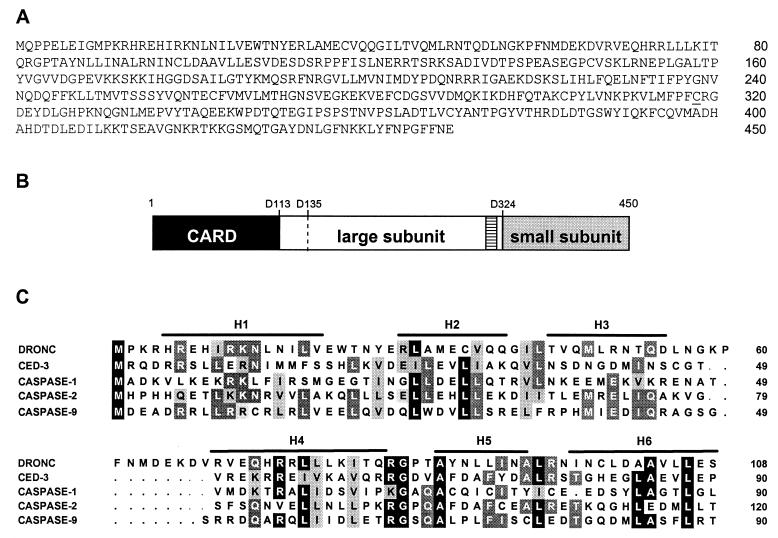
To identify CARD-containing molecules, we searched the GenBank database by using the prodomain of Nedd2 (mouse caspase-2). By using the tblastn program, we identified a Drosophila EST with a small ORF that shares 29% sequence identity with the caspase-2 prodomain over a 88-aa stretch. This EST belongs to a cluster of 11 ESTs in the Berkeley Fly Database. Sequencing of two independent cDNA clones revealed the presence of a complete ORF for a caspase-like molecule that we named DRONC (for Drosophila Nedd2-like caspase). The full-length dronc cDNA sequence of 2,090 nt has been deposited in GenBank. The putative DRONC protein consists of 450 aa residues (Fig. 1A). In vitro translation of mRNA generated from dronc cDNA produced a 50-kDa protein consistent with the expected size (data not shown). Over the entire length of the protein, DRONC shares 25% identity (40% similarity) with caspase-2. The region downstream of the prodomain, which encodes the two subunits of DRONC, shares highest homology (27–28% identity, 44–48% similarity) with all CPP32-like caspases, including caspase-3, -7, -8, -9, -10, and CED-3 (data not shown). Interestingly, DRONC shares <20% identity with the three known Drosophila caspases. The putative prodomain of DRONC contains a CARD (Fig. 1B) that is similar to the CARDs of caspase-1, -2, -9, and CED-3 (Fig. 1C). A unique feature of DRONC is the sequence PFCRG that encompasses the catalytic Cys (Cys-318) residue, which is distinct from the QAC(R/Q/G)(G/E) sequence found in all other known caspases.
Figure 1.
Sequence and structure of DRONC. (A) Deduced amino acid sequence of DRONC. The full-length nucleotide sequence of dronc cDNA comprises 2,090 bp. dronc ORF extends from base 370 to base 1722 (450 aa). The catalytic Cys residue is underlined. (B) Putative structure of DRONC. The potential cleavage sites (D113, D135, and D324) in the DRONC precursor are indicated. The position of pentapeptide sequence PFCRG containing the catalytic Cys residue is shown as a hatched box. (C) An alignment of the CARDs of various caspases. The locations of the six α-helices (H1-H6) are marked. Residues conserved in at least four proteins are marked in black, those conserved in three proteins are highlighted in dark gray and those showing conservative changes are shown in light gray. Note that the DRONC CARD contains an extended linker region between H3 and H4, not present in other CARDs.
Enzymatic Activity and Substrate Specificity of DRONC.
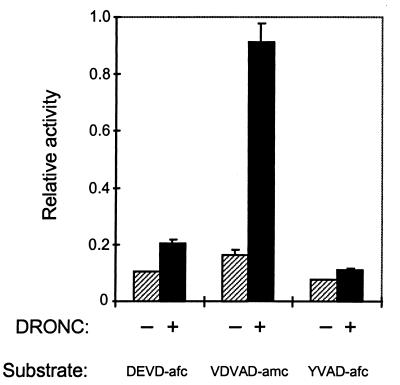
To check whether DRONC is indeed a caspase, we generated recombinant DRONC in E. coli and assessed its proteolytic activity on synthetic fluorogenic peptide substrates. Expression of both the full-length DRONC precursor or truncated DRONC lacking the putative prodomain (residues 1–113) generated enzyme that showed low-level activity on a caspase-3 substrate DEVD-afc (Fig. 2). However, DRONC activity on the caspase-2 pentapeptide substrate VDVAD-amc was 5-fold higher than on DEVD-afc (Fig. 2), suggesting that, similar to caspase-2 (25), the minimum substrate requirement for DRONC includes a P5 residue. Under identical conditions, recombinant DRONC and caspase-2 show approximately similar activities on VDVAD and DEVD substrates (data not shown). No significant cleavage by DRONC of caspase-1 substrate YVAD-afc was observed (Fig. 2).
Figure 2.
Activity of recombinant DRONC on fluorogenic peptide substrates. E. coli lysates containing recombinant DRONC were incubated with various fluorogenic caspase substrates at 37°C for 30 min and release of amc or afc was monitored by a fluorimeter. Equivalent amount of E. coli lysates lacking DRONC were used in control experiments.
Ectopic Expression of DRONC Induces Apoptosis.
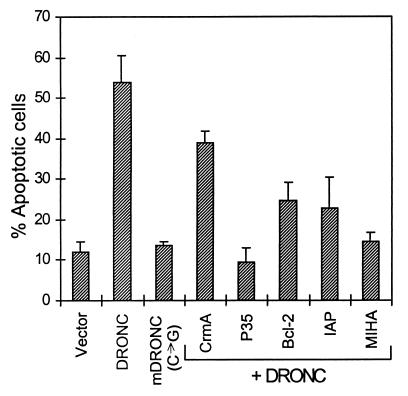
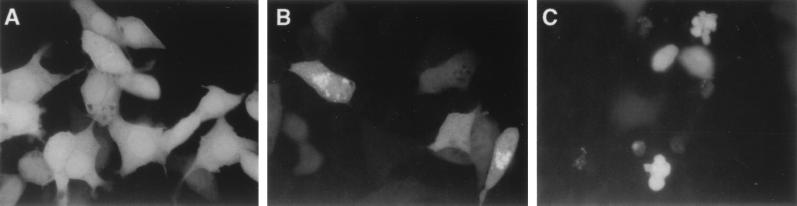
A number of caspases have been shown to induce apoptosis when overexpressed (3, 4, 23, 26). We transiently coexpressed DRONC with β-galactosidase in NIH 3T3 cells. At 48 hr posttransfection approximately 60% of the β-galactosidase positive cells had undergone apoptosis (Fig. 3). DRONC-induced cell death was almost completely abolished by coexpression of baculovirus P35 and inhibited to a lesser extent by MIHA, OpIAP, and Bcl-2 (Fig. 3). CrmA, an inhibitor of caspase-1, was least effective in inhibiting DRONC-induced apoptosis. A substitution mutation of the catalytic Cys-318 to Gly completely abolished the cell-killing activity of DRONC, suggesting that cysteine protease activity was responsible for the apoptotic function of DRONC (Fig. 3). We also monitored the localization of DRONC protein in transfected cells by using DRONC-GFP fusion constructs. Fusion of GFP to the carboxyl terminal of DRONC did not affect its cell-killing activity (Fig. 4, data not shown). At 24 hr, when most of the transfected cells appeared morphologically normal, DRONC was mostly localized in the cytoplasmic fraction of cells. In some cells, DRONC protein appeared to be concentrated asymmetrically near the cellular nucleus (Fig. 4B), possibly associated with some subcellular structures. Staining of transfected cells with mitochondrial markers suggested that DRONC did not localize to mitochondria (data not shown). At 48 hr after transfection, DRONC-GFP protein was uniformly distributed in apoptotic cells (Fig. 4C).
Figure 3.
DRONC induces apoptosis in transfected cells. Various expression constructs were cotransfected with pEF-β-galactosidase into NIH 3T3 cells by lipofection. At 48 hr posttransfection cells were fixed and stained with 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-gal), and blue cells were observed for apoptosis. Bars represent apoptotic cells as % of total β-galactosidase-positive cells ±SEM. At least 400 blue cells were scored for each dish. The data shown were derived from three independent experiments.
Figure 4.
Cellular localization of GFP-DRONC protein. Expression vectors containg (A) GFP or (B and C) GFP-DRONC fusion were transfected into NIH 3T3 cells. At (A and B) 24 hr or (C) 48 hr posttransfection, cells were fixed and GFP-positive cells were examined by using a fluorescence microscope and photographed.
Expression of dronc During Drosophila Development.
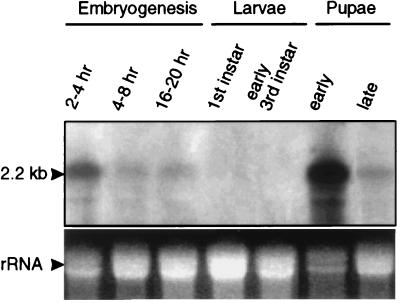
RNA blot analysis of dronc detected a 2.2-kb transcript, consistent with the size of cDNA, at all embryonic stages (Fig. 5). Between first and third instar larva stages dronc transcript was barely detectable, however, as discussed below, from late third instar larva stage, dronc mRNA was remarkably up-regulated. We further examined dronc expression by in situ hybridization to Drosophila embryos and larval tissues by using a digoxigenin-labeled antisense mRNA probe (Fig. 6). Dronc is expressed highly in stage 1–4 syncitial embryos (Fig. 6A). Because zygotic expression does not begin before stage 5 (27), this early expression of DRONC represents maternally derived mRNA. In stage 8 cellularized embyros, dronc mRNA is ubiquitously expressed (Fig. 6B), but as development proceeds expression levels generally are reduced (Fig. 6C). Expression of the Drosophila caspase dredd has been shown to be up-regulated in embryonic cells undergoing programmed cell death (15). However, unlike dredd, dronc expression was not up-regulated in apoptotic cells in embryos (Fig. 6 A–C).
Figure 5.
Expression of dronc mRNA during Drosophila development. Approximately 20 μg of total RNA isolated from various developmental stages was analyzed by Northern blotting. (Lower) A portion of the ethidium bromide stained gel before transfer to membrane.
Figure 6.
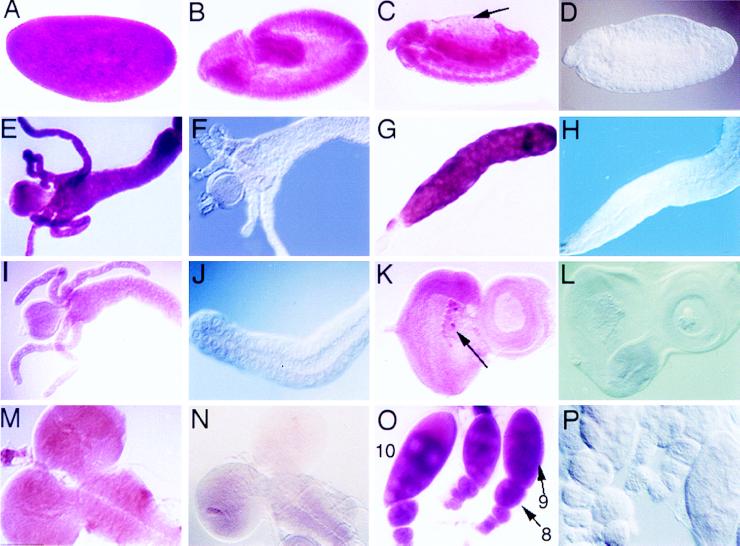
In situ analysis of dronc mRNA expression during Drosophila development. Expression of dronc transcript was detected by in situ hybridization with a digoxigenin-labeled antisense mRNA probe. (A) A stage 3 syncitial embyro showing high levels of dronc expression. (B) A stage 8 embryo with uniform dronc expression throughout. (C) By stage 13 dronc expression is reduced throughout. Arrow indicates amnioserosa. (D) A stage 13 embryo hybridized with the dronc sense control probe shows no background staining. (E) A late third instar larval midgut with high dronc expression. (F) A late third instar larval midgut hybridised with the sense control riboprobe. (G) A late third instar larval salivary gland showing high dronc expression. (H) dronc sense control on late third instar salivary gland. (I) A late second instar midgut showing low levels of dronc expression. (J) A second instar salivary gland tissue showing no dronc expression. (K) A late third instar eye imaginal disc shows ubiquitous low level of dronc mRNA and higher staining in a subset of blood cells associated with the eye disc (arrow). (L) dronc sense control on late third instar eye imaginal disc. (M) Brain lobes from late second instar larva showing ubiquitous low levels of dronc mRNA. (N) dronc sense control on late second instar brain lobes shows weak background staining. (O) Adult egg chambers showing high expression of dronc particularly at the later stages of oogenesis. The stages of oogenesis for each chamber are indicated. (P) dronc sense riboprobe control on adult egg chambers.
dronc expression was further examined in second and third instar larval tissues (Fig. 6 E–N). High levels of dronc expression was observed in midgut and salivary glands from late third instar larvae (Fig. 6 E and G), but not from second instar larvae (Fig. 6 I and J). Massive apoptosis of midgut tissues occurs at the onset of pupariation although small numbers of apoptotic cells can be detected in the gastric caeca in late second instar larvae, whereas apoptosis of the salivary glands begins 13.5 hr after pupariation (13). Thus, high expression of dronc precedes apoptosis in these tissues. Low levels of dronc expression were observed throughout third instar larval eye discs and brain lobes (Fig. 6 K and M), which contain apoptotic cells at this stage (28). However, up-regulation of dronc expression was not observed in eye disc or brain lobe cells that should be undergoing apoptosis (data not shown). The cells strongly staining with dronc in the eye disc are blood cells (Fig. 6K), which often associate with imaginal discs. High expression of dronc in a subset of blood cells is consistent with programmed cell death also occurring in these cells. We also checked dronc expression in ovaries (Fig. 6 O and P). During oogenesis in adult flies, nurse cells undergo apoptosis in stage 12 oocytes, which is required for the deposition of nurse cell cytoplasm into the oocytes (29). We observed strong dronc expression in egg chambers after stage 10, but also in earlier stages (Fig. 6O), indicating that dronc expression precedes apoptosis during oogenesis.
dronc mRNA Expression Is Induced by Ecdysone.
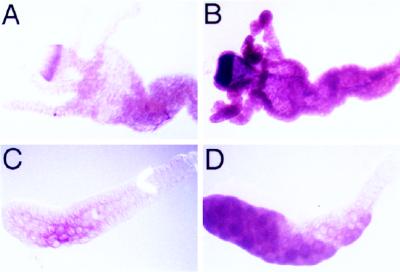
The steroid hormone ecdysone has been shown to mediate apoptosis of larval tissues during pupariation (13). To investigate whether ecdysone also induces expression of dronc, we examined the levels of dronc mRNA after the addition of ecdysone to second instar larval midgut and salivary gland tissues, which normally show only low levels of dronc mRNA (Fig. 7). After 1 hr exposure to ecdysone there was a several-fold increase in dronc mRNA levels in the early second instar larval midgut tissue (Fig. 7 A and B), indicating that ecdysone can induce dronc expression in the midgut. However, salivary glands from early second instar larvae did not show dronc induction after ecdysone treatment (data not shown). Because salivary glands normally undergo apoptosis later than midgut tissues, it was possible that the failure of ecdysone to induce dronc in salivary glands was the result of the absence of a developmentally controlled factor required for ecdysone-induced gene expression. For this reason we then examined whether ecdysone could induce dronc expression in salivary glands at a later developmental stage. Salivary glands from late second instar larvae, which normally only express very low levels of dronc, were found to strongly express dronc 1 hr after ecdysone treatment (Fig. 7 C and D). Thus, ecdysone induces dronc expression in both midgut and salivary gland tissues.
Figure 7.
dronc mRNA is up-regulated by ecdysone. An early second instar midgut incubated for 1 hr (A) without or (B) with ecdysone. A late second instar salivary gland incubated for 1 hr (C) without or (D) with ecdysone. Note massive induction of dronc expression on ecdysone treatment in both larval midgut and salivary gland. The sense control riboprobe did not show any staining on tissues with or without ecdysone treatment (data not shown).
Processing and Activation of DRONC.
Consistent with high expression of dronc, cell extracts prepared from late third instar larvae efficiently processed in vitro-translated DRONC into subunits of approximately 20 kDa and 14 kDa (data not shown). We then measured caspase activity in extracts prepared from larvae and pupae on VDVAD and DEVD substrates. There was at least a 5-fold increase in VDVAD cleavage activity in late third instar larvae as compared with second instar larvae, whereas, DEVD cleavage activity, which was comparable to VDVAD cleavage activity in second instar larvae, only increased by approximately 1.5-fold (data not shown). These results suggest that increased VDVAD cleavage activity in late third instar larvae may be attributable to increased levels of DRONC. In 16- to 18-hr pupae, both VDVAD and DEVD cleavage activities were reduced to about half of those observed at second instar larval stage (data not shown).
DISCUSSION
Caspases belong to two distinct classes. Caspases with long N-terminal prodomains (the class I caspases) are believed to be directly recruited to specific death complexes through the death effector domains (DED) or the CARDs present in their prodomain regions (3, 4, 26). This recruitment facilitates oligomerization and autoactivation of class I caspases. The class II caspases have very short or no prodomain and hence they lack the ability to be recruited to specific death complexes. It is thought that class II caspases require cleavage by upstream class I caspases for their activation. Among proapoptotic mammalian caspases, caspase-2 and -9 contain CARD domains, whereas caspase-8 and -10 carry two copies of DEDs in their prodomain region (3, 4, 26). The C. elegans CED-3 caspase also contains a CARD domain, whereas Drosophila DREDD contains two DEDs (3, 4, 15, 16, 26). The DED-containing caspases, such as caspase-8 and -10, are activated on adaptor-mediated recruitment to death receptors, whereas the CARD containing caspase-9 requires APAF-1 (the mammalian homologue of CED-4), cytochrome c released from mitochondria, and dATP for its activation (3, 4, 26, 30). The presence of a DED containing caspase DREDD in Drosophila implies that a death receptor-mediated pathway may exist in the fly (15, 16). Our identification of DRONC as a CARD-containing Drosophila caspase suggests that a pathway similar to CED-3/caspase-9 also may exist in Drosophila. This implies that CARD-containing adaptors such as CED-4/APAF-1, might be required for DRONC activation. No CED-4 homologues have been reported in Drosophila to date; however, it now might be possible to identify such proteins by using DRONC as an interacting partner.
An unusual feature of DRONC is the sequence PFCRG surrounding the catalytic Cys residue. All known mammalian caspases have a consensus QAC(R/Q/G)G sequence (1–6), whereas Drosophila DCP-2/DREDD differs somewhat with a QACQE sequence (15, 16). However, in all caspases except DRONC, the QAC sequence is completely conserved. The variation in the sequence may reflect unique substrate specificity of DRONC. Interestingly, recombinant DRONC shows little or no activity on caspase-1 and caspase-3 substrates, but has appreciable activity on the pentapeptide caspase-2 substrate (Fig. 2). This suggests that, like caspase-2 (25), DRONC requires a minimum of a pentapeptide sequence as a substrate. It is, however, unclear at present, whether the caspase-2 substrate is the optimal substrate for DRONC.
Insect metamorphosis is controlled by the production of waves of the steroid hormone, ecdysone, which regulates the deletion of obsolete larval tissues and their replacement by tissues of the adult animal (13, 31). A large transient increase in ecdysone levels occurs before puparium formation and prepupal development, and again 10 hr later just before pupation (32). Recent studies have shown that larval midgut and salivary glands undergo ecdysone-induced programmed cell death during prepupal and early pupal development (13). In late third instar larvae, VDVAD cleavage activity was significantly higher than in second instar larvae. This increased VDVAD cleavage activity is likely to be attributable to DRONC activity consistent with its high expression in third instar larvae. Our demonstration that dronc mRNA can be dramatically up-regulated by ecdysone suggests that this caspase may be the main effector in mediating programmed cell death in larval midgut and salivary glands. Our data suggest the possible transcription regulation of a caspase gene by a steroid hormone. This up-regulation may be the result of a direct interaction between the ecdysone receptor complex and the ecdysone response elements in the dronc promoter or indirectly through an ecdysone-induced transcription factor. Characterization of the dronc promoter region will provide information on whether dronc is directly regulated by ecdysone. Expression of dronc in other embryonic, larval, and adult tissues (Fig. 6) suggests that DRONC also may function in other cell death pathways. Examination of dronc mutant phenotypes will further establish the function of DRONC in developmentally programmed cell death in Drosophila.
Acknowledgments
We thank David Vaux and Vishva Dixit for the generous gift of cDNA clones. This work was supported by the Wellcome Trust and the Australian Research Council. L.D. is the recipient of a Commonwealth Postgraduate Award. S.K. is a Wellcome Senior Fellow. H.R. was supported by an Australian Research Council Fellowship and an Australian Research Council Special Investigator Award.
ABBREVIATIONS
- GFP
green fluorescent protein
- CARD
caspase recruitment domain
- EST
expressed sequence tag
- DED
death effector domains
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF104357).
References
- 1.Kumar S. Trends Biochem Sci. 1995;20:198–202. doi: 10.1016/s0968-0004(00)89007-6. [DOI] [PubMed] [Google Scholar]
- 2.Dorstyn L, Kinoshita M, Kumar S. In: Apoptosis: Mechanisms and Role in Disease. Kumar S, editor. Heidelberg: Springer; 1998. pp. 1–24. [Google Scholar]
- 3.Thornberry N A, Lazebnik Y. Science. 1998;281:312–316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 4.Cryns V, Yuan J. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- 5.Humke E W, Ni J, Dixit V M. J Biol Chem. 1998;273:15702–15707. doi: 10.1074/jbc.273.25.15702. [DOI] [PubMed] [Google Scholar]
- 6.Hu S, Snipas S J, Vincenz C, Salvesen G, Dixit V M. J Biol Chem. 1998;273:29648–29653. doi: 10.1074/jbc.273.45.29648. [DOI] [PubMed] [Google Scholar]
- 7.Hengartner M O. In: C. elegans II. Riddle D L, Blumenthal T, Meyer B J, Priess J R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. pp. 383–415. [Google Scholar]
- 8.Kuida K, Zheng T S, Na S, Kuan C-Y, Yang D, Karasuyama H, Rakic P, Flavell R A. Nature (London) 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 9.Kuida K, Haydar T F, Kuan C-Y, Gu Y, Taya C, Karasuyama H, Su M S-S, Rakic P, Flavell R A. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 10.Hakem R, Hakem A, Duncan G S, Henderson J T, Woo M, Soengas M S, Elia A, de la Pompa J L, Kagi D, Khoo W, et al. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 11.Bergeron L, Perez G I, Macdonald G, Shi L, Sun Y, Juriskova A, Vormuza S, Latham K E, Flaws J A, Salter J C, et al. Genes Dev. 1998;12:1304–1314. doi: 10.1101/gad.12.9.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrams J M, White K, Fessler L I, Steller H. Development (Cambridge, UK) 1993;117:29–43. doi: 10.1242/dev.117.1.29. [DOI] [PubMed] [Google Scholar]
- 13.Jiang C, Baehrecke E H, Thummel C S. Development (Cambridge, UK) 1997;124:4673–4683. doi: 10.1242/dev.124.22.4673. [DOI] [PubMed] [Google Scholar]
- 14.Song Z, McCall K, Steller H. Science. 1997;275:536–540. doi: 10.1126/science.275.5299.536. [DOI] [PubMed] [Google Scholar]
- 15.Chen P, Rodriguez A, Erskine R, Thach T, Abrams J M. Dev Biol. 1998;201:202–216. doi: 10.1006/dbio.1998.9000. [DOI] [PubMed] [Google Scholar]
- 16.Inohara N, Koseki T, Hu Y, Chen S, Nunez G. Proc Natl Acad Sci USA. 1997;94:10717–10722. doi: 10.1073/pnas.94.20.10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser A G, Evan G I. EMBO J. 1997;16:2805–2813. doi: 10.1093/emboj/16.10.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCall K, Steller H. Science. 1998;279:230–234. doi: 10.1126/science.279.5348.230. [DOI] [PubMed] [Google Scholar]
- 19.Fraser A G, McCarthy N J, Evan G I. EMBO J. 1997;16:6192–6199. doi: 10.1093/emboj/16.20.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvey N L, Butt A J, Kumar S. J Biol Chem. 1997;272:13134–13139. doi: 10.1074/jbc.272.20.13134. [DOI] [PubMed] [Google Scholar]
- 21.Butt A J, Harvey N L, Parasivam G, Kumar S. J Biol Chem. 1998;273:6763–6768. doi: 10.1074/jbc.273.12.6763. [DOI] [PubMed] [Google Scholar]
- 22.Dorstyn L, Kumar S. Cell Death Diff. 1997;4:570–579. doi: 10.1038/sj.cdd.4400281. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S, Kinoshita M, Noda M, Copeland N G, Jenkins N A. Genes Dev. 1994;8:1613–1626. doi: 10.1101/gad.8.14.1613. [DOI] [PubMed] [Google Scholar]
- 24.Lehner C F, O’Farrell P H. Cell. 1989;56:957–968. doi: 10.1016/0092-8674(89)90629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talanian R V, Quinlan C, Trautz S, Hackett M C, Mankovich J A, Banach D, Ghayur T, Brady K D, Wong W W. J Biol Chem. 1997;272:9677–9682. doi: 10.1074/jbc.272.15.9677. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Colussi P A. Trends Biochem Sci. 1999;24:1–4. doi: 10.1016/s0968-0004(98)01332-2. [DOI] [PubMed] [Google Scholar]
- 27.Edgar B A, Schubiger G. Cell. 1986;44:871–877. doi: 10.1016/0092-8674(86)90009-7. [DOI] [PubMed] [Google Scholar]
- 28.Wolff T, Ready D F. Development (Cambridge, UK) 1991;113:825–839. doi: 10.1242/dev.113.3.825. [DOI] [PubMed] [Google Scholar]
- 29.Foley K, Cooley L. Development (Cambridge, UK) 1998;125:1075–1082. doi: 10.1242/dev.125.6.1075. [DOI] [PubMed] [Google Scholar]
- 30.Li P, Nijhawan D, Budhihardjo I, Srinivasula S, Ahmad M, Alnemri E S, Wang X. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 31.Thummel C S. Trends Genet. 1996;12:306–310. doi: 10.1016/0168-9525(96)10032-9. [DOI] [PubMed] [Google Scholar]
- 32.Riddifird L M. In: The Development of Drosophila melanogaster. Bates M, Arias A M, editors. II. Plainview, NY: Cold Spring Harbor Lab. Press; 1993. pp. 899–939. [Google Scholar]