Abstract
The human somatic angiotensin converting enzyme (ACE) contains two homologous domains, each bearing a zinc-dependent active site. All of the synthetic inhibitors of this enzyme used in clinical applications interact with these two active sites to a similar extent. Recently, several lines of evidence have suggested that the N-terminal active site of ACE might be involved in specific hydrolysis of some important physiological substrates, like Acetyl-Seryl-Aspartyl-Lysyl-Proline, a negative regulator of hematopoietic stem cell differentiation and proliferation. These findings have stimulated studies aimed at identifying new ACE inhibitors able to block only one of the two active sites of this enzyme. By screening phosphinic peptide libraries, we discovered a phosphinic peptide Ac-Asp-(L)Pheψ(PO2-CH2)(L)Ala-Ala-NH2, called RXP 407, which is able to differentiate the two ACE active sites, with a dissociation constant three orders of magnitude lower for the N-domain of the enzyme. The usefulness of a combinatorial chemistry approach to develop new lead structures is underscored by the unusual chemical structure of RXP 407, as compared with classical ACE inhibitors. As a highly potent and selective inhibitor of the N-terminal active site of wild ACE (Ki = 12 nM), RXP 407, which is metabolically stable in vivo, may lead to a new generation of ACE inhibitors able to block in vivo only a subset of the different functions regulated by ACE.
Angiotensin converting enzyme (ACE) (peptidyl dipeptidase A, EC 3.4.15.1) is a key player in the regulation of blood pressure and cardiovascular function. ACE inhibitors efficiently block the renin-angiotensin cascade by inhibiting angiotensin II formation and are widely used for the treatment of patients with high blood pressure, cardiac failure, and diabetic nephropathy (1). cDNA cloning has been used to determine the primary structure of human endothelial somatic ACE, revealing the unexpected presence of two homologous domains (2). Each domain contains an active site, characterized by the presence of a zinc-metallopeptidase consensus sequence and whose function was demonstrated by site-directed mutagenesis (3).
The presence of two active sites in ACE has stimulated many attempts to establish whether they differ in function (4–8). The two ACE active sites hydrolyze with approximately the same catalytic efficiency angiotensin I and bradykinin, the two main physiological substrates of ACE involved in the control of blood pressure. The two active sites, however, differ in their sensitivity to chloride activation of the hydrolysis of some substrates and in their relative sensitivity to some ACE inhibitors (5, 6, 9, 10). Captopril is a 15-fold more potent inhibitor of the N-domain than of the C-domain (7) whereas Bz-PheΨ(CO-CH2)Gly-Pro is ≈40× more potent toward the C-domain (8). The presence of two functional active sites led to the hypothesis that there might be other substrates specifically cleaved by either domain. Indeed, ACE is known to hydrolyze many substrates besides angiotensin I and bradykinin. Angiotensin 1–7 (Asp1-Pro7 angiotensin) is preferentially cleaved by the N-domain but at the same time inhibits the angiotensin I hydrolysis by the C-domain (8). Another physiological and ACE domain-specific substrate has been recently discovered: Ac-Ser-Asp-Lys-Proline (AcSDKP), a negative regulatory factor of hematopoietic stem cell differentiation and proliferation (11), which is cleaved by ACE both in vitro (12) and in vivo (13) and, interestingly, 50× faster in vitro by the N-terminal active site of ACE, compared with the C-terminal active site (hereafter called N- and C-domain) (12). Therefore, the physiological functions of ACE are not limited to its cardiovascular role, and ACE also may be involved in hematopoietic stem cell regulation by constantly degrading AcSDKP (13).
The demonstration that a physiological substrate like AcSDKP is mostly cleaved by one ACE active site makes it imperative to develop potential inhibitors of ACE able to block only a single active site of this enzyme. Such highly selective inhibitors of ACE are not available because most known inhibitors of ACE exhibit only a modest selectivity (7, 8). To this end, we screened different phosphinic peptide libraries, using a systematic approach previously described (14, 15). Such phosphinic peptides were chosen because they are highly potent inhibitors of several zinc-metallopeptidases (14–18) and are good analogues of the substrate in the transition-state for this class of enzyme (19). They were screened on wild-type ACE and ACE mutants in which either the N- or the C-domain of ACE had been inactivated, as reported before (3). This report describes a potent ACE inhibitor able to discriminate between the N- and C-domains of ACE. This inhibitor, called RXP 407, displays a Ki value of 12 nM for the ACE N-domain and is three orders of magnitude less potent at the C-domain.
MATERIALS AND METHODS
Fluorenylmethoxycarbonyl amino acid derivatives, rink amide resin, and 2-chlorotrityl resin were from Nova Biochem. 7-methoxycoumarin-2-acetic acid (McaOH) and solvents were from Aldrich.
Chemistry.
Solid-phase peptide synthesis was performed on a model 357 Advanced ChemTech multiple peptide synthesizer by using the fluorenylmethoxycarbonyl-strategy. Peptides with a C-terminal amide group were synthesized on a rink amide resin. For C-terminal carboxylate peptides, the 2-chlorotrityl resin was used (20). The protocol used to synthesize the phosphinic peptides and the mixture of phosphinic peptides was similar to that described (14, 15). Optically pure (S) or (R) phenylalaninephosphinic amino acids were prepared according to Baylis et al. (21). The synthesis of the corresponding phosphinic blocks [fluorenylmethoxycarbonyl(S)PheΨ[PO(OAd)-CH2]Ala (Ad, adamantye; Ψ indicates that the peptide bond has been modified, and the formula of the group that has replaced the peptide Bond is in parentheses) and fluorenylmethoxycarbonyl(R)PheΨ[PO(OAd)-CH2]Ala] was similar to that described (22). Mca-Ala-Ser-Asp-Lys-DpaOH (Dpa, N3(2,4-dinitrophenyl) L-2,3-diamino propionyl) was synthesized according to Knight et al. (23).
All single peptides were purified by preparative reverse-phase HPLC (Vydac, Hesperia, CA, 218TP1022 column). All peptides were recovered by lyophilization. Peptide purities were checked by analytical HPLC (Vydac, 218TP104 column) and mass spectroscopy.
Enzymes.
Human wild-type somatic ACE and two ACE mutants, containing only one functional active site, were obtained through stable expression in Chinese hamster ovary cells transfected with appropriate ACE cDNA. The two ACE mutants were full-length enzymes with either the N- or the C-domain catalytic site inactivated by substitution of the two zinc-binding histidyl residues by lysine residues. The construction of the ACE cDNAs and their expression in Chinese hamster ovary cells, as well as the purification of the corresponding proteins, have been described (3).
Enzyme Assays and Inhibition Studies.
Assays carried out with Mca-Ala-Ser-Asp-Lys-DpaOH substrate were performed at 25°C in 50 mM Hepes (pH 6.8), 200 mM NaCl, and 10 μM ZnCl2. Continuous assays were performed by recording the fluorescence increase at 390 nm (λex = 340 nm), induced by the cleavage of the Mca-substrate (S = Km, 40 μM) by ACE, with a Fluorolite 1000 microassay fluorometer (Dynatech). The experiments were carried out in 96-well microassay plates. Assays and inhibition studies performed with angiotensin I, and Ac-Ser-Asp-Lys-Pro substrates were based on HPLC experiments, as described (7). For these substrates, assays were performed at 25°C in 50 mM Hepes [pH 7.5 (angiotensin I) or pH 7 (Ac-SDKP)], 50 mM NaCl, 10 and μM ZnCl2. For inhibition studies, inhibitors or mixtures of inhibitors were preincubated with the enzyme 90 min before the addition of the substrate.
Simulations.
The inhibition profile of ACE by the inhibitor RXP 407 was simulated by using the program dynafit from P. Kuzmic (24). This program represents the system under consideration by a set of kinetic equations that correspond to substrate cleavage and inhibitor binding. The enzyme was modeled as two independent sites, each site being able to cleave the same substrate, with specific kinetic parameters. The six equilibria used to model ACE are shown in Scheme 1.  Simulations using dynafit require explicit definition of all kinetic constants. For all equilibria, the kon value was set at 108⋅M−1⋅sec−1. The koff values for the Mca-substrate hydrolysis (koff1, koff2, equations 1 and 2 in Scheme 1) were set according to the Michaelis model [koff1,2 = (kon × Km1, 2) + kcat1,2]. Michaelis constants (Km1 = 37 μM, Km2 = 42 μM), corresponding to the degradation of the Mca-substrate, were determined experimentally for the N-domain and C-domain. The dissociation constants Ki of RXP 407 were 12 nM and 5 μM for sites 1 and site 2, respectively. The corresponding kinetic constants (koff3, 4) were calculated according to koff3,4 = kon × Ki1,2. Three inhibition profiles, corresponding to three different values of kcat2, were simulated. For each profile, 12 simulations were performed at inhibitor concentrations I = 0.001, 0.005, 0.010, 0.05, 0.10, 0.50, 1, 5, 10, 100, and 500 μM. For these simulations, substrate and enzyme concentrations were set at 20 μM and 15 nM, respectively. All other values for the kinetic constants appear in the Scheme 1.
Simulations using dynafit require explicit definition of all kinetic constants. For all equilibria, the kon value was set at 108⋅M−1⋅sec−1. The koff values for the Mca-substrate hydrolysis (koff1, koff2, equations 1 and 2 in Scheme 1) were set according to the Michaelis model [koff1,2 = (kon × Km1, 2) + kcat1,2]. Michaelis constants (Km1 = 37 μM, Km2 = 42 μM), corresponding to the degradation of the Mca-substrate, were determined experimentally for the N-domain and C-domain. The dissociation constants Ki of RXP 407 were 12 nM and 5 μM for sites 1 and site 2, respectively. The corresponding kinetic constants (koff3, 4) were calculated according to koff3,4 = kon × Ki1,2. Three inhibition profiles, corresponding to three different values of kcat2, were simulated. For each profile, 12 simulations were performed at inhibitor concentrations I = 0.001, 0.005, 0.010, 0.05, 0.10, 0.50, 1, 5, 10, 100, and 500 μM. For these simulations, substrate and enzyme concentrations were set at 20 μM and 15 nM, respectively. All other values for the kinetic constants appear in the Scheme 1.
Disposition of RXP 407 in Vivo.
Tritiated RXP 407 (4.62 mCi/μmol) in PBS solution was i.v administrated at a dose of 130 μg/kg to WKY rats. Blood samples were taken at 0.1, 0.25, 0.5, 1, 2, 4, 8, 16, and 24 h. The total radioactivity contained in the plasma samples was determined by liquid scintillation counting with a Tricard 1990 CA β-analyzer (Packard), after incorporation of scintillation mixture (Pico-Fluo, Packard). The determination of metabolites in plasma, urine, and feces was performed by HPLC on a waters apparatus equipped with a Floone radiodetector (Packard).
RESULTS
Identification of RXP 407 from Phosphinic Peptide Libraries.
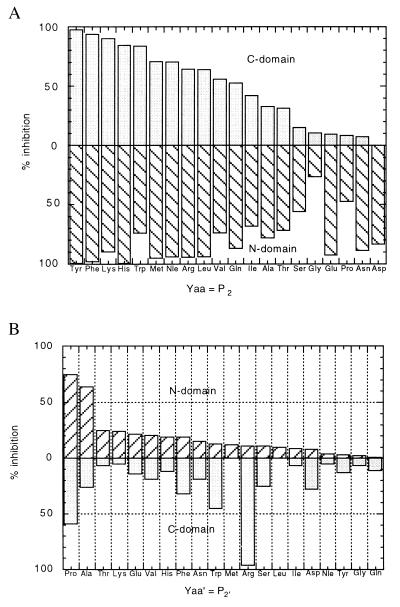
To facilitate the screening of inhibitors, a quenched-fluorogenic substrate of ACE (Mca-Ala-Ser-Asp-Lys-Dpa), partially based on the sequence of Ac-Ser-Asp-Lys-Pro, was developed. The cleavage of this substrate between aspartic and lysine by ACE produces a marked fluorescence signal that can be used to monitor the activity of many ACE samples in microassay plates. Among three mixtures of phosphinic peptides of general formula Ac-Pheψ(PO2-CH2)Ala-Yaa′-Zaa′-NH2, Ac-Yaa-Pheψ(PO2-CH2)Ala-Yaa′-NH2, and Ac-Zaa-Yaa-Pheψ(PO2-CH2)Ala-NH2, potent inhibition of ACE was only observed with the second mixture (data not shown and Fig. 1a). To check the presence of selective inhibitors in this mixture, 20 different peptide mixtures, of general formula Ac-Yaa-Pheψ(PO2-CH2)Ala-Yaa′-NH2, each containing a single amino acid in the Yaa position, with a mixture of 20 different amino acids in the Yaa′ position, were synthesized, and their activities were evaluated both for the N- and C-domain. The data reported in Fig. 2a suggest that the S2 subsite of both the N- and C-domains of ACE exhibits rather broad selectivity, as several residues are accepted in the inhibitor P2 position. Of interest, the mixture of general formula Ac-Asp-Pheψ(PO2-CH2)Ala-Yaa′-NH2 markedly inhibited the N-domain of ACE but appeared inactive on the C-domain. This observation led us to prepare 20 different phosphinic peptides of general formula, Ac-Asp-Pheψ(PO2-Ch2)Ala-Yaa′-NH2, with Yaa′ representing 20 different amino acids. These inhibitors were tested at concentrations of 100 nM and 5 μM for the N- and C-domains, respectively, reflecting the selectivity of these inhibitors for the N-domain (Fig. 2b). The molecules with a proline or an alanine residue in the P2′ position were the most potent inhibitors of this domain.
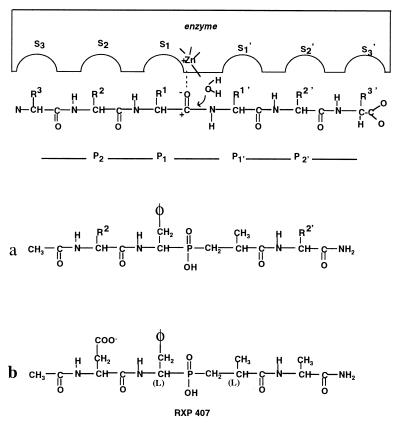
Figure 1.
Schematic model of the ACE active site showing the relationships between the enzymes subsites (… S2, S1, S1′, S2′… ) and the substrate/phosphinic peptide inhibitor residues (… P2, P1, P1′, P2′… ).
Figure 2.
(a) Influence of the P2 position on the inhibition of the C-domain and N-domain by inhibitor mixtures of the general formula Ac-Yaa-PheΨ(PO2-CH2)Ala-Yaa′-NH2 (Yaa′ contains a mixture of 20 different amino acids). The concentration of phosphinic peptides was 200 nM. The concentration of the Mca-Ala-Ser-Asp-Lys-Dpa substrate was 25 μM. (b) Influence of the P2′ position on the inhibition of the C-domain and the N-domain by inhibitors of the general formula Ac-Asp-PheΨ(PO2-CH2)Ala-Yaa′-NH2. The concentration of phosphinic peptides was 5 μM for the C-domain and 100 nM for the N-domain. The concentration of the Mca-Ala-Ser-Asp-Lys-Dpa substrate was 25 μM.
Structural Determinants of RXP 407.
Because of the presence of both S and R configurations of the pseudophenylalanine and pseudoalanine in the phosphinic block, each peptide synthesis afforded a mixture of four diastereoisomers. To establish the potency of each diastereoisomer, Ac-Asp-(L)Pheψ(PO2-CH2)Ala-Ala-NH2 and Ac-Asp-(D)Pheψ(PO2-CH2)Ala-Ala-NH2 peptides were synthesized. Only the preparation containing a (L) pseudophenylalanine amino phosphinic residue was able to inhibit the N-domain. This sample, containing the diastereoisomers Ac-Asp-(L)Pheψ(PO2-CH2)(L)Ala-Ala-NH2 and Ac-Asp-(L)Pheψ(PO2-CH2)(D)Ala-Ala-NH2, was resolved into two fractions by means of reverse-phase HPLC. Only one fraction actively inhibited the N-domain. Because of the recognized preference of ACE for inhibitors with a pseudo (L) amino in their P1′ position (25), we hypothesized that the active fraction contains the following molecule: Ac-Asp-(L)Pheψ(PO2-CH2)(L)Ala-Ala-NH2. This compound, called RXP 407 (Fig. 1b), has a Ki value of 12 nM for the N-domain and is more than three orders of magnitude less potent at the C-domain (Table 1).
Table 1.
Substrate, Mca-Ala-Ser-Asp-Lys-Dpa (pH 6.8), 50 mM Hepes, and 200 mM NaCl, at 25°C
| ki, ACE-N | ki, ACE-C | |
|---|---|---|
| RXP 407, Ac-Asp-(l)Phe(PO2CH2)(l)Ala-Ala-NH2 | 12 nM | 25 μM |
| Compound I, Ac-Asp-Phe(PO2CH2)Ala-Ala-NH2 | 25 nM | 25 μM |
| Compound II, Ac-Asp-Phe(PO2CH2)Ala-AlaOH | 2 nM | 7 nM |
| Compound III, H2N-Asp-Phe(PO2CH2)Ala-Ala-NH2 | 5 nM | 800 nM |
| Compound IV, Ac-Ala-Phe-(PO2CH2)Ala-Ala-NH2 | 15 nM | 200 nM |
Structure–function relationships [compounds II, III, and IV, as compared with I (Table 1)] demonstrate that a C terminus carboxamide group in RXP 407 plays a crucial role in the selectivity of this inhibitor. Furthermore, the presence of an N-acetyl group, as well as an aspartic side-chain in the inhibitor P2 position, also contributes to the selectivity of RXP 407.
Inhibition of ACE by RXP 407.
Mca-Ala-Ser-Asp-Lys-Dpa substrate. The inhibition profiles of the N-domain, ACE, and the C-domain, respectively, by RXP 407, using Mca-Ala-Ser-Asp-Lys-Dpa as substrate, are reported in Fig. 3a. The curves corresponding to the titration of the N- and the C-domains show only one point of inflexion whereas the curve of ACE exhibits two inflexion points. Taking into consideration the different potencies of RXP 407 for the two active sites of ACE, the presence of two titration domains in the inhibition profile of ACE could be expected for an enzyme containing two active sites. However, it can seen from Fig. 3a that the curve corresponding to ACE inhibition is not simply the sum of the curves obtained for the N- and C-domains. In fact, in addition to the inhibitor selectivity exhibited by the two active sites of ACE, the inhibition profile of ACE also will depend on the specificity of each active site in cleaving the substrate used for the titration experiment. To develop this intuitive interpretation more formally, a theoretical model of ACE inhibition was developed to perform simulations.
Figure 3.
(a) Inhibition profiles of the N-domain, ACE, and the C-domain by RXP 407. The substrate used was Mca-Ala-Ser-Asp-Lys-Dpa (25 μM). (b) Simulations of the inhibition profiles of the N-domain, ACE, and the C-domain ACE by RXP 407. S = 10, S = 1, and S = 0.1 refer to a substrate being cleaved respectively 10× better by the N-domain, as compared with the C-domain, equally well by the two actives sites of ACE, and 10× better by the C-domain, as compared with the N-domain. (c) Inhibition profiles of the N-domain and ACE by RXP 407. The substrate used was Ac-Ser-Asp-Lys-Pro (25 μM). (d) Inhibition profiles of the N-domain and ACE by RXP 407. The substrate used was angiotensin I (50 μM).
Theoretical model of ACE inhibition.
In this model, the two ACE active sites were considered independent, and ACE was treated as two distinct enzymes (N- and C-domains). These two enzymes, E1 and E2, are able to cleave the same substrate, with a specificity peculiar to each enzyme. The inhibition constants (Ki values) of RXP 407 for E1 and E2 were those determined for the N- and C-domains, respectively. Three theoretical cases of substrate specificity were considered: a substrate equally cleaved by E1 and E2 (S = 1, Fig. 3b), a substrate 10× better hydrolyzed by E1 than by E2 (S = 10, Fig. 3b), and the reverse situation (S = 0.1, Fig. 3b) (see Materials and Methods for details).
These simulations confirm that the shape of the inhibition profile depends on the relative specificity of E1 and E2 in cleaving the substrate (Fig. 3b). When the substrate is preferentially cleaved by E1, the inhibition profile is shifted to the left, and when the substrate is cleaved by E2, the profile is shifted to the right. When the substrate is equally cleaved by the two active sites, the curve displays two inflexion points. Thus, according to these simulations, the shape of the inhibition profile obtained with RXP 407 is indicative of the specificity with which E1 and E2 cleave the substrate used for the inhibition titration.
Natural substrates of wild-type ACE.
The inhibition of ACE by RXP 407 was determined by using two natural substrates of ACE, AcSDKP and angiotensin I (Fig. 3 c and d, respectively). For comparison, the profiles corresponding to the inhibition of the N-domain with these substrates also are reported. For AcSDKP, the inhibition profiles determined for ACE and the N-domain are only slightly different. Thus, ACE behaves like the N-domain, indicating that AcSDKP is mostly cleaved by the N-terminal active site of ACE. The left-side inhibition profile of ACE is also consistent with preferential cleavage of AcSDKP by the N-terminal active site of ACE (see theoretical curves). For angiotensin I, the inhibition profiles obtained for ACE and the N-domain are very different. This observation, as well as our simulations, are indicative of a substrate cleaved by both ACE active sites.
Metabolism and Pharmacokinetics of RXP 407.
After a single i.v. dose of tritiated RXP 407 in the rat, the 0- to 48-h urine contained an average of almost 98% of the injected radioactivity as intact RXP 407, indicating preferential renal clearance of this inhibitor and the absence of any metabolism of this phosphinic peptide. Pharmacokinetic profiles corresponding to the clearance of RXP 407 from the rat plasma exhibited a two-time domain, characterized by a rapid phase of RXP 407 elimination followed by a slower clearance. Nevertheless, after 2 h, the plasma concentration of RXP 407 was still above its Ki value for the N-domain of ACE.
DISCUSSION
RXP 407 is a potent inhibitor of somatic ACE able to differentiate the two active sites of this enzyme with a difference in specificity of three orders of magnitude. It is quite likely that this inhibitor would not have been found without the use of combinatorial chemistry. Structure–activity relationships demonstrate the peculiar characteristics of this inhibitor: The inhibition depends on the presence of a C-terminal amide group, an aspartic side chain in the P2 position of the inhibitor, and an N-acetyl group. Data reported in Table 1 indicate that these three structural features act repulsively in the binding of the inhibitor to the C-terminal active site of ACE but are well tolerated by the N-terminal active site. These results suggest that the N-terminal active site of ACE possesses a broader selectivity than the C-terminal active site. In view of structure–activity relationships, it has long been claimed that a free C-terminal carboxylate group in the P2′ position of the inhibitor is an absolute requisite in preparing potent inhibitors of ACE (26, 27), which explains why a free carboxylate group in the P2′ position has been routinely incorporated in the ACE inhibitor structures over the last two decades. This has probably impeded the discovery of selective inhibitors of the N-terminal active site of ACE. Another difference between RXP 407 and the usual ACE inhibitors is molecular size. Most ACE inhibitors interact with the S1′, S2′ subsites, and among these, a few project a residue in the S1 subsite (25). RXP 407, in addition to S1′, S2′, and S1 subsites, also involves the S2 subsite (Fig. 1b). This feature is extremely important because the aspartic side chain and the N-acetyl group, located in the P2 position of RXP 407, also contribute to the selectivity of this inhibitor (Table 1).
With a selectivity factor of three orders of magnitude between the two ACE active sites, RXP 407 is an inhibitor whose inhibition titration curve demonstrates that ACE contains two functional active sites. Mixed potent inhibitors of the two ACE active sites do not easily permit demonstration of the occurrence of two active sites in this enzyme, and before the cloning of ACE many authors had concluded that ACE contained only one active site (28–31). As clearly shown by the theoretical model developed in this study, RXP 407 also can be used to determine the specificity of the N- and C-terminal active sites of ACE in cleaving a particular substrate. In this respect, the results reported in Fig. 3c constitute direct evidence that Ac-SDKP is indeed selectively cleaved by the N-domain of ACE. Previous conclusions regarding AC-SDKP degradation by ACE were in fact only based on the use of the N- and C-domain mutants of this enzyme (5, 7). Similarly, RXP should be useful in studying the influence of pH and chloride concentration on the relative specificity of the two active sites of ACE.
This inhibitor makes it possible to block only the N-terminal active site of ACE and to examine in vivo its effects. One immediate application should be to show that, in vivo, only the N-terminal active site of ACE contributes to the cleavage of AcSDKP. In this connection, it is of interest to remark that, according to our in vitro results, despite the blockade of the N-domain by RXP 407, efficient hydrolysis of angiotensin I into angiotensin II by the C-domain should still occur under these conditions (Fig. 3d). Thus, one can envisage controlling plasma AcSDKP levels without interfering with blood pressure control. Taking into consideration the important role of AcSDKP in the control of the hematopoietic cycle (11), inhibition of the N-terminal ACE active site may have important clinical applications in facilitating hematopoietic recovery after aggressive cancer chemotherapy (32–35). The in vivo stability of RXP 407, as well as its pharmacokinetic properties, should allow us to address this issue in the near future. Besides their possible clinical applications, the development of selective ACE inhibitors may help to uncover still unknown physiological functions of ACE and may contribute to the efforts aimed at discovering whether the ACE gene duplication is associated with particular functions of this enzyme in mammalian species. The present work, based on a combinatorial chemistry approach, shows that it is possible to develop selective inhibitors of ACE and should stimulate further research in this direction.
ABBREVIATIONS
- ACE
angiotensin converting enzyme
- AcSDKP
Ac-Ser-Asp-Lys-Proline
References
- 1.Weaber B, Nussberger J, Brunner H R. In: Hypertension. Laragh J H, Brenner B M, editors. New York: Raven; 1995. pp. 2861–2875. [Google Scholar]
- 2.Soubrier F, Alhenc-Gelas F, Hubert C, Allegrini J, John M, Tregear G, Corvol P. Proc Natl Acad Sci USA. 1988;85:9386–9390. doi: 10.1073/pnas.85.24.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei L, Alhenc-Gelas F, Corvol P, Clauser E. J Biol Chem. 1991;266:9002–9008. [PubMed] [Google Scholar]
- 4.Jaspard E, Wei L, Alhenc-Gelas F. J Biol Chem. 1993;268:9496–9503. [PubMed] [Google Scholar]
- 5.Deddish P A, Wang L-X, Jackman H L, Michel B, Wang J, Skidgel R A, Erdös E G. J Pharmacol Exp Ther. 1996;279:1582–1589. [PubMed] [Google Scholar]
- 6.Deddish P A, Jackman H L, Skidgel R A, Erdös E G. Biochem Pharmacol. 1997;53:1459–1463. doi: 10.1016/s0006-2952(97)00087-7. [DOI] [PubMed] [Google Scholar]
- 7.Michaud A, Williams T A, Chauvet M-T, Corvol P. Mol Pharmacol. 1997;51:1070–1076. doi: 10.1124/mol.51.6.1070. [DOI] [PubMed] [Google Scholar]
- 8.Deddish P A, Marcic B, Jackman H L, Wang H-Z, Skidgel R A, Erdös E G. Hypertension. 1998;31:912–917. doi: 10.1161/01.hyp.31.4.912. [DOI] [PubMed] [Google Scholar]
- 9.Wei L, Clauser E, Alhenc-Gelas F, Corvol P. J Biol Chem. 1992;267:13398–13405. [PubMed] [Google Scholar]
- 10.Perich R B, Jackson P, Johnston C I. Eur J Pharmacol. 1994;266:201–211. doi: 10.1016/0922-4106(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 11.Lenfant M, Wdzieczak-Bakala J, Guittet E, Prome J-C, Sotty D, Frindel E. Proc Natl Acad Sci USA. 1989;86:779–782. doi: 10.1073/pnas.86.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rousseau A, Michaud A, Chauvet M-T, Lenfant M, Corvol P. J Biol Chem. 1995;270:3636–3661. doi: 10.1074/jbc.270.8.3656. [DOI] [PubMed] [Google Scholar]
- 13.Azizi M, Rousseau A, Ezan E, Guyene T-T, Michelet S, Grognet J-M, Lenfant M, Corvol P, Ménard J. J Clin Invest. 1996;97:839–844. doi: 10.1172/JCI118484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiracek J, Yiotakis A, Vincent B, Lecoq A, Nicolaou A, Checler F, Dive V. J Biol Chem. 1995;270:21701–21706. doi: 10.1074/jbc.270.37.21701. [DOI] [PubMed] [Google Scholar]
- 15.Jiracek J, Yiotakis A, Vincent B, Checler F, Dive V. J Biol Chem. 1996;271:19606–19611. doi: 10.1074/jbc.271.32.19606. [DOI] [PubMed] [Google Scholar]
- 16.Krapcho J, Turk C, Cushman D W, Powel J R, DeForrest J M, Spitzmiller E R, Karanewsky D S, Duggan M, Rovnvak G, Schwartz J, et al. J Med Chem. 1988;31:1148–1160. doi: 10.1021/jm00401a014. [DOI] [PubMed] [Google Scholar]
- 17.Yiotakis A, Lecoq A, Nicolaou A, Labadie J, Dive V. Biochem J. 1994;303:323–327. doi: 10.1042/bj3030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yiallouros I, Vassiliou S, Yiotakis A, Zwilling R, Stöcker S, Dive V. J Biochem. 1998;331:375–379. doi: 10.1042/bj3310375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grams F, Dive V, Yiotakis A, Yiallouros I, Vassiliou S, Zwilling R, Bode W, Stocker W. Nat Struct Biol. 1996;3:671–675. doi: 10.1038/nsb0896-671. [DOI] [PubMed] [Google Scholar]
- 20.Barlos K, Chatzi O, Gatos D, Stavropoulos G. Int J Pept Protein Res. 1991;37:513–520. [PubMed] [Google Scholar]
- 21.Baylis, E. K., Campbell, C. D. & Dingwall, J. G. (1984) J. Chem. Soc. Perkin. Trans. 1, 2845–2849.
- 22.Yiotakis A, Vassiliou S, Jiracek J, Dive V. J Org Chem. 1996;61:6601–6605. doi: 10.1021/jo9603439. [DOI] [PubMed] [Google Scholar]
- 23.Knight C G, Willenbrock F, Murphy G. FEBS Lett. 1992;296:263–266. doi: 10.1016/0014-5793(92)80300-6. [DOI] [PubMed] [Google Scholar]
- 24.Kuzmic P. Anal Biochem. 1996;237:260–273. doi: 10.1006/abio.1996.0238. [DOI] [PubMed] [Google Scholar]
- 25.Patchett A A, Cordes E H. Adv Enzymol Relat Areas Mol Biol. 1985;57:1–84. doi: 10.1002/9780470123034.ch1. [DOI] [PubMed] [Google Scholar]
- 26.Ondetti M A, Rubin B, Cushman D W. Science. 1977;196:441–444. doi: 10.1126/science.191908. [DOI] [PubMed] [Google Scholar]
- 27.Petrillo E W, Ondetti M A. Med Res Rev. 1982;2:1–41. doi: 10.1002/med.2610020103. [DOI] [PubMed] [Google Scholar]
- 28.Bünning P, Riordan J F. J Inorg Biochem. 1985;24:183–198. doi: 10.1016/0162-0134(85)85002-9. [DOI] [PubMed] [Google Scholar]
- 29.Strittmatter S M, Snyder S H. Mol Pharmacol. 1986;29:142–148. [PubMed] [Google Scholar]
- 30.Cumin F, Vellaud V, Corvol P, Alhenc-Gelas F. Biochem Biophys Res Commun. 1989;163:718–725. doi: 10.1016/0006-291x(89)92282-1. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y-N P, Riordan J F. Biochemistry. 1990;29:10413–10498. [Google Scholar]
- 32.Bodgen A E, Carde P, Deschamps de Paillette E, Moreau J P, Tubiana M, Frindel E. Ann N Y Acad Sci. 1991;628:126–139. doi: 10.1111/j.1749-6632.1991.tb17230.x. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe T, Brown G S, Kelsey L S, Yan Y, Jackson J D, Ewel C, Kessinger A, Talmadge J E. Exp Hematol (Charlottesville, Va) 1996;24:713–721. [PubMed] [Google Scholar]
- 34.Bodgen A E, Moreau J P, Gamba-Vitalo C, Deschamps de Paillette E, Tubiana M, Frindel E, Carde P. Int J Cancer. 1998;76:38–46. doi: 10.1002/(sici)1097-0215(19980330)76:1<38::aid-ijc8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 35.Massé A, Ramirez L H, Bindoula G, Grillon C, Wdzieczak-Bakala J, Raddassi K, Deschamps de Paillette E, Mencia-Huerta J M, Koscielny S, Potier P, et al. Blood. 1998;91:441–449. [PubMed] [Google Scholar]