Abstract
Rhodobacter species are useful model organisms for studying the structure and function of c type cytochromes (Cyt c), which are ubiquitous electron carriers with essential functions in cellular energy and signal transduction. Among these species, Rhodobacter capsulatus has a periplasmic Cyt c2Rc and a membrane-bound bipartite Cyt cyRc. These electron carriers participate in both respiratory and photosynthetic electron-transfer chains. On the other hand, until recently, Rhodobacter sphaeroides was thought to have only one of these two cytochromes, the soluble Cyt c2Rs. Recent work indicated that this species has a gene, cycYRs, that is highly homologous to cycYRc, and in the work presented here, functional properties of its gene product (Cyt cyRs) are defined. It was found that Cyt cyRs is unable to participate in photosynthetic electron transfer, although it is active in respiratory electron transfer, unlike its R. capsulatus counterpart, Cyt cyRc. Chimeric constructs have shown that the photosynthetic incapability of Cyt cyRs is caused, at least in part, by its redox active subdomain, which carries the covalently bound heme. It, therefore, seems that this domain interacts differently with distinct redox partners, like the photochemical reaction center and the Cyt c oxidase, and allows the bacteria to funnel electrons efficiently to various destinations under different growth conditions. These findings raise an intriguing evolutionary issue in regard to cellular apoptosis: why do the mitochondria of higher organisms, unlike their bacterial ancestors, use only one soluble electron carrier in their respiratory electron-transport chains?
Keywords: bacterial energy transduction, protein–protein interactions, photochemical reaction center, cytochrome c oxidase
Mitochondria of higher eukaryotes contain a single, soluble, and highly conserved cytochrome (Cyt c) that functions as an electron carrier between the membrane-associated Cyt bc1 complex and the aa3-type Cyt c oxidase in the linear electron-transfer chain (ETC) of aerobic respiration. On the other hand, in α-proteobacteria, which are used widely for functional and structural studies on biological energy transduction, several aerobic and anaerobic ETCs are used under different growth conditions. In these bacteria, the presence of multiple ETCs creates a physiological need to funnel electrons specifically to appropriate destinations, depending on the growth conditions. Thus, in species like Rhodobacter capsulatus and Paracoccus denitrificans, unlike mitochondria, multiple Cyt c function in parallel as electron carriers with seemingly overlapping functions (1).
Photosynthetic (Ps) and respiratory (Res) ETCs of the purple, nonsulfur facultative phototrophs of Rhodobacter species provide an excellent model system for studies on cellular energy transduction (2). In these species, Ps growth depends on the cyclic electron transfer between the photochemical reaction center and the Cyt bc1 complex. In R. capsulatus, either a soluble Cyt c2, or a membrane bound Cyt cy, reduces the photooxidized reaction center from the periplasmic side of the cytoplasmic membrane (3). The same electron carriers also transfer electrons from the Cyt bc1 complex to the cbb3-type Cyt c oxidase (4), thereby participating in a mitochondrial-like Res ETC of this species (5). However, in wild-type strains of the closely related Rhodobacter sphaeroides, Ps growth strictly depends on the presence of the soluble Cyt c2 (6). Thus, it was believed that a Cyt cy homolog is not present in R. sphaeroides. In the absence of any other suitable candidate, participation of Cyt c2 as an electron carrier from the Cyt bc1 complex to the aa3-type (7) and to the cbb3-type (8) Cyt c oxidase also has been proposed (6). Recently, a gene highly homologous to Rc cycY (encoding Cyt cyRc) was encountered in R. sphaeroides (9). This finding suggested to us that R. sphaeroides Cyt cy (Cyt cyRs) might not be functional in Ps electron transfer, because a Cyt c2− R. sphaeroides strain is Ps− (6) and also is complemented readily to a Ps+ phenotype with Cyt cyRc (10). Herein, we have identified the polypeptide corresponding to Cyt cyRs and characterized its functional properties. We show that Cyt cyRs is a membrane-attached electron carrier that participates only in Res electron transfer, unlike its R. capsulatus counterpart, which is functional for both Ps and Res electron transport. Furthermore, using chimeric Cyt cy constructs, we establish that the differential Ps capability of these cytochromes is related, at least partly, to their redox active domains interacting with their partners. It therefore seems that, although the Rhodobacter species simultaneously express several highly homologous electron carriers, they also endow them with pronounced specificity toward their redox partners to direct electron flow efficiently to the different branches of their ETCs, thereby maximally supporting their physiological needs.
MATERIALS AND METHODS
Bacterial Strains and Growth Conditions.
R. capsulatus and R. sphaeroides strains were grown under chemoheterotrophic or photoheterotrophic conditions in mineral-peptone-yeast extract (MPYE)-enriched (11) and yeast-extract-casamino acid-enriched (12) media, respectively, or in minimal medium A, as described (11). Photoheterotrophic growth on solid medium occurred in anaerobic jars with H2/CO2-generating gas packs (BBL). Res and Ps growth rates were determined as described (13, 14). Escherichia coli strains CJ236 [dut1 ung1 thi-1 relA1/pCJ105 (F′ CamR)] (15), HB101 [F−proA2 hsdS20 (rB−, mB−) recA13 rpsL20] (15), S17.1 [ara recA rpoL RP4-2-2-Tet∷Mu-Kan∷Tn7] (16), and XL1-Blue [recA1 endA1 gyrA96 thi-1, hsdR17 lac/F′ proAB lacIqZΔM15 Tn10 (TetR)] (15) were grown on Luria–Bertani broth, supplemented, where appropriate, with antibiotics, as described (11).
Molecular Genetic Techniques.
Uracil-containing single-stranded DNA isolated from E. coli CJ236 containing pKD2 (cycYRs) and the primer 5′-CTGGCCTCGCTCCAGGACTACAAGGACGACGATGACAAGTAAGGGCAGAACCCCCTG-3′ was used to extend by site-directed mutagenesis the C terminus of Cyt cyRs with an in-frame octapeptide corresponding to the FLAG epitope (N-Asp–Tyr–Lys–Asp–Asp–Asp–Asp–Lys-C; Kodak) followed by a stop codon, as it has been described for Cyt cyRc (11). This process yielded pHM118 (cycYRs-FLAG). For mutagenesis of pHM6 carrying cycYRc-FLAG (11), the QuikChange kit (Stratagene) was used as recommended by the manufacturer, with the following modifications. All reaction mixtures contained 15% (vol/vol) glycerol and were incubated at 98°C for 30 s before cycling (18 cycles of 97°C for 30 s, 55°C for 10 s, and 68°C for 12 min). The mutagenic primers NdeI.1 (5′-CGCCGGGGGACATATGCTCGTCAAGACGCAC-3′) and NheI.1 (5′-CGAAGTGGACCCGGCTAGCATCACCGGCGAC-3′) were used to create an NdeI site at base-pair position 87 of the insert (corresponding to methionine 1 of Cyt cyRc; pHM110) and an NheI site at base-pair position 371 (changing Thr-96 of Cyt cyRc to Ser; pHM112), respectively. Plasmid pHM113 was obtained by using pHM110 as a template and had both of the aforementioned NdeI and NheI sites.
DNA Sequence Analysis.
Automated DNA sequencing with a dye terminator cycle sequencing kit (AmpliTaq FS, Applied Biosystems) was performed as specified by the manufacturer. The nucleotide sequence of the 1.25-kb insert on pKD2 was determined on both strands by using the M13 universal primers and the following internal primers obtained through GIBCO/BRL: scycY1, 5′-CGTCACATCCTCGCCGTG-3′; scycY4, 5′-CGCCGAGGCGCCGACCTGGA-3′; scycY2, 5′-TGGAAAGCGATTCGGCCGGT-3′; scycY5, 5′-GGCCGAATCGCTTTCCAGGG-3′; scycY3, 5′-TCGCCAAATGCGCCGCCTGTC-3′; and scycY6, 5′-GGCCACCGCGCGGTCGAC-3′. Homology searches, sequence alignments, and predictions for transmembrane helices were performed as described (11).
Construction of Chimeric Cyt cy Derivatives.
PCRs were performed by using AmpliTaq DNA polymerase in the presence of 200 ng of each primer and with 20–50 ng of plasmid DNA as a template (11). For the “Cyt c exchange” construct, the Cyt c domain of Cyt cyRs-FLAG from pHM118 was amplified (30 cycles of 97°C for 30 s, 55°C for 10 s, and 72°C for 2 min) by using the universal M13 forward primer and the primer 5′-GGAAGCGAATTCGCCAAATGCGCCGCC-3′ carrying an EcoRI site. The 0.8-kb PCR product thus obtained was digested with XbaI and EcoRI restriction enzymes and used to replace the corresponding fragments of pHM4 containing the “anchor-linker” domain of Cyt cyRc fused in-frame to the mature form of Cyt c2 (11), yielding pHM124. This plasmid encoded a chimeric Cyt cy where the first 108 amino acid residues of Cyt cyRc are fused in-frame to the Cyt c domain (residues 82–174) of Cyt cyRs-FLAG. For the anchor-linker exchange construct, residues 1–68 of Cyt cyRs were amplified by using pKD2 as a template and the primers 5′-CGAATCCATATGTTCGACACGATGACCCTG-3′ and 5′-GTAACTGCTAGCTTCCGGAAACGGCACCGCTT-3′. The PCR product was digested with NdeI and NheI and ligated to the 3.9-kb NheI–NdeI fragment of pHM113, yielding pHM121. Because the NdeI mutation overlaps the ATG start codon of cycYRc, it affected the expression of Cyt cyRc. Thus, the NdeI mutation in pHM121 was converted back to the wild-type sequence by using the mutagenic primer 5′-AACCGCCGGGGGAGCCATGTTCGACACGATG-3′. The resulting plasmid pHM125 encoded a chimeric Cyt cy in which the first 68 amino acid residues of Cyt cyRs are fused in-frame to the Cyt c domain (residues 95–199) of Cyt cyRc-FLAG. All constructions were confirmed by restriction digestions and DNA sequencing analyses, and the plasmids pHM124 and pHM125 were cloned into the unique HindIII site of pRK415 (17) to yield their transferable derivatives pHM126 and pHM127, respectively (Table 1).
Table 1.
Bacterial strains and plasmids
| Strain | Relevant characteristics | Refs.178 |
|---|---|---|
| R. capsulatus | ||
| MT1131† | crtD121 RifR, wild-type | 13 |
| Y262 | Overproducer of gene transfer agent | 18 |
| FJ2 | crtD121 Δ(cycARc∷kan) Δ (cycYRc∷spe); cyt c2Rc−cyRc−,Ps− | 3 |
| M6G-G4/S4 | crtD121 qox-260 Δ(cycA∷kan); Qox− cyt c2−,Ps+ | 14 |
| R. sphaeroides | ||
| Ga† | crt, wild-type | 10 |
| Gadc2 | crt, Δ(cycARs∷spe); cyt c2Rs−,Ps− | 19 |
| Gadcy | crt, cycYRs∷kan; cyt cyRs−,Ps+ | |
| Gadc2cy | crt, cycYRs∷kan Δ (cycARs∷spe); cyt c2Rs−, cyt cyRs−,Ps− | |
| Plasmids | ||
| pRK2013 | KanR, helper plasmid | 17 |
| pRK415 | TetR broad host-range plasmid | 17 |
| pSUP202 | AmpR, CmR, TetR, suicide plasmid | 16 |
| pBSII | pBluescript II KS(+), AmpR | 11 |
| pFJ66 | Δ(cycY∷spe); SpeR, TetR | 3 |
| pKD2 | cycYRs on a 1.25-kb EcoRI/BlgII fragment of pUI8180 in pBSII | |
| pHM4 | cyt MA-c2 on a 1.2-kb BamHI/HindIII fragment in pBSII | 11 |
| pHM6 | cycYRc-FLAG on a 1.2-kb BamHI/HindIII fragment in pBSII | 11 |
| pHM8 | cyt MA-c2 on a 1.2-kb KpnI/BamHI fragment in pRK415 | 11 |
| pHM13 | cycYRs on a 2.0-kb XbaI/HindIII fragment in pRK415 | |
| pHM14 | cycARc on a 1.25-kb BamHI/HindIII fragment in pRK415 | |
| pHM15 | cycYRs on a 1.6-kb EcoRI/XbaII fragment of pU1942 (see ref. 9) in pBSII | |
| pHM16 | cycYRs∷kan on a 2.9-kb EcoRI fragment in pBSII | |
| pHM17 | cycYRs∷kan on a 2.9-kb EcoRI fragment in pSUP202 | |
| pHM100 | Like pHM126 but without a FLAG epitope | |
| pHM110 | Like pHM6 but contains an NdeI site at position 87 | |
| pHM112 | Like pHM6 but contains an NheI site at position 371 | |
| pHM113 | Like pHM6 but contains both an NdeI and an NheI site at positions 87 and 371, respectively | |
| pHM118 | Like pKD2 but contains cycYRs-FLAG | |
| pHM119 | pHM118 at HindIII site of pRK415 | |
| pHM121 | cycYRs68∷cycYRc95-FLAG on a 1.2-kb KpnI/XbaI fragment in pBSII (“anchor-linker exchange chimera”) | |
| pHM123 | pHM121 at HindIII site of pRK415 | |
| pHM124 | cycYRc108∷cycYRs82-FLAG on a 1.2-kb KpnI/XbaI fragment in pBSII (“cyt c exchange chimera”) | |
| pHM125 | Like pHM121 but does not contain an NdeI site | |
| pHM126 | pHM124 at HindIII site of pRK415 | |
| pHM127 | pHM125 at HindIII site of pRK415 |
If not otherwise indicated, all strains and plasmids were obtained during this work.
The R. capsulatus strain MT1131 (RifR crtD) and R. sphaeroides strain Ga (crt) are referred to as wild-type strains, because they are wild-type in respect to their Cyt c profile and growth properties.
Chromosomal Inactivation of cycYRc and cycYRs.
The null allele of cycYRc [Δ(cycYRc∷spe)] carried by pFJ66 was introduced into the chromosome of appropriate R. capsulatus strains by using the gene transfer agent (18) as described (3). Chromosomal location of the inserted spectinomycin resistance (SpeR) cartridge was confirmed by PCR, by using the primers 5′-GAAAGCCGGCGAGGAAAAGT-3′ and 5′-CTCGGTTTTCTGGAAGGCGA-3′ as described (11). The 1.6-kb BamHI fragment of pUC4-Kixx (Amersham Pharmacia) carrying the kanamycin resistance (KanR) gene was cloned into the unique BamHI site, overlapping with the glycine 24 codon of Cyt cyRs on pHM15 (Table 1). The 2.9-kb EcoRI fragment of pHM16 thus obtained, transcribing kan and cycYRs in the opposite orientation, was cloned into the unique EcoRI site of pSUP202 (16), yielding pHM17 (cycYRs∷kan). Conjugal transfer of pHM17 from E. coli S17.1 into R. sphaeroides was done as described (19), and KanR transconjugates were screened for tetracycline sensitivity (TetS). The chromosomal location of kan in the retained KanR TetS isolates was confirmed by PCR amplification by using the primers scycY1 and scycY2 described above.
Biochemical Techniques.
Isolation of chromatophore vesicles, SDS/10% or 16.5% PAGE, and immunoblot analyses with anti-FLAG antibodies were performed as described (11). Protein concentrations were determined by the method of Lowry et al. (20), and the presence of Cyt c was indicated by the intrinsic peroxidase activity of the Cyt c heme group by using 3,3′,5,5′-tetramethylbenzidine (TMBZ) and H202 (21). Oxygen uptake measurements with various substrates and inhibitors were performed as described (5).
RESULTS
Physical Organization of the Genes Surrounding cycYRs.
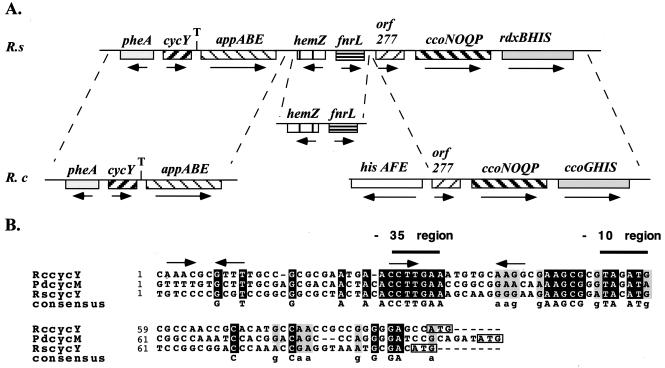
The cosmid pUI8180 contains chromosomal DNA from the region 443 kb clockwise from the chosen zero position on the physical map of chromosome I of R. sphaeroides (9). Determination of the DNA sequence of its 1.25–kb EcoRI–BglII fragment on pKD2 (Table 1) identified three ORFs (Fig. 1). The largest one of these ORFs (522 bp), encoding a Cyt c of 17.7 kDa (for the apoprotein), with a conserved heme binding motif (Cys–Ala–Ala–Cys–His) between its residues 85 and 89, had pronounced homology to cycYRc. Hereafter, this ORF is called cycYRs, and the properties of its product, Cyt cyRs, are described below. Genetic organization and sequence of the ORFs either preceding (pheA encoding chorismate mutase/prephenate dehydratase) or following (appA involved in oligopeptide transport, formerly called ORF2) cycYRs are well conserved between R. sphaeroides and R. capsulatus (ref. 11; Fig. 1).
Figure 1.
(A) Physical and genetic maps of the chromosomal regions surrounding R. sphaeroides and R. capsulatus cycY. Only cycYRs is physically linked to the ccoNOQP–ccoGHIS/rdxBHIS cluster required for the biosynthesis of the cbb3-type Cyt c oxidase in both organisms (9, 30). Arrows and “T” refer to transcription direction and termination signal at the 3′ downstream region of cycY, respectively. (B) Sequence comparison of the 5′ upstream regions of cycY from R. capsulatus (RccycY), R. sphaeroides (RscycY), and P. denitrificans (PdcycM). Putative −35 (TTGAA) and −10 (TAg/cAT) regions for a σ70-like promoter are indicated. Additional conserved elements of unknown significance (indicated by arrows) and putative ribosome binding sites rich in adenosine and guanine residues in the vicinity of the boxed translational start (ATG) codons also are shown.
Primary Structure of Cyt cyRs.
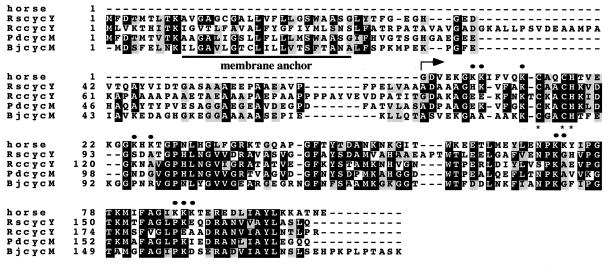
Alignment of the amino acid sequence of Cyt cyRs with Cyt cyRc and its homologs in other species (22, 23) indicated that members of Cyt cy family have conserved their bipartite domain structure (Fig. 2). Each member contains a carboxyl-terminal Cyt c subdomain and an amino terminal “linker” subdomain preceded by an unprocessed signal sequence-like stretch anchoring it to the membrane (11). Several Lys residues in the Cyt c subdomain of Cyt cyRs (e.g., Lys-8, -13, -72, and -87, in equine Cyt c numbering) thought to be important for the interactions of Cyt c with its redox partners (24, 25) are well conserved, whereas several others such residues (e.g., Lys-7, -25, -27, -73, -86, and -88) are not. In addition, a single residue deletion (corresponding to position 111 of Cyt cyRc) and a three-residue insertion (Ala–Pro–Thr at positions 127–130 of Cyt cyRs) are noticeable. Moreover, major differences are seen in the linker regions connecting the membrane anchor to the Cyt c subdomain. In particular, the linker portion of Cyt cyRc is considerably longer than those of R. sphaeroides, P. denitrificans, and B. japonicum because of the 15- and 7-residue insertions located between positions 45–60 and 84–91 of Cyt cyRc, respectively (Fig. 2).
Figure 2.
Alignment of the amino acid sequences for horse-heart Cyt c: R. capsulatus (RccycY), R. sphaeroides (RscycY), P. denitrificans (PdcycM), and Bradyrhizobium japonicum (BjcycM) Cyt cy homologs. Black and gray boxes correspond to identical residues and conserved substitutions, respectively. The Cyt c domains and CXYCH heme binding motifs are indicated by an arrow and asterisks, respectively. Dots represent the key Lys residues known to be important for the interaction of equine Cyt c with its various redox partners (24, 25).
Cyt cyRs Does Not Function in Ps Electron Transfer.
R. sphaeroides strains bearing a null allele of cycYRs in the presence and absence of Cyt c2 were sought to probe the function of Cyt cyRs. The strains Gadcy (Cyt cy−) and Gadc2cy (Cyt c2− and Cyt cy−), obtained as described in Materials and Methods, were Ps+ and Ps− like their isogenic parents Ga (wild type) and Gadc2 (Cyt c2−), respectively. Thus, consistent with earlier works (6, 19), inactivation of cycYRs had no effect on Ps growth of R. sphaeroides either on minimal or enriched growth media. Furthermore, the R. sphaeroides strain pHM13/Gadc2 and the R. capsulatus strain pHM13/FJ2 (Cyt c2− and Cyt cy−), carrying a plasmid-borne copy of cycYRs, also were Ps−. These findings indicated that Cyt cyRs does not participate in Ps electron transfer in either R. sphaeroides or R. capsulatus. Finally, all R. sphaeroides strains used here were capable of wild-type-like Res growth (doubling times between 144 and 162 min; data not shown). However, the presence of a branched Res pathway, which depended on a poorly characterized quinol oxidase (Qox; refs. 26 and 27), precluded us from assessing the role of the Cyt c2 and Cyt cy on the Res ETC of R. sphaeroides.
Identification of Cyt cyRs and Its Expression Under Res and Ps Growth Conditions.
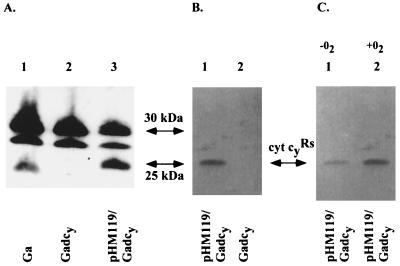
To identify the gene product of cycYRs readily, its epitope-tagged derivative (Cyt cyRs-FLAG) was constructed. TMBZ-SDS/PAGE and immunoblot analyses of various R. sphaeroides strains producing Cyt cyRs-FLAG indicated that, among the membrane-associated TMBZ stained bands, only a band of 24 kDa was shifted to a molecular mass of 25 kDa and recognized with anti-FLAG antibodies (Fig. 3). Further, this 24-kDa band was absent in Gadcy (cycYRs∷kan), lacking Cyt cyRs. These findings identified the 24-kDa and 25-kDa bands as native Cyt cyRs and its FLAG-epitope-tagged derivative, respectively (Fig. 3 A and B). They also showed that Cyt cyRs is expressed under both Ps and Res growth conditions in R. sphaeroides, although its steady-state level was lower under Ps growth conditions (Fig. 3C). Moreover, the data also indicated that under Ps growth conditions the 30-kDa and 28-kDa bands, proposed to correspond to the Cyt cp (with a calculated molecular mass of 31 kDa) and Cyt co (27 kDa) subunits of the cbb3-type Cyt c oxidase (10), were expressed at lower levels in comparison to aerobic growth conditions (data not shown).
Figure 3.
(A) TMBZ-SDS/PAGE analysis of Cyt c of various R. sphaeroides strains. Chromatophore membranes were prepared from cells grown on yeast-extract-casamino acid-enriched medium by respiration, and ≈75 μg of proteins were loaded per lane. Lane 1, Ga (wild type); lane 2, Gadcy (lacking Cyt cyRs); and lane 3, pHM119/Gadcy (containing Cyt cyRs-FLAG). The addition of the FLAG epitope changes the molecular mass of Cyt cyRs by ≈1 kDa. (B) Immunoblot analysis of a similar gel with anti-FLAG M2 antibody. Lanes 1 and 2 correspond to pHM119/Gadcy (Cyt cyRs-FLAG) and Gadcy, respectively, and contained 25 μg of membrane proteins prepared from cells grown as for A. (C) Immunoblot analysis with anti-FLAG M2 antibody as for B, except that chromatophore membranes (25 μg of total proteins per lane) were obtained from cells of pHM119/Gadcy (Cyt cyRs-FLAG) grown in yeast-extract-casamino acid-enriched medium in the absence (lane 1) and presence (lane 2) of oxygen.
Cyt cyRs Can Support Res Growth, but Not Ps Growth, in R. capsulatus.
Res growth of R. capsulatus becomes restricted to the Cyt bc1 → Cyt c oxidase branch solely (hence, growth is sensitive to Cyt bc1 inhibitors like myxothiazol) when the Qox-dependent branch is eliminated by a mutation (14, 28). Simultaneous inactivation of Cyt c2 and Cyt cy in such a Qox− background is fatal for cells (5), strongly implying that these cytochromes are the sole electron carriers capable of connecting the Cyt bc1 complex to the Cyt c oxidase. Thus, various Qox− R. capsulatus strains carrying either the Cyt cyRc or the Cyt cyRs as the sole electron carrier were constructed to assess their electron-transfer capabilities. Growth properties of these strains showed that, although the Cyt cyRc, Cyt c2, and its membrane-bound derivative, Cyt MA-c2 (11), were able to support both Ps and Res growth of R. capsulatus, Cyt cyRs was functional only during Res growth (Table 2). The Res electron carrier ability of Cyt cyRs was confirmed further by direct O2 uptake (Table 3), as well as NADH-induced cyanide-sensitive Cyt c reduction measurements (data not shown). It was noticed that the endogenous O2-uptake activity of pHM13/SL3 (Cyt cyRs) was relatively low, but it could reach a level similar to that seen with M6G-G4/S4 (Cyt cyRc) on addition of exogenous Cyt c (Table 3). This finding correlated well the low amount of Res electron-transfer activity found in pHM13/SL3 with the low amount of Cyt cyRs present in pHM119/SL3 (Fig. 4B).
Table 2.
R. sphaeroides Cyt cy is able to support electron transfer from the Cyt bc1 complex to the Cyt cbb3 oxidase in R. capsulatus
| Strain | Electron carrier (Cyt c2 or Cyt cy) | Ps | Res, min | Myx178 |
|---|---|---|---|---|
| MT1131 | Cyt c2Rc + Cyt cyRc | +† | +, 142 | + |
| pRK415/M6G-G4/S4 | Cyt cyRc | + | +, 199 | − |
| pHM13/SL3‡ | Cyt cyRs | −† | +, 344 | − |
| pHM14/SL3 | Cyt c2Rc | + | +, 265 | − |
| pHM8/SL3 | Cyt MA-c2Rc | + | +, 264 | − |
| pHM100/SL3§ | Cyt c exchange chimera | − | +, 321 | − |
Myx corresponds to respiratory growth in the presence of 10 μM myxothiazol in MPYE medium.
MT1131 has a doubling time of approximately 120 min and forms colonies 1.7–1.9 mm in diameter after 2 days of incubation on MPYE medium under the growth conditions used. “+” and “−” indicate Ps growth similar to that of MT1131 on solid medium and no visible colony formation after 5 days of incubation under the same conditions, respectively. Growth rates were not redetermined in SL3 (Qox−) background, because, in the more appropriate R. capsulatus strain FJ2 (Cyt c2− and Cyt cy−), plasmid-borne Cyt cyRc, Cyt c2Rc, and its membrane-bound derivative (Cyt MA-c2Rc) support Ps growth in MPYE medium with doubling times of 205, 137, and 154 min, respectively, whereas Cyt cyRs (pHM13) and cyt c exchange chimera (pHM100) do not confer any appreciable Ps growth.
SL3 was obtained by introducing Δ(cycY∷spe) allele into the R. capsulatus strain M6G-G4/S4 (Qox− Cyt c2−) carrying the desired electron carrier on a plasmid, as described in Materials and Methods.
No SpeR derivative of pHM127/M6G-G4/S4 carrying the linker-anchor exchange chimera was obtained on introduction of cycY∷spe under Res or Ps growth conditions, unlike pHM100/M6G-G4/S4 (or pHM126/M6G-G4/S4; not shown) carrying the Cyt c exchange chimera.
Table 3.
Respiratory electron transport activities of various R. capsulatus strains measured by using isolated membrane fragments
| Substrates, concentration | Moles of O2 consumed per h per mg of protein
|
||
|---|---|---|---|
| pRK415/M6G-G4/S4 (Cyt cyRc) | pHM13/SL3 (Cyt cyRs) | pHM8/SL3 (Cyt MA-c2Rc) | |
| NADH, 0.5 mM | 9.6 | 2.2 | 15.2 |
| Rotenone, 5 μM | 0.4 | 0.4 | 0.3 |
| Myxothiazol, 2 μM | 0.4 | 0.3 | 0.2 |
| Antimycin A, 2 μM | 1.7 | 0.5 | 1.8 |
| Cyanide, 50 μM | 0.2 | 0.1 | 0.2 |
| NADH + Cyt c, 50 μM | 19.2 | 19.0 | 21.4 |
Numbers are the mean value of two sets of independent experiments. Cyt MA-c2Rc, membrane-bound derivative of Cyt c2.
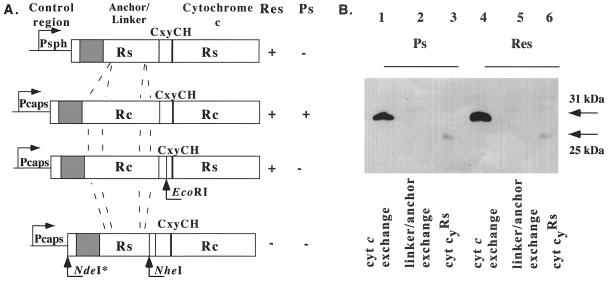
Figure 4.
(A) Schematic representation of the chimeric Cyt c exchange (pHM124/126) and linker-anchor exchange (pHM125/127) cytochromes along the native Cyt cyRs and Cyt cyRc. Control region, Anchor/Linker, and Cytochrome c refer to the various subdomains of these cytochromes. The restriction sites used to create these chimeras are indicated. (B) Immunoblot analysis of various R. capsulatus strains carrying chimeric Cyt cy-FLAG derivatives with anti-FLAG M2 antibody. Chromatophores were prepared from cells grown in enriched MPYE medium under either Ps (lanes 1–3) or Res (lanes 4–6) growth conditions, and 25 μg membrane proteins were loaded per lane. Lane 1, pHM126/M6G-G4/S4 (Cyt c exchange); lane 2 and 5, pHM127/M6G-G4/S4 (linker-anchor exchange); lane 3, pHM119/M6G-G4/S4 (Cyt cyRs-FLAG); lane 4, pHM126/SL3 (Cyt c exchange); and lane 6, pHM119/SL3 (Cyt cyRs-FLAG).
Why Is Cyt cyRs Unable to Support Ps Electron Transfer?
Chimeric Cyt cy derivatives were constructed to determine why Cyt cyRs is unable to function as an electron carrier between the reaction center and the Cyt bc1 complex (Fig. 4A). First, the Cyt c domain from Cyt cyRs-FLAG was fused to the anchor-linker domain of Cyt cyRc, yielding a Cyt c exchange chimera carried by pHM126. Second, the anchor-linker domain of Cyt cyRs was fused to the Cyt c domain of Cyt cyRc-FLAG, yielding an anchor-linker exchange chimera carried by pHM127 (Table 1). In all cases, caution was taken to reassure that expression of the chimeric cytochromes was mediated by an intact cycYRc control region known to be active under both Ps and Res growth conditions in both R. capsulatus and R. sphaeroides (3, 11). Interestingly, the Cyt c exchange chimera encoded by pHM126 was unable to support as the sole electron carrier the Ps but able to support the Res growth of a Qox− mutant of R. capsulatus lacking both Cyt c2 and Cyt cy (Table 2). However, no derivative of M6G-G4/S4 (Qox−) lacking both the Cyt c2 and Cyt cy but carrying the linker-anchor exchange chimera encoded by pHM127 was obtained (Table 2), indicating that such a strain was unable to grow by either Ps or Res growth. Immunoblot analyses of appropriate strains indicated that pHM126/SL3 readily produced the Cyt c exchange chimera under both Ps and Res conditions. On the other hand, no linker exchange chimera was detectable in strains harboring pHM127, including M6G-G4/S4 (Fig. 4B). Data similar to those presented here (Table 2 and Fig. 4) also were obtained with similar chimeric cytochrome constructs with different fusion junctions (data not shown). The findings, therefore, indicate that the Ps-growth inability of Cyt cyRs seems to be an intrinsic property of its Cyt c domain.
DISCUSSION
This work identified a heme-stainable polypeptide with a molecular mass of 24 kDa as the mature form of Cyt cyRs encoded by cycYRs as the R. sphaeroides homolog of R. capsulatus Cyt cyRc. The DNA sequences adjacent to the translational start and stop sites of cycY homologs from several species indicated that the homology was not limited only to their coding portions (Fig. 1). The presence of conserved sequences similar to the −35 and −10 regions and closely resembling consensus promoter sequences recognized by the housekeeping RNA polymerase (29) at the 5′ upstream and the presence of a strong transcription terminator sequence at the 3′ end downstream of cycY suggest that these genes form monocistronic units. It is noteworthy that only in R. sphaeroides is the pheA-appABE cluster containing cycY physically linked to hemZ (encoding an anaerobic coproporphyrinogen III oxidase), fnrL (oxygen-response regulator), and ccoNOQP (Cyt cbb3 oxidase) genes (9, 30). In R. capsulatus, these genes are dispersed into three different chromosomal loci (31).
The finding that Cyt cyRs does not play a significant role in Ps growth of R. sphaeroides also is consistent with earlier studies performed with mutants lacking Cyt c2Rs (6, 10). Indeed, these mutants do not have any appreciable electron-transfer activity to the reaction center even though they harbor Cyt cyRs (10). Considering that Cyt cy homologs participate in Res electron transfer in some other species (22, 23) and that an unknown membrane-bound Cyt c has been proposed as an electron donor to the aa3-type Cyt c oxidase in R. sphaeroides (32), we studied whether Cyt cyRs mediates electron transfer to the Cyt c oxidase(s). Growth phenotypes and biochemical assays of appropriate strains showed that Cyt cyRs is able to replace R. capsulatus endogenous Cyt cy and Cyt c2 only for Res electron transfer. In addition, its Cyt c domain was sufficient for this activity as indicated by the Cyt c exchange chimera encoded by pHM126 (Table 1). Thus, the reason why Cyt cyRs, which is able to donate electrons efficiently to the Cyt c oxidase, is unable to do so to the reaction center is intriguing. It is noteworthy that only some of the Lys residues of equine Cyt c thought to be important for interactions with its redox partners are conserved in Cyt cyRs (Fig. 2). In addition, the Cyt c2 binding surface of the reaction center is negatively charged (33, 34), and there is a marked difference between the calculated isoelectric points of Cyt cyRs (pI = 4.74) and Cyt cyRc (pI = 7.76). It may be that the precise location of the charged residues affects the ability of Cyt cyRs to form a productive complex with the reaction center under physiological conditions. This has been proposed to be the case for acidic isocytochrome c2 (pI = 4.87) of R. sphaeroides, which has ≈40-fold lower affinity for the reaction center in vitro compared with Cyt c2Rs (pI = 7.01). It is thought that the negative charge of isocytochrome c2 brings about its lowered affinity toward the reaction center by changing the orientation of the dipole moment at the isocytochrome c2 docking surface (35). In any event, a relatively low affinity for binding to the reaction center of the Cyt c domain of the Cyt cyRs may explain, at least partially, the Ps incapability of Cyt cyRs and suggests that its interactions with the Cyt c oxidase may be different than those with the reaction center. In this regard, it remains to be seen whether Cyt cyRs is a preferred electron carrier either for the aa3-type or the cbb3-type Cyt c oxidase in R. sphaeroides.
It is interesting to note that the linker-anchor exchange chimera encoded by pHM127 is not detected in R. capsulatus cells grown by either photosynthesis or respiration. Because the transcriptional and translational regulatory regions of this construct are identical to those of cycYRc, which is known to be active under both growth conditions (36), it is likely that the absence of this chimera is caused by posttranslational events. If so, it seems that the structure of the linker regions of various Cyt cy homologs plays an important role in determining their relative steady-state stability and also specificity, possibly by mediating appropriate interactions with their redox partners (22, 23, 36).
Finally, it is widely believed that the ancestor of the mitochondria was a proteobacterium, similar to those investigated herein, carrying both the Cyt c2 and Cyt cy (37). Interestingly, a homolog of Cyt cyRs, but not of Cyt c2, has been uncovered recently in the genome of Rickettsia prowazekii (38) that is related closely to mitochondria, as determined by phylogenetic analyses. However, no homolog of Cyt cy has been detected in mitochondria, which suggests that soluble Cyt c have been favored during the evolution of eukaryotic cells. Excitingly, in addition to the electron-transfer role, Cyt c recently has been implicated as an activator of apoptotic proteases and nucleases (reviewed in ref. 39). This unexpected function of Cyt c requires its diffusion out of the mitochondria, a process that is not readily feasible for membrane-attached Cyt cy. Thus, the dual function of eukaryotic Cyt c implies why mitochondria might have conserved soluble electron carriers over their membrane-attached relatives.
The work described here shows that, although Cyt cyRc can support both Ps and Res growth of R. capsulatus, its R. sphaeroides homolog, Cyt cyRs, can participate only in the Res electron transfer, at least partly because of the intrinsic properties of its Cyt c subdomain. Therefore, it seems that the different functional properties of various electron carriers clearly endow facultative phototrophs with an excellent way to regulate the flux of electrons through the respiratory and Ps branches of their ETCs.
Acknowledgments
We thank K. Duyck for the construction of pKD2 and J. Zeilstra-Ryalls and S. Kaplan for providing us with a plasmid carrying cycYRs. This work was supported by Department of Energy Grant DE-FG02-91ER20052 to F.D. and Ministero Università, Ricerca Scientifica e Technologica of Italy Grant CO-FIN 1997 to D.Z.
ABBREVIATIONS
- ETC
electron-transfer chain
- Ps
photosynthetic
- Res
respiratory
- Cyt c
cytochrome c
- Qox
quinol oxidase
- TMBZ
3,3′,5,5′-tetramethylbenzidine
- MPYE
mineral-peptone-yeast extract
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (the accession no. for the 1.25-kb EcoRI–BglII fragment of pKD2 encompassing cycYRs is AF076611).
References
- 1.Nicholls D G, Ferguson S J. Bioenergetics 2. London: Academic; 1992. [Google Scholar]
- 2.Zannoni D, Daldal F. Arch Microbiol. 1993;160:413–423. doi: 10.1007/BF00245301. [DOI] [PubMed] [Google Scholar]
- 3.Jenney F E, Daldal F. EMBO J. 1993;12:1283–1292. doi: 10.1002/j.1460-2075.1993.tb05773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray K A, Grooms M, Myllykallio H, Moomaw C R, Slaughter C A, Daldal F. Biochemistry. 1994;33:3120–3127. doi: 10.1021/bi00176a047. [DOI] [PubMed] [Google Scholar]
- 5.Hochkoeppler A, Jenney F E, Lang S E, Zannoni D, Daldal F. J Bacteriol. 1995;177:608–613. doi: 10.1128/jb.177.3.608-613.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donohue T J, McEwan A G, Van D S, Crofts A R, Kaplan S. Biochemistry. 1988;27:1918–1925. doi: 10.1021/bi00406a018. [DOI] [PubMed] [Google Scholar]
- 7.Gennis R B, Casey R P, Azzi A, Ludwig B. Eur J Biochem. 1982;125:189–195. doi: 10.1111/j.1432-1033.1982.tb06667.x. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Horsman J A, Berry E, Shapleigh J P, Alben J O, Gennis R B. Biochemistry. 1994;33:3113–3119. doi: 10.1021/bi00176a046. [DOI] [PubMed] [Google Scholar]
- 9.Zeillstra-Ryalls J H, Kaplan S. J Bacteriol. 1995;177:6422–6431. doi: 10.1128/jb.177.22.6422-6431.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenney F E, Prince R C, Daldal F. Biochim Biophys Acta. 1996;1273:159–164. doi: 10.1016/0005-2728(95)00137-9. [DOI] [PubMed] [Google Scholar]
- 11.Myllykallio H, Jenney F E, Moomaw C R, Slaughter C A, Daldal F. J Bacteriol. 1997;179:2623–2631. doi: 10.1128/jb.179.8.2623-2631.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paddock M, Rongey S, Feher G, Okamura M. Proc Natl Acad Sci USA. 1989;86:6602–6606. doi: 10.1073/pnas.86.17.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daldal F, Cheng S, Applebaum J, Davidson E, Prince R C. Proc Natl Acad Sci USA. 1986;83:2012–2016. doi: 10.1073/pnas.83.7.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daldal F. J Bacteriol. 1988;170:2388–2391. doi: 10.1128/jb.170.5.2388-2391.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 16.Simons R, Priefer U, Puhler A. Bio/Technology. 1980;1:784–791. [Google Scholar]
- 17.Ditta G, Schmidhauser T, Yakobson E, Lu P, Liang X-W, Finlay D R, Guiney D, Helinski D R. Plasmid. 1985;13:149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- 18.Yen H C, Hu N T, Marrs B L. J Mol Biol. 1979;131:157–168. doi: 10.1016/0022-2836(79)90071-8. [DOI] [PubMed] [Google Scholar]
- 19.Caffrey M, Davidson E, Cusanovich M, Daldal F. Arch Biochem Biophys. 1992;292:419–426. doi: 10.1016/0003-9861(92)90011-k. [DOI] [PubMed] [Google Scholar]
- 20.Lowry O, Rosebrough N, Farr A, Randall R. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 21.Thomas P E, Ryan D, Levin W. Anal Biochem. 1976;75:168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- 22.Turba A, Jetzek M, Ludwig B. Eur J Biochem. 1995;231:259–265. [PubMed] [Google Scholar]
- 23.Bott M, Ritz D, Hennecke H. J Bacteriol. 1991;173:6766–6772. doi: 10.1128/jb.173.21.6766-6772.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margoliash E, Bosshard H. Trends Biochem Sci. 1983;8:316–320. [Google Scholar]
- 25.Moore G R, Pettigrew G W. Cytochromes c: Evolutionary, Structural & Physicochemical Aspects. Berlin: Springer; 1990. [Google Scholar]
- 26.Whale F R, Jones O T G. Biochim Biophys Acta. 1970;223:146–157. doi: 10.1016/0005-2728(70)90139-8. [DOI] [PubMed] [Google Scholar]
- 27.Zannoni D. In: Anoxygenic Photosynthetic Bacteria. Blankenship R E, Madigan M T, Bauer C E, editors. Norwell, MA: Kluwer; 1995. pp. 949–971. [Google Scholar]
- 28.Marrs B, Gest H. J Bacteriol. 1973;114:1045–1051. doi: 10.1128/jb.114.3.1045-1051.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cullen P J, Kaufman C K, Bowman W C, Kranz R G. J Biol Chem. 1997;272:27266–27273. doi: 10.1074/jbc.272.43.27266. [DOI] [PubMed] [Google Scholar]
- 30.Koch H, Hwang O, Daldal F. J Bacteriol. 1998;180:969–978. doi: 10.1128/jb.180.4.969-978.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vlcek C, Paces V, Maltsev N, Paces J, Haselkorn R, Fonstein M. Proc Natl Acad Sci USA. 1997;94:9384–9388. doi: 10.1073/pnas.94.17.9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosler J P, Fetter J, Tecklenburg M J, Espe M, Lerma C, Ferguson-Miller S. J Biol Chem. 1992;267:24264–24272. [PubMed] [Google Scholar]
- 33.Tiede D M, Vashishta A C, Gunner M R. Biochemistry. 1993;32:4515–4531. doi: 10.1021/bi00068a006. [DOI] [PubMed] [Google Scholar]
- 34.Adir N, Axelrod H L, Beroza P, Isaacson R A, Rongey S H, Okamura M Y, Feher G. Biochemistry. 1997;35:2535–2547. doi: 10.1021/bi9522054. [DOI] [PubMed] [Google Scholar]
- 35.Witthuhn V C, Goa J, Hong S, Halls S, Rott M A, Wraight C A, Crofts A R, Donohue T J. Biochemistry. 1997;36:903–911. doi: 10.1021/bi961648k. [DOI] [PubMed] [Google Scholar]
- 36.Myllykallio H, Drepper F, Mathis P, Daldal F. Biochemistry. 1998;37:5501–5510. doi: 10.1021/bi973123d. [DOI] [PubMed] [Google Scholar]
- 37.Margulis L. Symbiosis in Cell Evolution. New York: Freeman; 1981. [Google Scholar]
- 38.Andersson S G, Zomorodipour A, Andersson J O, Sicheritz-Pontèn T, Alsmark U C M, Podowski R M, Näslund A K, Eriksson A-S, Winkler H H, Kurland C G. Nature (London) 1998;396:133–140. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- 39.Reed J C. Cell. 1997;91:559–562. doi: 10.1016/s0092-8674(00)80442-0. [DOI] [PubMed] [Google Scholar]