Abstract
Solid state nuclear magnetic resonance (NMR) spectroscopy is particularly useful in structural studies of amyloid fibrils because solid state NMR techniques have unique capabilities as site-specific, molecular-level structural probes of noncrystalline materials. These techniques provide experimental data that strongly constrain the secondary, tertiary, and quaternary structures of amyloid fibrils, permitting the development of experimentally-based structural models. Examples of techniques that are applicable to amyloid samples prepared with isotopic labeling of specific sites and to samples prepared with uniform isotopic labeling of selected residues are presented, illustrating the utility of the various techniques and labeling schemes. Information regarding the preparation of amyloid samples for solid state NMR measurements is also included.
I. Introduction
The phrase “solid state NMR” simply means nuclear magnetic resonance (NMR) techniques that are applicable to samples that are solids (crystalline or noncrystalline) or solid-like (e.g., phospholipid bilayer membranes, precipitated protein aggregates). As demonstrated by recent studies in our laboratory (Antzutkin et al., 2000; Antzutkin et al., 2003; Antzutkin et al., 2002; Balbach et al., 2000; Balbach et al., 2002; Gordon et al., 2004; Oyler and Tycko, 2004; Petkova et al., 2004; Petkova et al., 2002; Petkova et al., 2005; Tycko and Ishii, 2003) and in other laboratories (Benzinger et al., 1998; Benzinger et al., 2000; Burkoth et al., 2000; Gregory et al., 1998; Heller et al., 1996; Jaroniec et al., 2002b; Jaroniec et al., 2004; Kammerer et al., 2004; Lansbury et al., 1995; Laws et al., 2001; Naito et al., 2004; Siemer et al., 2005), amyloid fibrils are excellent systems for solid state NMR investigations. This is because (i) amyloid fibrils, although noncrystalline, do have well-defined molecular structures and therefore yield solid state NMR data that are of high quality and have clear interpretations, (ii) amyloid fibrils can be prepared with selective or uniform isotopic labeling in the multimilligram quantities required for most solid state NMR measurements, (iii) amyloid fibrils can be prepared in high concentrations by lyophilization or centrifugation, leading to good signal-to-noise ratios in the solid state NMR data, (iv) the structural information available from solid state NMR measurements is arguably more direct and specific than information available from other measurements, and (v) the molecular structures of amyloid fibrils are of great current interest in the biomedical, biophysical, and biochemical research communities.
Based on x-ray fiber diffraction data, we know that the principal structural motifs in amyloid fibrils are cross-β motifs, i.e., ribbon-like β-sheets extending over the length of the fibrils, comprised of β-strand peptide segments oriented approximately perpendicular to the long fibril axis and linked by backbone hydrogen bonds oriented approximately parallel to the long axis (Sunde and Blake, 1998; Tycko, 2004). The reality of the cross-β motif in fibrils formed by the β-amyloid peptide associated with Alzheimer's disease (Aβ) has been confirmed by electron microscopy (EM) (Serpell and Smith, 2000) and by recent solid state NMR measurements on oriented fibrils (Oyler and Tycko, 2004). Given that amyloid fibrils are constructed from β-sheets, the molecular structures of amyloid fibrils can be discussed at the levels of primary, secondary, tertiary, and quaternary structure, in analogy to the classification of structure in globular proteins and protein complexes. Primary structure refers to the amino acid sequence. For amyloid fibrils, secondary structure refers to the locations of β-strand and non-β-strand segments and of ordered and disordered segments. Tertiary structure refers to the arrangement of β-strands into parallel or antiparallel β-sheets. Quaternary structure refers to the relative orientation of and contacts between β-sheets. As explained below, solid state NMR data can be used to determine or place strong constraints on secondary, tertiary, and quaternary structure in amyloid fibrils. These constraints permit the development of complete structural models based entirely on experimental data (Burkoth et al., 2000; Jaroniec et al., 2004; Kammerer et al., 2004; Lansbury et al., 1995; Petkova et al., 2002).
II. Determination of secondary structure
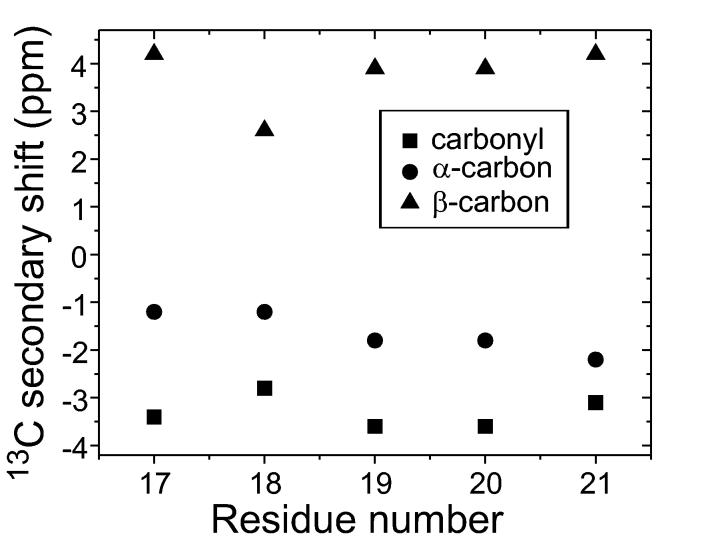
Constraints on secondary structure come from at least two types of solid state NMR measurements. One approach is simply to measure isotropic 13C NMR chemical shifts in fibrils that are prepared with 13C labels at specific carbonyl, α-carbon, or β-carbon si tes (Balbach et al., 2002; Heller et al., 1996; Laws et al., 2001; Naito et al., 2004) or with uniformly 13C-labeled residues (Balbach et al., 2000; Jaroniec et al., 2002b; Jaroniec et al., 2004; Petkova et al., 2004; Petkova et al., 2002; Petkova et al., 2005; Siemer et al., 2005). In β-strand segments, carbonyl and α-carbon lines are shifted upfield relative to “random coil” chemical shift values, while β-carbon lines are shifted downfield (Balbach et al., 2000; Petkova et al., 2002; Saito, 1986; Spera and Bax, 1991; Wishart et al., 1991). Observation of β-strand-like 13C chemical shifts for several sequential residues provides strong evidence for a β-strand encompassing these residues. As an example, 13C chemical shift data for Aβ16-22 fibrils (where Aβn-m means residues n through m of the Aβ peptide) are plotted in Fig. 1 as “secondary shifts”, or the differences between observed chemical shifts and the corresponding random coil shifts.
Figure 1:
13C NMR secondary shifts (i.e., differences between observed chemical shifts and random coil shifts) for amyloid fibrils formed by Aβ16-22 (N-acetyl-KLVFFAE-amide). Negative secondary shifts for β-carbons and carbonyl carbons and positive secondary shifts for β-carbons indicate a β-strand containing residues 17-21 (Balbach et al., 2000).
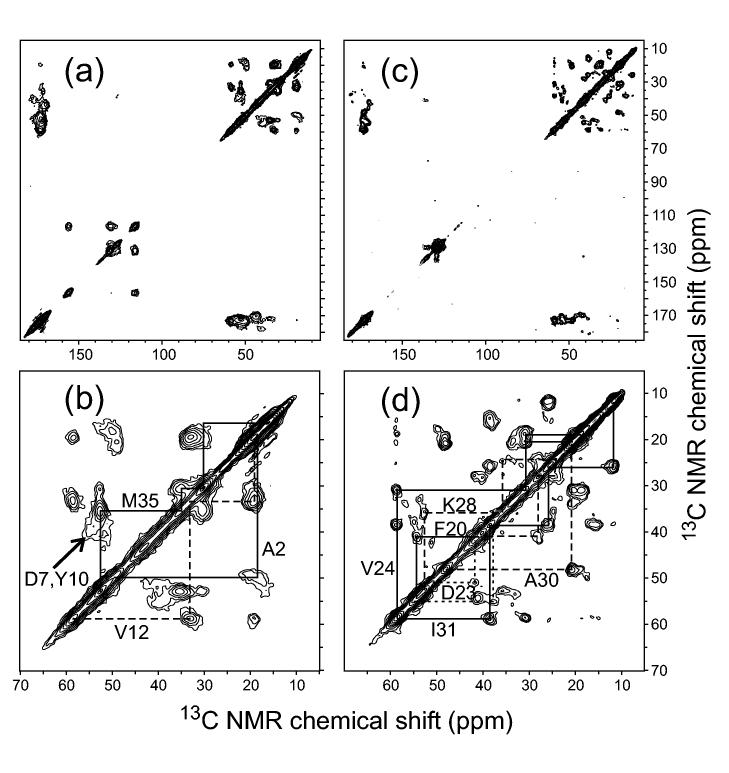
The chemical shift measurements are carried out with magic-angle spinning (MAS), a standard solid state NMR technique in which samples are placed in cylindrical capsules (called MAS rotors) and spun rapidly about an axis that makes an angle with the magnetic field of the NMR spectrometer, using a pneumatic turbine system. MAS at 5-25 kHz frequencies averages out both chemical shift anisotropies (CSA) and nuclear magnetic dipole-dipole couplings (e.g., between 13C-13C and 15N-13C pairs), resulting in relatively sharp NMR lines at the isotropic chemical shift positions. Dipole-dipole couplings to protons are not removed efficiently by MAS at readily achievable spinning frequencies and therefore must be removed by high-power proton decoupling. In the case of samples with uniformly labeled residues, two-dimensional (2D) spectroscopy (or higher-dimensional spectroscopy) is usually required for resolution and assignment of the 13C MAS NMR lines (Balbach et al., 2000; Jaroniec et al., 2002b; Jaroniec et al., 2004; Petkova et al., 2004; Petkova et al., 2002; Petkova et al., 2005; Siemer et al., 2005). Fig. 2 shows examples of 2D solid state 13C-13C NMR spectra of Aβ1-40 fibrils, synthesized with uniform 15N and 13C labeling of selected residues.
Figure 2:
Two-dimensional 13C NMR spectra of Aβ1-40 fibrils in lyophilized form, obtained with magic-angle spinning under conditions that produce strong crosspeaks for directly bonded 13C pairs (Ishii, 2001; Petkova et al., 2002). Full spectra (a,c) and expansions of the aliphatic regions (b,d) are shown for fibrils that are uniformly 15N,13C-labeled at A2, D7, G9, Y10, V12, and M35 (a,b) or F10, D23, V24, K28, G29, A30, and I31 (c,d). Solid and dashed lines indicate crosspeak connectivities used for resonance assignment. Note the broad crosspeaks for A2 and D7, which support the existence of a disordered N-terminal segment in Aβ1-40 fibrils. Spectra were recorded in a 9.4 T field, with magic-angle spinning at approximately 23 kHz.
A more sophisticated and potentially more powerful approach is to use a class of solid state NMR techniques that are collectively called “tensor correlation methods” (Blanco and Tycko, 2001; Chan and Tycko, 2003; Costa et al., 1997a; Costa et al., 1997b; Dabbagh et al., 1994; Feng et al., 1997; Feng et al., 1996; Ishii et al., 1996; McDermott et al., 1994; Reif et al., 2000; SchmidtRohr, 1996a; SchmidtRohr, 1996b; Takegoshi et al., 2000; Tycko and Dabbagh, 1991; Weliky et al., 1993; Weliky and Tycko, 1996). These techniques provide quantitative constraints on the relative orientations of pairs of chemical bonds (e.g., the relative orientation of a 15N-H bond and a 13Cα-H bond within one residue, which depends on the backbone ϕ torsion angle for this residue) or pairs of functional groups (e.g., the relative orientation of sequential 13C-labeled backbone carbonyl sites, which depends on the backbone ϕ and ψ torsion angles between the labeled sites). Tensor correlation methods are particularly useful for determining sidechain conformations or backbone conformations at residues where the chemical shift data suggest a non-β-strand conformation (Antzutkin et al., 2003).
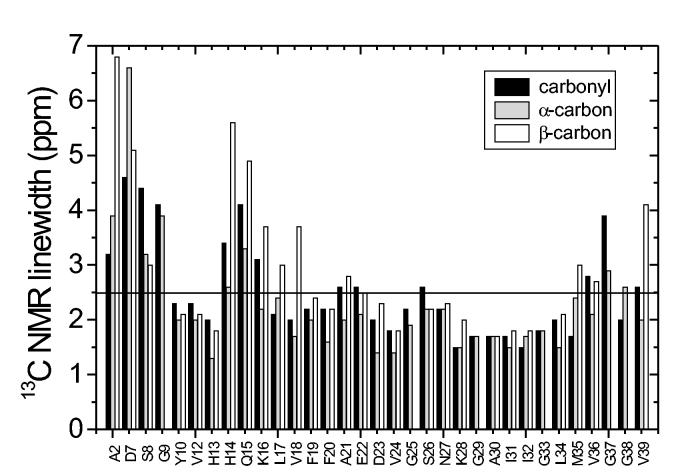
Structurally ordered segments in amyloid fibrils can be distinguished from disordered segments by measurements of 13C MAS NMR linewidths. In lyophilized samples, ordered segments exhibit linewidths of roughly 2 ppm or less for backbone sites, while disordered segments exhibit linewidths greater than 3 ppm (Balbach et al., 2000; Petkova et al., 2004; Petkova et al., 2002). For comparison, solid state 13C MAS NMR linewidths for peptides that are tightly bound to antibodies, and are therefore structurally ordered, are 1.5-2.5 ppm in noncrystalline frozen solutions (Sharpe et al., 2004; Weliky et al., 1999). Fully hydrated amyloid samples may exhibit narrower lines for ordered segments (although not necessarily), and may exhibit missing signals for disordered segments that undergo large-amplitude motions when hydrated (Jaroniec et al., 2002b; Jaroniec et al., 2004; Petkova et al., 2004; Siemer et al., 2005). Fig. 3 shows 13C NMR linewidth data extracted from 2D spectra of Aβ1-40 fibrils.
Figure 3:
13C NMR linewidths (full width at half maximum) extracted from two-dimensional spectra as in Fig. 2. These data indicate conformational disorder in the N-terminal segment, in the vicinity of Q15, and (to a lesser extent) in residues 35-39. Other residues are well ordered.
To date, helical secondary structure has not been observed in amyloid fibrils by solid state NMR, although helical segments in peptides and proteins can be identified by either 13C NMR chemical shift measurements (Havlin and Tycko, 2005) or tensor correlation methods (Blanco et al., 2001; Blanco and Tycko, 2001; Chan and Tycko, 2003; Long and Tycko, 1998). Solid state NMR can also be used to characterize β-turn conformations (Ashida et al., 2002; Sharpe et al., 2004; Weliky et al., 1999; Yao and Hong, 2004). Recent models for amyloid fibrils derived from solid state NMR and other data (Guo et al., 2004; Jimenez et al., 2002; Petkova et al., 2002; Williams et al., 2004) assign the observed non-β-strand segments to “bend” or “loop” structures, rather than the standard β-turns invoked in some earlier models (George and Howlett, 1999; Lazo and Downing, 1998; Li et al., 1999).
III. Determination of tertiary structure
As reflected in structural models for Aβ fibrils published before 2000 (Chaney et al., 1998; George and Howlett, 1999; Lazo and Downing, 1998; Li et al., 1999), a consensus once existed that the cross-β motifs in amyloid fibrils were comprised of antiparallel β-sheets. This belief was overturned by solid state NMR studies of Aβ10-35 fibrils by Lynn, Meredith, Botto, and coworkers (Benzinger et al., 1998; Benzinger et al., 2000; Burkoth et al., 2000; Gregory et al., 1998), which showed that Aβ10-35 fibrils contain only in-register parallel β-sheets (i.e., β-sheets in which residue k in one β-strand forms backbone hydrogen bonds with residues k-1 and k+1 of a neighboring β-strand). Subsequently, in-register parallel β-sheets have been discovered in other amyloid fibrils, including full-length Aβ fibrils (Antzutkin et al., 2000; Antzutkin et al., 2002; Balbach et al., 2002; Petkova et al., 2005; Torok et al., 2002), by solid state NMR (Antzutkin et al., 2000; Antzutkin et al., 2002; Balbach et al., 2002; Gordon et al., 2004) and other techniques (Der-Sarkissian et al., 2003; Jayasinghe and Langen, 2004; Torok et al., 2002). However, certain amyloid fibrils have been shown to contain antiparallel β-sheets, particularly fibrils formed by relatively short peptides (Balbach et al., 2000; Gordon et al., 2004; Kammerer et al., 2004; Lansbury et al., 1995; Petkova et al., 2004), implying that there is no truly universal molecular structure for amyloid fibrils. Results obtained to date suggest that parallel β-sheets are favored in amyloid fibrils because parallel alignment of β-strand segments maximizes hydrophobic contacts (Antzutkin et al., 2000; Petkova et al., 2002) and permits favorable interactions among polar sidechains, dubbed “polar zippers” by M.F. Perutz (Perutz et al., 1994), within a single β-sheet. Antiparallel β-sheets can be favored by electrostatic interactions, but have been found in amyloid fibrils comprised of peptides with hydrophobic segments only when the requirement for optimal hydrophobic contacts can also be met with antiparallel alignment of β-strand segments (Balbach et al., 2000; Gordon et al., 2004; Lansbury et al., 1995; Petkova et al., 2004).
Constraints on tertiary structure are obtained from measurements of nuclear magnetic dipole-dipole couplings, whose strength is proportional to , where γI and γS are the gyromagnetic ratios of coupled nuclei I and S and RIS is the internuclear distance. For a 13C-13C pair at RIS = 4.8 Å (the interstrand distance in a β-sheet), the coupling strength is 69 Hz, implying a 15 ms time scale for evolution of NMR signals from 13 C nuclei with 4.8 Å separations. For a 15N-13C pair at RIS = 4.1 Å (the approximate distance between an amide nitrogen and a carbonyl carbon in an interstrand hydrogen bond), the coupling strength is 45 Hz, implying a 22 ms time scale.
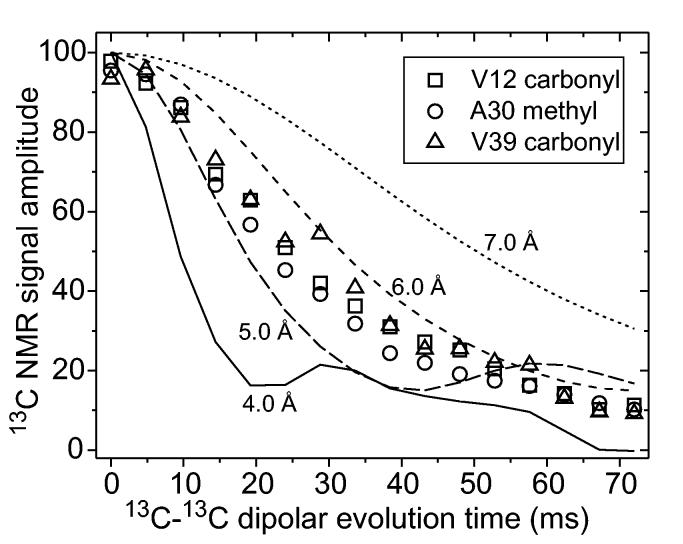
In-register parallel β-sheets can be identified by synthesizing a series of peptide samples with single 13C labels at backbone or sidechain sites (Antzutkin et al., 2000; Balbach et al., 2002; Benzinger et al., 1998; Gregory et al., 1998). Because carbonyl and methyl carbon sites have relatively weak dipole-dipole couplings to proteins and hence exhibit relatively long transverse spin relaxation times (T2 values) under typical experimental conditions, it has proven most useful to label backbone carbonyl and alanine methyl sites. Measurements of intermolecular 13C-13C dipole-dipole couplings, as in Fig. 4 for Aβ1-40 fibrils, permit intermolecular distances up to at least 6 Å to be measured with approximately ±10% accuracy (limited by effects of T2 relaxation and pulse sequence imperfections). Measurements of intermolecular distances between backbone carbonyl sites alone may be incapable of distinguishing in-register alignment of β-strands from a one-residue shift in alignment, as examination of molecular models for parallel β-sheets shows that a one-residue shift only increases the nearest-neighbor intermolecular distances for backbone carbonyl labels from 4.8 Å to approximately 5.2 Å. However, nearest-neighbor intermolecular distances for alanine methyl carbon labels increase from 4.8 Å to approximately 6.7 Å with a one-residue shift. Therefore, data such as those in Fig. 4 can only be explained by an in-register parallel β-sheet structure.
Figure 4:
Experimental constraints on intermolecular distances in Aβ1-40 fibrils, using the fpRFDR-CT technique (Balbach et al., 2002; Ishii et al., 2001) to measure 13C-13C nuclear magnetic dipole-dipole couplings in samples that are 13C labeled at single sites in V12, A30, or V39. Simulations for linear chains of 13C nuclei with nearest-neighbor spacings from 4.0 Å to 7.0 Å are shown. These data indicate that the labeled residues participate in in-register parallel β-sheets. Data were obtained in 9.4 T field, with magic-angle spinning at 20.0 kHz.
Data in Fig. 4 were obtained under MAS, using a pulse sequence technique called “constant-time finite-pulse radiofrequency-driven recoupling” (fpRFDR-CT) (Balbach et al., 2002; Ishii et al., 2001). This technique is one example of a class of solid state NMR methods called “recoupling” techniques, in which radiofrequency pulses are applied in synchrony with sample rotation to restore dipole-dipole couplings or other nuclear spin interactions that would otherwise be averaged out by MAS (Bennett et al., 1998; Gullion and Schaefer, 1989; Gullion and Vega, 1992; Hohwy et al., 1998; Ishii, 2001; Mehta et al., 1996; Meier and Earl, 1986; Tycko and Dabbagh, 1990; Tycko et al., 1989). The fpRFDR-CT technique has the advantages of being relatively insensitive to T2 effects, compatible with high-speed MAS, and effective even in the presence of the large 13C CSA characteristic of carbonyl carbons.
NMR signal decay curves recorded under fpRFDR-CT or other dipolar recoupling techniques are primarily determined by the shortest internuclear distances, i.e., the strongest dipole-dipole couplings. Thus, these data do not readily distinguish a propagating in-register parallel β-sheet structure from, for example, a structure that alternates between in-register parallel and antiparallel (or shifted) alignment of neighboring β-strands. Multiple quantum 13C NMR measurements, which are sensitive to the entire network of dipole-dipole couplings among many 13C spins, can distinguish among these and other structures (Antzutkin et al., 2000). Multiple quantum 13C NMR data show that the in-register parallel alignment of peptide chains in Aβ1-40 fibrils extends over at least four successive peptide chains in the β-sheets (and probably hundreds of β-strands).
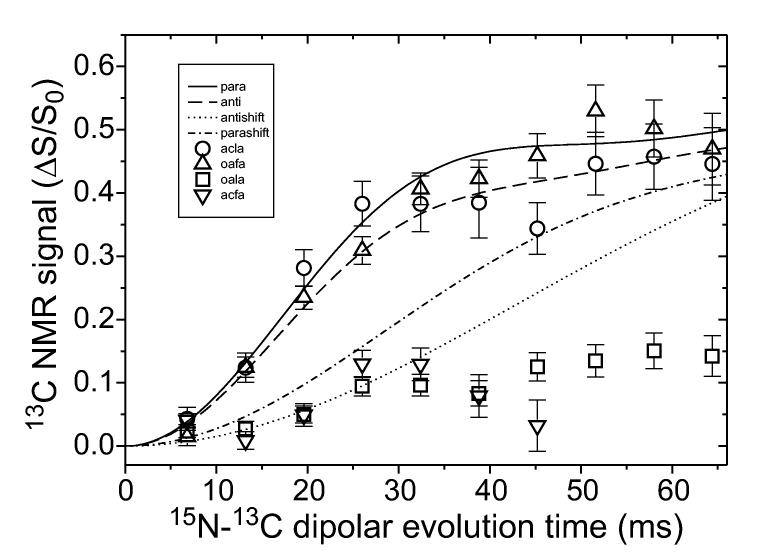
Antiparallel β-sheets have also been demonstrated by solid state NMR to exist in amyloid fibrils. Measurements of either 13C-13C (Lansbury et al., 1995) or 15N-13C (Balbach et al., 2000; Gordon et al., 2004; Kammerer et al., 2004; Petkova et al., 2004) intermolecular dipole-dipole couplings can be used to identify antiparallel β-sheets in samples with selective isotopic labeling. Measurements of intermolecular couplings between 15N-labeled backbone amide sites and 13C-labeled backbone carbonyl sites, using the rotational echo double resonance (REDOR) recoupling technique (Anderson et al., 1995; Gullion and Schaefer, 1989), are particularly powerful because the 4.1 Å internuclear distance for hydrogen bonded backbone amide and carbonyl sites is significantly shorter than other intermolecular distances in β-sheets. REDOR data such as those in Fig. 5 establish the registry of hydrogen bonds unambiguously.
Figure 5:
Measurements of intermolecular dipole-dipole couplings between 15N labels at backbone amide sites and 13C labels at backbone carbonyl sites in amyloid fibrils formed by Aβ16-22 with either acetyl (circles, downward triangles) or octanoyl (upward triangles, squares) groups at the N-terminus. These measurements use the REDOR solid state NMR technique (Anderson et al., 1995; Gullion and Schaefer, 1989). Fibrils are formed from mixtures of molecules that are 15N-labeled at A21 and molecules that are 13C-labeled at L17 (circles, squares) or F20 (triangles), with an approximate 1.6:1.0 ratio of 15N-labeled to 13C-labeled molecules. Curves are simulations in which the labeled residues are hydrogen bonded in parallel (solid line) or antiparallel (dashed line) β-sheets, or shifted from hydrogen bonded positions by one residue in parallel (dash-dotted line) or antiparallel (dotted line) β-sheets. These data indicate an antiparallel β-sheet structure with hydrogen bonds between residues 17+k and 21-k (k = 0, 1, 2, 3) for the acetyl peptide, and an in-register parallel β-sheet structure for the octanoyl peptide (Gordon et al., 2004). Data were obtained in a 9.4 T field, with magic-angle spinning at 5.00 kHz.
Constraints on secondary and tertiary structure from fpRFDR-CT, multiple quantum NMR, REDOR, and related solid state NMR measurements can be analyzed quantitatively by comparison of experimental data with accurate numerical simulations of the solid state NMR measurements. Such simulations contain no adjustable parameters other than the geometric parameters that describe the molecular structure, such as internuclear distances.
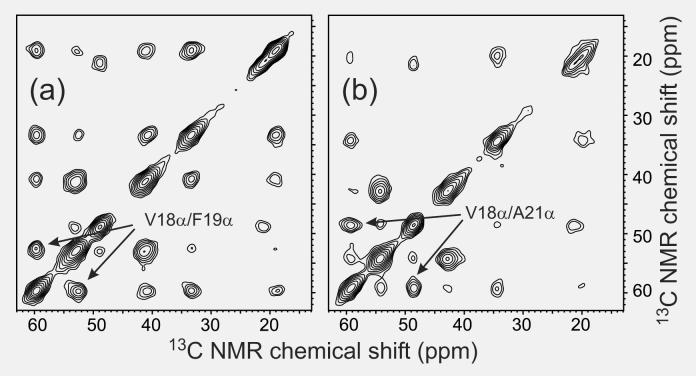
In samples with multiple uniformly labeled residues (or multiple residues 13C-labeled at α-carbons), the hydrogen bond registry in antiparallel β-sheets can also be established from 2D solid state 13C NMR spectra in which transfer of nuclear spin polarization between 13C-labeled sites occurs in three steps: (i) transfer from 13C nuclei to directly bonded protons; (ii) transfer among protons that are within approximately 3 Å of one another; (iii) transfer from protons to directly bonded 13C nuclei. Such “proton-mediated” transfers are particularly efficient for α-carbons that are directly opposite one another in antiparallel β-sheets, because the corresponding interstrand β-proton distances are approximately 2.1 Å. Proton-mediated 2D 13C-13C exchange spectra of antiparallel β-sheet structures therefore show strong, nonsequential crosspeaks connecting NMR lines of α-carbons that align with one another (Petkova et al., 2004; Tycko and Ishii, 2003). Fig. 6 shows examples of proton-mediated 2D spectra for Aβ11-25 fibrils prepared at pH 2.4 and pH 7.4. These spectra reveal that the hydrogen bond registry is pH-dependent, presumably due to variations in electrostatic interactions.
Figure 6:
Proton-mediated two-dimensional 13C-13C exchange spectra of Aβ11-25 fibrils grown at pH 7.4 (a) or pH 2.4 (b), with uniform 15N and 13C labeling of V18, F19, F20, and A21. Strong crosspeaks between α-carbon chemical shifts of V18 and α-carbon chemical shifts of either F19 (a) or A21 (b) indicate the hydrogen bond registry in antiparallel β-sheets (Petkova et al., 2004; Tycko and Ishii, 2003). At pH 7.4, residue 17+k is hydrogen bonded to residue 20-k. At pH 2.4, residue 17+k is hydrogen bonded to residue 22-k. Spectra were recorded in a 14.1 T field, with magic-angle spinning at 21.40 kHz.
IV. Determination of quaternary structure
Experimental constraints on quaternary structure from solid state NMR take the form of measurements of approximate distances, or “contacts”, between sidechains that project above or below the β-sheets, or between sidechain and backbone sites. Because the shortest distances between backbone atoms in different β-sheets are roughly 8 Å or more, corresponding to 13C-13C dipole-dipole couplings of 15 Hz or less, constraints on quaternary structure can not be obtained readily from backbone-backbone couplings.
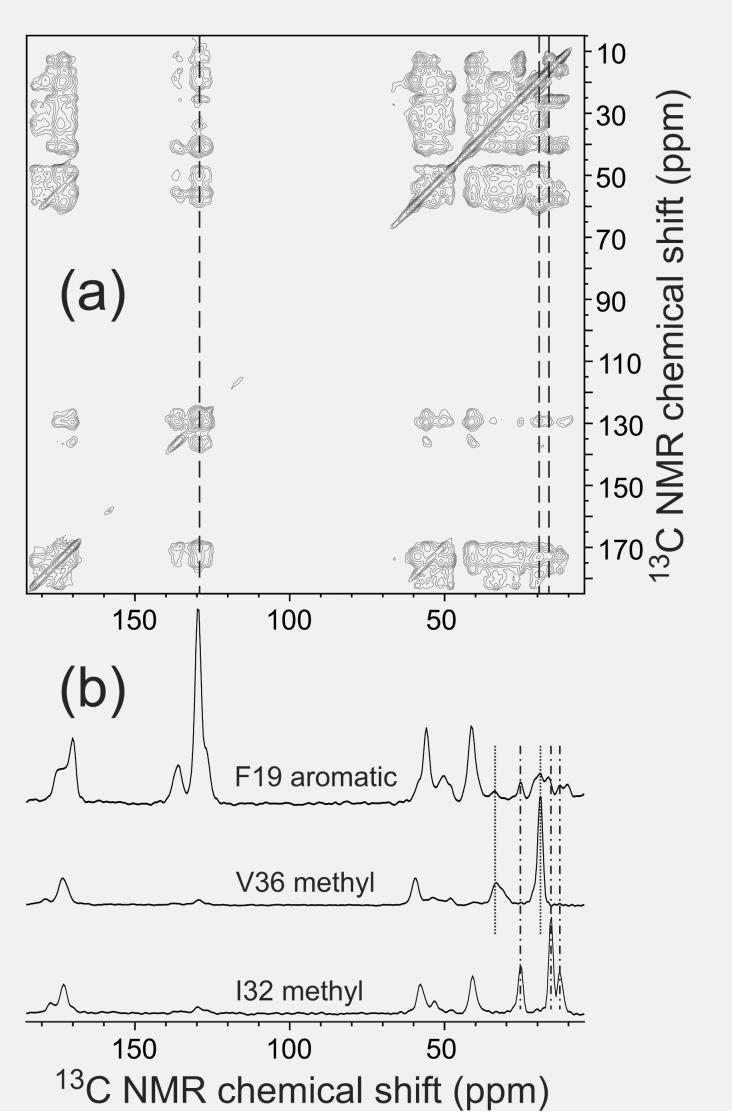
Semi-quantitative information about sidechain-sidechain distances can be obtained from 2D 13C-13C exchange spectra of amyloid fibril samples with multiple uniformly labeled residues, obtained under MAS and with exchange periods greater than 100 ms. An example is shown in Fig. 7. Under these conditions, all possible intraresidue crosspeaks are observed (rather than only the one-bond or two-bond crosspeaks that appear with shorter exchange periods, as in Fig. 2), and many inter-residue crosspeaks are observed for sequential pairs of labeled residues. In addition, nonsequential crosspeaks that can only arise from quaternary contacts may be observed. Because of the complexity of the dipole-dipole coupling networks in such measurements, internuclear distances can not be determined precisely from the crosspeak intensities. However, basic principles of nuclear spin interactions (Tycko and Dabbagh, 1992) imply that detectable crosspeaks in 2D 13C exchange spectra with exchange periods less than one second can only occur if the internuclear distances are less than 10 Å.
Figure 7:
(a) Two-dimensional 13C-13C exchange spectra of Aβ1-40 fibrils, prepared with uniform 15N and 13C labeling of K16, F19, A21, E22, I32, and V36, obtained with a 500 ms exchange period. (b) One-dimensional slices taken at positions indicated by the dashed lines in the two-dimensional spectrum. The slice at the chemical shift of F19 aromatic carbons shows crosspeaks at the chemical shifts of V36 aliphatic carbons (dotted lines) and I32 aliphatic carbons (dotdashed lines). These crosspeaks indicate contacts between aromatic and aliphatic sidechains, placing constraints on quaternary structure. The spectrum was recorded in a 14.1 T field, with magic-angle spinning at 18.00 kHz.
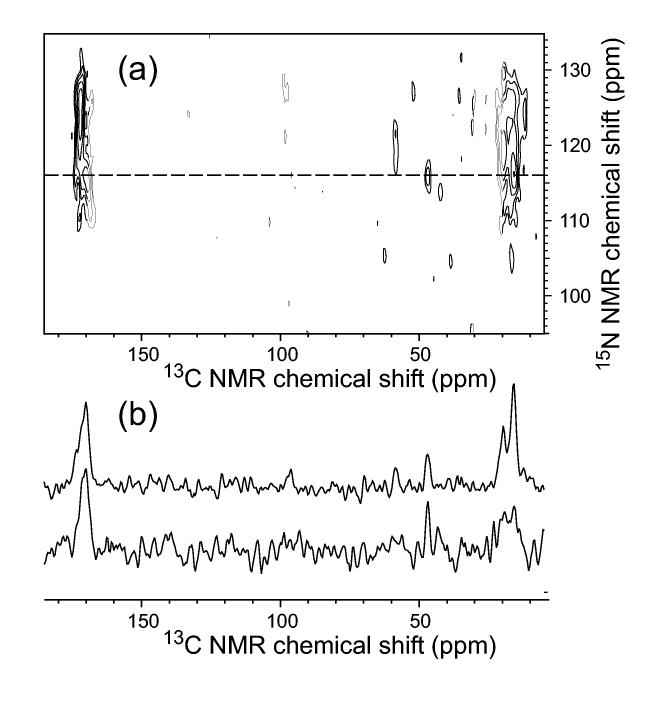
Constraints on quaternary structure in amyloid fibrils can also be obtained from measurements of 15N-13C dipole-dipole couplings. Two-dimensional 15N-13C transferred echo double resonance (TEDOR) techniques (Hing et al., 1992; Jaroniec et al., 2002a; Michal and Jelinski, 1997) are particularly useful for detecting the proximity of specific sidechain carbon sites to specific backbone nitrogen sites in samples with multiple uniformly labeled residues. An example is shown in Fig. 8. By comparing 2D 15N-13C TEDOR spectra of samples in which all molecules are isotopically labeled with spectra of samples in which labeled molecules are diluted in unlabeled molecules (prior to fibril formation), one can distinguish intermolecular 15N-13C contacts from intramolecular contacts.
Figure 8:
(a) Two-dimensional 15N-13C TEDOR spectrum of Aβ1-40 fibrils, prepared with uniform 15N and 13C labeling of I31, G33, M35, G37, and V39, obtained with 5.745 ms 15N-13C recoupling periods (Jaroniec et al., 2002a). Crosspeaks indicate the proximity of particular 13C-labeled backbone and sidechain sites to 15N-labeled backbone amide sites. (b) One-dimensional slices taken at the 15N chemical shift of G33, indicated by the dashed line in the two-dimensional spectrum, for samples grown from labeled Aβ1-40 (top) and from a 2:1 mixture of unlabeled and labeled Aβ1-40. The reduced amplitude of the M35 methyl crosspeak relative to the G33 carbonyl crosspeak in the isotopically diluted sample indicates that the M35 methyl/G33 amide contact is primarily intermolecular and therefore represents a quaternary constraint. Spectra were recorded in a 14.1 T field, with magic-angle spinning at 11.140 kHz.
V. Construction of molecular models based on solid state NMR data
In principle, the molecular structures of amyloid fibrils can be determined completely from solid state NMR data, with no assistance from other experimental techniques, in analogy to approaches commonly employed to determined complete structures of soluble proteins from solution NMR data. However, this approach would require solid state NMR constraints on many torsion angles and many internuclear distances (both intramolecular and intermolecular) for each residue in the amyloid-forming peptide or protein. The limited resolution and signal-to-noise in solid state NMR spectra and the difficulty of preparing fibril samples with arbitrary isotopic labeling patterns make this approach impractical, except possibly in the case of fibrils formed by relatively short peptides (Jaroniec et al., 2004).
Fortunately, information from other experimental techniques greatly simplifies the task of constructing realistic models for amyloid fibril structures. The fact that amyloid fibrils contain cross-β motifs implies that the number of possible quaternary structures is not large, once solid state NMR data that constrain the secondary and tertiary structures are available. This is because the cross-β motif requires that all β-strand segments be approximately perpendicular to a single axis (the long axis of the fibrils), with their backbone carbonyl and amide groups orientated to permit hydrogen bonds that are approximately parallel to the same axis. The number of possible quaternary structures is also strongly restricted by measurements of fibril dimensions in EM or atomic force microscope (AFM) images and by measurements of the mass-per-length of amyloid fibrils by scanning transmission electron microscopy (Antzutkin et al., 2002; Goldsbury et al., 2000; Petkova et al., 2005). In addition, the structural model should make sense from the standpoint of physical chemistry (e.g., exposure of hydrophobic groups to aqueous solvent should be minimized and burial of unpaired charges in the fibril core should be avoided). Detection of a small number of unambiguous contacts between β-sheets by solid state NMR may then be sufficient to determine the correct quaternary structure and hence the principal features of the full molecular structure. The precise details of sidechain conformations and backbone conformations at certain sites may remain undetermined, but these can be constrained by subsequent solid state NMR measurements as required.
These remarks apply to the determination of structural models for the cores of amyloid fibrils. As demonstrated definitively by biochemical experiments on yeast prion protein fibrils (Baxa et al., 2002), amyloid fibrils formed by large proteins can contain highly structured globular domains outside the fibril core. Determination of the structures of these domains in the context of amyloid fibrils would depend on solid state NMR strategies demonstrated recently in studies of microcrystalline globular proteins (Castellani et al., 2003; Lange et al., 2005).
Atomic coordinates for molecular models derived from solid state NMR constraints can be generated with molecular modeling and molecular dynamics (MD) software (Petkova et al., 2002). A relatively simple approach to the generation of atomic coordinates that reflect the experimental information consists of three steps: (i) construction of a peptide in a conformation that is approximately consistent with available data by manual assignment of backbone torsion angles, using molecular modeling software; (ii) construction of an initial model for a segment of the amyloid structure by manual combination of multiple copies of this peptide with appropriate translations and rotations; (iii) refinement of the model by alternating cycles of energy minimization and MD simulations, using appropriate software. In the energy minimization and MD simulations, experimental constraints on internuclear distances and on backbone and sidechain torsion angles are enforced by artificial harmonic potential functions. If the simulations are performed without explicit solvent, electrostatic interactions are turned off or attenuated by setting the dielectric constant to an artificially large value. To identify aspects of the structural model that are not restricted by experimental constraints, the MD simulations can be run at elevated temperatures and the results of multiple independent refinement attempts can be compared. Portions of the structure that are not constrained by experiments will appear to be disordered.
VI. Sample preparation for solid state NMR
Amyloid fibrils are typically prepared by incubation of peptides or proteins in aqueous or mixed solvents under controlled conditions of concentration, pH, ionic strength, and temperature. Formation of fibrils can be monitored by EM or AFM imaging of aliquots of the incubating solution. However, it is important to recognize that EM and AFM images do not necessarily show all aggregated structures (nonfibrillar as well as fibrillar) in the solution. Ultimately, only the absence of signals attributable to nonfibrillar components in the solid state NMR spectra themselves is proof that the sample is fully fibrillized. Complete conversion to fibrils is essential because all immobilized molecules in the sample will contribute approximately equally to the solid state NMR signals. Unlike EM and AFM, solid state NMR sees the entire sample.
Structural studies of amyloid fibrils using the techniques discussed above require roughly 1 μmole of isotopically labeled molecules to achieve an adequate signal-to-noise ratio in the data. With 1 μmole samples, 2D spectra are typically obtained in 0.5-10 days (depending on the details of the measurements) when the experiments are performed at room temperature and in 9.4 T or 14.1 T magnetic fields. Solid state NMR measurements can be performed either on centrifuged pellets of fibrils or on lyophilized samples. We find that pelleted and lyophilized samples have identical chemical shifts in 13C MAS NMR spectra (Petkova et al., 2004), indicating that lyophilization does not perturb the structures of amyloid fibrils. Certain sidechain 13C NMR linewidths are reduced in hydrated samples (either pelleted or lyophilized and subsequently rehydrated) relative to the linewidths in dry lyophilized samples, most likely reflecting sidechain motions in hydrated samples that are quenched in dry samples. Lyophilization permits a denser packing of fibrils into the MAS rotors, increasing the signal-to-noise ratio and facilitating high-speed MAS. Lyophilization also minimizes sample heating by radiofrequency pulses during NMR experiments.
EM images often show several distinct morphologies for fibrils that are formed by a single peptide or protein (Goldsbury et al., 2000; Jimenez et al., 2002). The distinct morphologies may differ in diameter, twist periodicity, and apparent propensity for lateral association. The sensitivity of 13C NMR chemical shifts to subtle variations in molecular structure has allowed us to demonstrate that distinct fibril morphologies have different underlying molecular structures (Petkova et al., 2005). The molecular structure propagates with the morphology when sonicated fibril fragments are used to seed the growth of new fibrils. Existing data suggest that fibrils with different morphologies have different quaternary structures and different conformations in non-β-strand segments. These findings imply that careful attention must be paid to fibril growth conditions, which can affect the relative abundances of various fibril polymorphs, and to the detailed appearance of fibrils in EM images before comparisons of structural data from different research groups are made. The most definitive proof that two different groups are studying the same fibril structure would be that the two groups observe the same sets of NMR chemical shifts in their samples.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases and by the Intramural AIDS Targeted Antiviral Program of the National Institutes of Health. Data presented in the figures were obtained by Drs. Yoshitaka Ishii, Aneta T. Petkova, and John J. Balbach. Additional contributions to this work by Drs. Oleg N. Antzutkin, Richard D. Leapman, Stephen C. Meredith, David J. Gordon, Nathan A. Oyler, Jerry C.C. Chan, Gerd Buntkowsky, Anant K. Paravastu, Simon J. Sharpe, and Wai-Ming Yau are gratefully acknowledged.
Footnotes
prepared for Methods in Enzymology volume on “Amyloid, Prions, and Other Protein Aggregates, Part B”, edited by R. Wetzel and I. Kheterpal
References
- Anderson RC, Gullion T, Joers JM, Shapiro M, Villhauer EB, Weber HP. Conformation of 1-13C, 15N acetyl-l-carnitine: Rotational echo double resonance nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 1995;117:10546–10550. [Google Scholar]
- Antzutkin ON, Balbach JJ, Leapman RD, Rizzo NW, Reed J, Tycko R. Multiple quantum solid state NMR indicates a parallel, not antiparallel, organization of β-sheets in Alzheimer's β-amyloid fibrils. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13045–13050. doi: 10.1073/pnas.230315097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzutkin ON, Balbach JJ, Tycko R. Site-specific identification of non-β-strand conformations in Alzheimer's β-amyloid fibrils by solid state NMR. Biophys. J. 2003;84:3326–3335. doi: 10.1016/S0006-3495(03)70057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzutkin ON, Leapman RD, Balbach JJ, Tycko R. Supramolecular structural constraints on Alzheimer's β-amyloid fibrils from electron microscopy and solid state nuclear magnetic resonance. Biochemistry. 2002;41:15436–15450. doi: 10.1021/bi0204185. [DOI] [PubMed] [Google Scholar]
- Ashida J, Ohgo K, Asakura T. Determination of the torsion angles of alanine and glycine residues of bombyx mori silk fibroin and the model peptides in the silk I and silk II forms using 2D spin diffusion solid state NMR under off magic angle spinning. J. Phys. Chem. B. 2002;106:9434–9439. [Google Scholar]
- Balbach JJ, Ishii Y, Antzutkin ON, Leapman RD, Rizzo NW, Dyda F, Reed J, Tycko R. Amyloid fibril formation by Aβ16-22, a seven-residue fragment of the Alzheimer's β-amyloid peptide, and structural characterization by solid state NMR. Biochemistry. 2000;39:13748–13759. doi: 10.1021/bi0011330. [DOI] [PubMed] [Google Scholar]
- Balbach JJ, Petkova AT, Oyler NA, Antzutkin ON, Gordon DJ, Meredith SC, Tycko R. Supramolecular structure in full-length Alzheimer's β-amyloid fibrils: Evidence for a parallel β-sheet organization from solid state nuclear magnetic resonance. Biophys. J. 2002;83:1205–1216. doi: 10.1016/S0006-3495(02)75244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxa U, Speransky V, Steven AC, Wickner RB. Mechanism of inactivation on prion conversion of the Saccharomyces cerevisiae Ure2 protein. Proc. Natl. Acad. Sci. U. S. A. 2002;99:5253–5260. doi: 10.1073/pnas.082097899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AE, Rienstra CM, Griffiths JM, Zhen WG, Lansbury PT, Griffin RG. Homonuclear radiofrequency-driven recoupling in rotating solids. J. Chem. Phys. 1998;108:9463–9479. [Google Scholar]
- Benzinger TLS, Gregory DM, Burkoth TS, Miller-Auer H, Lynn DG, Botto RE, Meredith SC. Propagating structure of Alzheimer's β-amyloid(10-35) is parallel β-sheet with residues in exact register. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13407–13412. doi: 10.1073/pnas.95.23.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzinger TLS, Gregory DM, Burkoth TS, Miller-Auer H, Lynn DG, Botto RE, Meredith SC. Two-dimensional structure of β-amyloid(10-35) fibrils. Biochemistry. 2000;39:3491–3499. doi: 10.1021/bi991527v. [DOI] [PubMed] [Google Scholar]
- Blanco FJ, Hess S, Pannell LK, Rizzo NW, Tycko R. Solid-state NMR data support a helix-loop-helix structural model for the N-terminal half of HIV-1 Rev in fibrillar form. J. Mol. Biol. 2001;313:845–859. doi: 10.1006/jmbi.2001.5067. [DOI] [PubMed] [Google Scholar]
- Blanco FJ, Tycko R. Determination of polypeptide backbone dihedral angles in solid state NMR by double quantum 13C chemical shift anisotropy measurements. J. Magn. Reson. 2001;149:131–138. [Google Scholar]
- Burkoth TS, Benzinger TLS, Urban V, Morgan DM, Gregory DM, Thiyagarajan P, Botto RE, Meredith SC, Lynn DG. Structure of the β-amyloid(10-35) fibril. J. Am. Chem. Soc. 2000;122:7883–7889. [Google Scholar]
- Castellani F, van Rossum BJ, Diehl A, Rehbein K, Oschkinat H. Determination of solid state NMR structures of proteins by means of three-dimensional 15N-13C-13C dipolar correlation spectroscopy and chemical shift analysis. Biochemistry. 2003;42:11476–11483. doi: 10.1021/bi034903r. [DOI] [PubMed] [Google Scholar]
- Chan JCC, Tycko R. Solid state NMR spectroscopy method for determination of the backbone torsion angle psi in peptides with isolated uniformly labeled residues. J. Am. Chem. Soc. 2003;125:11828–11829. doi: 10.1021/ja0369820. [DOI] [PubMed] [Google Scholar]
- Chaney MO, Webster SD, Kuo YM, Roher AE. Molecular modeling of the Aβ1-42 peptide from Alzheimer's disease. Protein Eng. 1998;11:761–767. doi: 10.1093/protein/11.9.761. [DOI] [PubMed] [Google Scholar]
- Costa PR, Gross JD, Hong M, Griffin RG. Solid state NMR measurement of psi in peptides: A NCCN 2Q-heteronuclear local field experiment. Chem. Phys. Lett. 1997a;280:95–103. [Google Scholar]
- Costa PR, Kocisko DA, Sun BQ, Lansbury PT, Griffin RG. Determination of peptide amide configuration in a model amyloid fibril by solid state NMR. J. Am. Chem. Soc. 1997b;119:10487–10493. [Google Scholar]
- Dabbagh G, Weliky DP, Tycko R. Determination of monomer conformations in noncrystalline solid polymers by two-dimensional NMR exchange spectroscopy. Macromolecules. 1994;27:6183–6191. [Google Scholar]
- Der-Sarkissian A, Jao CC, Chen J, Langen R. Structural organization of β-synuclein fibrils studied by site-directed spin labeling. J. Biol. Chem. 2003;278:37530–37535. doi: 10.1074/jbc.M305266200. [DOI] [PubMed] [Google Scholar]
- Feng X, Eden M, Brinkmann A, Luthman H, Eriksson L, Graslund A, Antzutkin ON, Levitt MH. Direct determination of a peptide torsional angle psi by double-quantum solid state NMR. J. Am. Chem. Soc. 1997;119:12006–12007. [Google Scholar]
- Feng X, Lee YK, Sandstrom D, Eden M, Maisel H, Sebald A, Levitt MH. Direct determination of a molecular torsional angle by solid state NMR. Chem. Phys. Lett. 1996;257:314–320. [Google Scholar]
- George AR, Howlett DR. Computationally derived structural models of the β-amyloid found in Alzheimer's disease plaques and the interaction with possible aggregation inhibitors. Biopolymers. 1999;50:733–741. doi: 10.1002/(SICI)1097-0282(199912)50:7<733::AID-BIP6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Goldsbury CS, Wirtz S, Muller SA, Sunderji S, Wicki P, Aebi U, Frey P. Studies on the in vitro assembly of Aβ1-40: Implications for the search for Aβ fibril formation inhibitors. J. Struct. Biol. 2000;130:217–231. doi: 10.1006/jsbi.2000.4259. [DOI] [PubMed] [Google Scholar]
- Gordon DJ, Balbach JJ, Tycko R, Meredith SC. Increasing the amphiphilicity of an amyloidogenic peptide changes the β-sheet structure in the fibrils from antiparallel to parallel. Biophys. J. 2004;86:428–434. doi: 10.1016/S0006-3495(04)74119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory DM, Benzinger TLS, Burkoth TS, Miller-Auer H, Lynn DG, Meredith SC, Botto RE. Dipolar recoupling NMR of biomolecular self-assemblies: Determining inter- and intrastrand distances in fibrillized Alzheimer's β-amyloid peptide. Solid State Nucl. Magn. Reson. 1998;13:149–166. doi: 10.1016/s0926-2040(98)00086-1. [DOI] [PubMed] [Google Scholar]
- Gullion T, Schaefer J. Rotational-echo double-resonance NMR. J. Magn. Reson. 1989;81:196–200. doi: 10.1016/j.jmr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Gullion T, Vega S. A simple magic angle spinning NMR experiment for the dephasing of rotational echoes of dipolar coupled homonuclear spin pairs. Chem. Phys. Lett. 1992;194:423–428. [Google Scholar]
- Guo JT, Wetzel R, Ying X. Molecular modeling of the core of Aβ amyloid fibrils. Proteins. 2004;57:357–364. doi: 10.1002/prot.20222. [DOI] [PubMed] [Google Scholar]
- Havlin RH, Tycko R. Probing site-specific conformational distributions in protein folding with solid state NMR. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3284–3289. doi: 10.1073/pnas.0406130102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J, Kolbert AC, Larsen R, Ernst M, Bekker T, Baldwin M, Prusiner SB, Pines A, Wemmer DE. Solid-state NMR studies of the prion protein H1 fragment. Protein Sci. 1996;5:1655–1661. doi: 10.1002/pro.5560050819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hing AW, Vega S, Schaefer J. Transferred-echo double-resonance NMR. J. Magn. Reson. 1992;96:205–209. [Google Scholar]
- Hohwy M, Jakobsen HJ, Eden M, Levitt MH, Nielsen NC. Broadband dipolar recoupling in the nuclear magnetic resonance of rotating solids: A compensated C7 pulse sequence. J. Chem. Phys. 1998;108:2686–2694. [Google Scholar]
- Ishii Y. 13C-13C dipolar recoupling under very fast magic angle spinning in solid state nuclear magnetic resonance: Applications to distance measurements, spectral assignments, and high-throughput secondary-structure determination. J. Chem. Phys. 2001;114:8473–8483. [Google Scholar]
- Ishii Y, Balbach JJ, Tycko R. Measurement of dipole-coupled lineshapes in a many-spin system by constant-time two-dimensional solid state NMR with high-speed magic-angle spinning. Chem. Phys. 2001;266:231–236. [Google Scholar]
- Ishii Y, Terao T, Kainosho M. Relayed anisotropy correlation NMR: Determination of dihedral angles in solids. Chem. Phys. Lett. 1996;256:133–140. doi: 10.1016/s0926-2040(98)00038-1. [DOI] [PubMed] [Google Scholar]
- Jaroniec CP, Filip C, Griffin RG. 3D TEDOR NMR experiments for the simultaneous measurement of multiple carbon-nitrogen distances in uniformly 13C, 15N-labeled solids. J. Am. Chem. Soc. 2002a;124:10728–10742. doi: 10.1021/ja026385y. [DOI] [PubMed] [Google Scholar]
- Jaroniec CP, MacPhee CE, Astrof NS, Dobson CM, Griffin RG. Molecular conformation of a peptide fragment of transthyretin in an amyloid fibril. Proc. Natl. Acad. Sci. U. S. A. 2002b;99:16748–16753. doi: 10.1073/pnas.252625999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaroniec CP, MacPhee CE, Bajaj VS, McMahon MT, Dobson CM, Griffin RG. High-resolution molecular structure of a peptide in an amyloid fibril determined by magic angle spinning NMR spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 2004;101:711–716. doi: 10.1073/pnas.0304849101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasinghe SA, Langen R. Identifying structural features of fibrillar islet amyloid polypeptide using site-directed spin labeling. J. Biol. Chem. 2004;279:48420–48425. doi: 10.1074/jbc.M406853200. [DOI] [PubMed] [Google Scholar]
- Jimenez JL, Nettleton EJ, Bouchard M, Robinson CV, Dobson CM, Saibil HR. The protofilament structure of insulin amyloid fibrils. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9196–9201. doi: 10.1073/pnas.142459399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer RA, Kostrewa D, Zurdo J, Detken A, Garcia-Echeverria C, Green JD, Muller SA, Meier BH, Winkler FK, Dobson CM, Steinmetz MO. Exploring amyloid formation by a de novo design. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4435–4440. doi: 10.1073/pnas.0306786101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange A, Becker S, Seidel K, Giller K, Pongs O, Baldus M. A concept for rapid protein-structure determination by solid state NMR spectroscopy. Angew. Chem.-Int. Edit. 2005;44:2089–2092. doi: 10.1002/anie.200462516. [DOI] [PubMed] [Google Scholar]
- Lansbury PT, Costa PR, Griffiths JM, Simon EJ, Auger M, Halverson KJ, Kocisko DA, Hendsch ZS, Ashburn TT, Spencer RGS, Tidor B, Griffin RG. Structural model for the β-amyloid fibril based on interstrand alignment of an antiparallel-sheet comprising a C-terminal peptide. Nat. Struct. Biol. 1995;2:990–998. doi: 10.1038/nsb1195-990. [DOI] [PubMed] [Google Scholar]
- Laws DD, Bitter HML, Liu K, Ball HL, Kaneko K, Wille H, Cohen FE, Prusiner SB, Pines A, Wemmer DE. Solid state NMR studies of the secondary structure of a mutant prion protein fragment of 55 residues that induces neurodegeneration. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11686–11690. doi: 10.1073/pnas.201404298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo ND, Downing DT. Amyloid fibrils may be assembled from β-helical protofibrils. Biochemistry. 1998;37:1731–1735. doi: 10.1021/bi971016d. [DOI] [PubMed] [Google Scholar]
- Li LP, Darden TA, Bartolotti L, Kominos D, Pedersen LG. An atomic model for the pleated β-sheet structure of Aβ amyloid protofilaments. Biophys. J. 1999;76:2871–2878. doi: 10.1016/S0006-3495(99)77442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long HW, Tycko R. Biopolymer conformational distributions from solid state NMR: β-helix and 310-helix contents of a helical peptide. J. Am. Chem. Soc. 1998;120:7039–7048. [Google Scholar]
- McDermott AE, Creuzet F, Gebhard R, Vanderhoef K, Levitt MH, Herzfeld J, Lugtenburg J, Griffin RG. Determination of internuclear distances and the orientation of functional-groups by solid state NMR: Rotational resonance study of the conformation of retinal in bacteriorhodopsin. Biochemistry. 1994;33:6129–6136. doi: 10.1021/bi00186a012. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Gregory DM, Kiihne S, Mitchell DJ, Hatcher ME, Shiels JC, Drobny GP. Distance measurements in nucleic acids using windowless dipolar recoupling solid state NMR. Solid State Nucl. Magn. Reson. 1996;7:211–228. doi: 10.1016/s0926-2040(96)01267-2. [DOI] [PubMed] [Google Scholar]
- Meier BH, Earl WL. Excitation of multiple quantum transitions under magic angle spinning conditions: Adamantane. J. Chem. Phys. 1986;85:4905–4911. [Google Scholar]
- Michal CA, Jelinski LW. REDOR 3D: Heteronuclear distance measurements in uniformly labeled and natural abundance solids. J. Am. Chem. Soc. 1997;119:9059–9060. [Google Scholar]
- Naito A, Kamihira M, Inoue R, Saito H. Structural diversity of amyloid fibril formed in human calcitonin as revealed by site-directed 13C solid state NMR spectroscopy. Magn. Reson. Chem. 2004;42:247–257. doi: 10.1002/mrc.1323. [DOI] [PubMed] [Google Scholar]
- Oyler NA, Tycko R. Absolute structural constraints on amyloid fibrils from solid state NMR spectroscopy of partially oriented samples. J. Am. Chem. Soc. 2004;126:4478–4479. doi: 10.1021/ja031719k. [DOI] [PubMed] [Google Scholar]
- Perutz MF, Johnson T, Suzuki M, Finch JT. Glutamine repeats as polar zippers: Their possible role in inherited neurodegenerative diseases. Proc. Natl. Acad. Sci. U. S. A. 1994;91:5355–5358. doi: 10.1073/pnas.91.12.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova AT, Buntkowsky G, Dyda F, Leapman RD, Yau WM, Tycko R. Solid state NMR reveals a pH-dependent antiparallel β-sheet registry in fibrils formed by a β-amyloid peptide. J. Mol. Biol. 2004;335:247–260. doi: 10.1016/j.jmb.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Petkova AT, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F, Tycko R. A structural model for Alzheimer's β-amyloid fibrils based on experimental constraints from solid state NMR. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16742–16747. doi: 10.1073/pnas.262663499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova AT, Leapman RD, Guo ZH, Yau WM, Mattson MP, Tycko R. Self-propagating, molecular-level polymorphism in Alzheimer's β-amyloid fibrils. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- Reif B, Hohwy M, Jaroniec CP, Rienstra CM, Griffin RG. NH-NH vector correlation in peptides by solid state NMR. J. Magn. Reson. 2000;145:132–141. doi: 10.1006/jmre.2000.2067. [DOI] [PubMed] [Google Scholar]
- Saito H. Conformation-dependent 13C chemical-shifts: A new means of conformational characterization as obtained by high-resolution solid state 13C NMR. Magn. Reson. Chem. 1986;24:835–852. [Google Scholar]
- Schmidt-Rohr K. A double-quantum solid state NMR technique for determining torsion angles in polymers. Macromolecules. 1996a;29:3975–3981. [Google Scholar]
- Schmidt-Rohr K. Torsion angle determination in solid 13C-labeled amino acids and peptides by separated-local-field double-quantum NMR. J. Am. Chem. Soc. 1996b;118:7601–7603. [Google Scholar]
- Serpell LC, Smith JM. Direct visualisation of the β-sheet structure of synthetic Alzheimer's amyloid. J. Mol. Biol. 2000;299:225–231. doi: 10.1006/jmbi.2000.3650. [DOI] [PubMed] [Google Scholar]
- Sharpe S, Kessler N, Anglister JA, Yau WM, Tycko R. Solid state NMR yields structural constraints on the V3 loop from HIV-1 gp120 bound to the 447-52d antibody Fv fragment. J. Am. Chem. Soc. 2004;126:4979–4990. doi: 10.1021/ja0392162. [DOI] [PubMed] [Google Scholar]
- Siemer AB, Ritter C, Ernst M, Riek R, Meier BH. High-resolution solid state NMR spectroscopy of the prion protein Het-s in its amyloid conformation. Angew. Chem. Int. Ed. 2005;44:2441–2444. doi: 10.1002/anie.200462952. [DOI] [PubMed] [Google Scholar]
- Spera S, Bax A. Empirical correlation between protein backbone conformation and Cα and Cβ13C nuclear magnetic resonance chemical shifts. J. Am. Chem. Soc. 1991;113:5490–5492. [Google Scholar]
- Sunde M, Blake CCF. From the globular to the fibrous state: Protein structure and structural conversion in amyloid formation. Q. Rev. Biophys. 1998;31:1–39. doi: 10.1017/s0033583598003400. [DOI] [PubMed] [Google Scholar]
- Takegoshi K, Imaizumi T, Terao T. One- and two-dimensional 13C-1H/15N-1H dipolar correlation experiments under fast magic-angle spinning for determining the peptide dihedral angle phi. Solid State Nucl. Magn. Reson. 2000;16:271–278. doi: 10.1016/s0926-2040(00)00076-x. [DOI] [PubMed] [Google Scholar]
- Torok M, Milton S, Kayed R, Wu P, McIntire T, Glabe CG, Langen R. Structural and dynamic features of Alzheimer's Aβ peptide in amyloid fibrils studied by site-directed spin labeling. J. Biol. Chem. 2002;277:40810–40815. doi: 10.1074/jbc.M205659200. [DOI] [PubMed] [Google Scholar]
- Tycko R. Progress towards a molecular-level structural understanding of amyloid fibrils. Curr. Opin. Struct. Biol. 2004;14:96–103. doi: 10.1016/j.sbi.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Tycko R, Dabbagh G. Measurement of nuclear magnetic dipole-dipole couplings in magic angle spinning NMR. Chem. Phys. Lett. 1990;173:461–465. [Google Scholar]
- Tycko R, Dabbagh G. Nuclear magnetic resonance crystallography: Molecular orientational ordering in three forms of solid methanol. J. Am. Chem. Soc. 1991;113:3592–3593. [Google Scholar]
- Tycko R, Dabbagh G. A simple theory of 13C nuclear spin diffusion in organic solids. Isr. J. Chem. 1992;32:179–184. [Google Scholar]
- Tycko R, Dabbagh G, Mirau PA. Determination of chemical shift anisotropy lineshapes in a two-dimensional magic-angle-spinning NMR experiment. J. Magn. Reson. 1989;85:265–274. [Google Scholar]
- Tycko R, Ishii Y. Constraints on supramolecular structure in amyloid fibrils from two-dimensional solid state NMR spectroscopy with uniform isotopic labeling. J. Am. Chem. Soc. 2003;125:6606–6607. doi: 10.1021/ja0342042. [DOI] [PubMed] [Google Scholar]
- Weliky DP, Bennett AE, Zvi A, Anglister J, Steinbach PJ, Tycko R. Solid state NMR evidence for an antibody-dependent conformation of the V3 loop of HIV-1 gp120. Nat. Struct. Biol. 1999;6:141–145. doi: 10.1038/5827. [DOI] [PubMed] [Google Scholar]
- Weliky DP, Dabbagh G, Tycko R. Correlation of chemical bond directions and functional group orientations in solids by two-dimensional NMR. J. Magn. Reson. Ser. A. 1993;104:10–16. [Google Scholar]
- Weliky DP, Tycko R. Determination of peptide conformations by twodimensional magic angle spinning NMR exchange spectroscopy with rotor synchronization. J. Am. Chem. Soc. 1996;118:8487–8488. [Google Scholar]
- Williams AD, Portelius E, Kheterpal I, Guo JT, Cook KD, Xu Y, Wetzel R. Mapping Aβ amyloid fibril secondary structure using scanning proline mutagenesis. J. Mol. Biol. 2004;335:833–842. doi: 10.1016/j.jmb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Wishart DS, Sykes BD, Richards FM. Relationship between nuclear magnetic resonance chemical shift and protein secondary structure. J. Mol. Biol. 1991;222:311–333. doi: 10.1016/0022-2836(91)90214-q. [DOI] [PubMed] [Google Scholar]
- Yao XL, Hong M. Structure distribution in an elastin-mimetic peptide (VPGVG)3 investigated by solid state NMR. J. Am. Chem. Soc. 2004;126:4199–4210. doi: 10.1021/ja036686n. [DOI] [PubMed] [Google Scholar]