Abstract
Although an association between the metabolic syndrome and hyperandrogenism has been suggested in women with polycystic ovarian syndrome, few studies have investigated this relationship in postmenopausal women. We measured estradiol, testosterone, and sex hormone binding globulin (SHBG) and calculated the free androgen index (FAI) in 212 postmenopausal women not using hormone therapy in the Women's Health Study. A modified ATP III definition of the metabolic syndrome (3 or more of the following: abdominal obesity, hypertriglyceridemia, low HDL, elevated blood pressure, and abnormal glucose metabolism) was used. Women with the metabolic syndrome had higher mean levels of estradiol, testosterone, and FAI values, and lower SHBG levels. Higher FAI and lower SHBG were associated with all components of the metabolic syndrome. After adjustment for BMI and other factors, women in the highest tertile of FAI had an OR of 12.6 (95% CI: 3.8, 41.6) for the metabolic syndrome, while those in the lowest SHBG tertile had an OR of 7.3 (95% CI: 2.7, 19.8). When stratified by BMI, the associations with high FAI and low SHBG remained significant even in women with BMI < 26.7 kg/m2. An androgenic hormone profile is associated with both the individual components of the metabolic syndrome and clustering of metabolic abnormalities in postmenopausal women.
Keywords: estradiol, gonadal steroid hormones, metabolic syndrome X, sex hormone binding globulin, testosterone
The metabolic syndrome identifies a cluster of metabolic abnormalities that place affected individuals at increased risk for developing diabetes mellitus (1) and cardiovascular disease (CVD) (2), as well as increased mortality from all causes (3). The Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP-III) provides a clinically useful, working definition of the metabolic syndrome that includes the presence of at least three of the following characteristics: abdominal obesity, elevated triglycerides, reduced levels of HDL cholesterol, high blood pressure, and elevated fasting glucose (4).
An association between the metabolic syndrome and hyperandrogenism has been suggested in premenopausal women with polycystic ovarian syndrome (PCOS). These women have an increased rate of metabolic abnormalities including central obesity, impaired glucose tolerance or diabetes, hypertension, and dyslipidemia (5, 6). The relationships of both androgens and estrogens with individual features of the metabolic syndrome such as hypertension, insulin resistance, and dyslipidemia have been investigated in both pre- and postmenopausal women (7-16); however, few studies (17-19) have examined the relationship between endogenous sex hormone levels and the metabolic syndrome as recently defined, and these reports have not provided definitive results in postmenopausal women.
Whether the association between sex hormones and the metabolic syndrome observed in premenopausal women persists through menopause has important public health implications, since the prevalence of both the metabolic syndrome (20) and cardiovascular disease increase after menopause. We examined the relationship of sex hormones and SHBG with the metabolic syndrome in a cross-sectional analysis of a nested case-control study of women in the Women's Health Study.
MATERIALS AND METHODS
Participants
The relationship of endogenous sex hormones and the metabolic syndrome was evaluated among a subset of postmenopausal women participating in the Women's Health Study (WHS), an ongoing, randomized, double-blind, placebo-controlled study of low-dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer (21). Apparently healthy women with no known cardiovascular disease or cancer (except nonmelanoma skin cancer) were enrolled between November 1992 and July 1995. Among the 39,876 female health professionals age 45 years or older at baseline randomized into the study, 28,345 (71 percent) provided baseline fasting blood samples which were stored in liquid nitrogen freezers at −170 degrees Celsius until the time of analysis.
Data on traditional cardiac risk factors were gathered in the WHS at baseline. WHS participants complete yearly questionnaires on demographic, behavioral, and lifestyle factors, as well as the occurrence of any medical illnesses. They are also asked to consent to release of relevant medical records, which are reviewed by an end points committee of physicians. Postmenopausal status was defined by the report of no menses for more than twelve months in the presence of natural menopause or at the time of bilateral salpingoophorectomy. Women who had undergone hysterectomy with either one or no ovaries removed were considered postmenopausal at age 56 (the age by which 95 percent of the cohort reached natural menopause).
For the current analysis, we utilized sex hormone values from a prior case-control study of 200 postmenopausal women who developed cardiovascular disease (CVD) and 200 control subjects (22). Our analysis excluded all subjects who were currently using hormone therapy, since exogenous hormone use has dramatic effects on levels of the endogenous hormones studied (22). Of the 230 women remaining, six participants were excluded due to unclear menopausal status. Twelve additional women were excluded because one or more variables in the metabolic syndrome definition were missing, leaving 212 women for our analyses. All participants were free from known CVD at baseline when the blood samples were collected. A composite endpoint of CVD, defined as the first occurrence of nonfatal MI, coronary revascularization, nonfatal stroke, coronary disease, or stroke death, was used in the primary study (22).
Sex hormone assays
All hormone assays were conducted in 2000. Total estradiol levels were measured at Quest Diagnostics (Capistrano, CA) by radioimmunoassay preceded by extraction and purification by Celite column chromatography. The lower limit of detection for the assay was 5 pg/mL. Total testosterone and SHBG levels were assayed at the Massachusetts General Hospital Reproductive Endocrine Laboratory. Testosterone was measured using a solid-phase radioimmunoassay (Diagnostic Products Corporation). Since all testosterone measurements were above the lower limit of detection of 4.0 ng/dL, the extraction method was not necessary. SHBG was measured using a fully automated system (Immunolite; Diagnostic Products Corporation), which used a solid-phase, two-site immunometric assay. Coefficients of variation from interspersed quality-controlled specimens were 9.5 percent for estradiol, 4.8 percent for testosterone, and 4.5 percent for SHBG. To estimate free (non-protein-bound) testosterone, we calculated the free androgen index (FAI), the molar ratio of total testosterone/SHBG multiplied by 100, which is highly correlated with free testosterone (23, 24).
Definition of the Metabolic Syndrome
According to the ATP III guidelines, the metabolic syndrome for women is defined as having three or more of the following: (1) abdominal obesity identified by a waist circumference > 88 cm; (2) triglycerides ≥ 150 mg/dL; (3) HDL cholesterol < 50 mg/dL; (4) blood pressure ≥ 130/85 mm Hg; and (5) abnormal glucose metabolism as identified by a fasting blood glucose ≥ 110 mg/dL (4).
A modified ATP definition, validated in prior work in the WHS (25), was used. Obesity. Since waist circumference was not available at baseline, a cutpoint for obesity of body mass index (BMI) ≥ 26.7 kg/m2 was used as a surrogate. This value corresponded to the same percentile for BMI as did a waist circumference of 88 cm when it was measured at year six of follow-up. Self-reported values for height and weight were used to calculate BMI. In a validation study of weight among 184 women in a similar cohort of female health professionals, the measured weights averaged only 1.5 kg (corresponding to an average difference in BMI of 0.5 kg/m2) higher than the self-reported weights with a Spearman correlation of 0.96 (26). Triglycerides and HDL cholesterol. Triglyceride and HDL cholesterol levels were directly measured utilizing stored baseline blood samples (Roche Diagnostics). Elevated blood pressure. Subjects with elevated blood pressure included those who reported a diagnosis of hypertension by a clinician on either the baseline or the run-in questionnaires. Those reporting systolic blood pressure (SBP) ≥ 130 mm Hg or diastolic blood pressure (DBP) ≥ 85 mm Hg on the run-in questionnaire (if blood pressure had been measured during the previous two years, chosen from nine categories for SBP and seven categories for DBP) were also included. A single measurement of self-reported blood pressure has been shown to be highly correlated with measured SBP (r = 0.72) and DBP (r = 0.60) in health professionals (27). Glucose intolerance. Because fasting glucose levels were not available, we used the diagnosis of diabetes at either baseline or during follow-up to identify individuals with baseline impairment of glucose metabolism. The diagnosis of diabetes was determined by self-report on the basis of annual questionnaires. The validity of self-reported diabetes has been demonstrated in the WHS (28). This modified ATP definition has been previously used in the WHS and has been shown to predict cardiovascular events (25).
Statistical analysis
Since hormone levels were skewed, continuous values were log-transformed to achieve normality. Geometric means of baseline endogenous hormones, adjusted for age and CVD case-control status, were compared by the General Linear Models (GLM) procedure for the metabolic syndrome and each of its components. In order to assess potential confounding and effect modification, geometric mean hormone levels were additionally compared within strata of BMI (<26.7 kg/m2 and ≥26.7 kg/m2) for those with and without the metabolic syndrome. In addition, we classified all subjects as having 0, 1, 2, 3 or more components of the metabolic syndrome and assessed for a trend across the groups using GLM procedure. Additional analyses were performed stratified according to the presence or absence of CVD during follow-up. The stratified analyses yielded similar results in women who remained free of CVD and those who developed CVD during follow-up; therefore, we present primary results on the entire group of 212 women. However, since results tended to be more significant in women who later developed CVD, analyses were adjusted for the subsequent development of CVD.
Logistic regression models were performed in order to determine the odds ratio (OR) of the metabolic syndrome by tertile of endogenous sex hormone level. Tertiles were based on the distribution in women who did not meet the definition of the metabolic syndrome described above. Models were adjusted for age, smoking, physical activity, alcohol use, and presence or absence of CVD during follow-up. Since elevated BMI is strongly associated with high estrogen levels and low SHBG, as well as many components of the metabolic syndrome, analyses were repeated with additional adjustment for BMI as a continuous variable to control for confounding. Analyses were also stratified by BMI < 26.7 kg/m2 to evaluate potential effect modification by BMI and verify that associations were present even for women who did not have obesity by the metabolic syndrome criteria.
RESULTS
Of the 212 postmenopausal women not using hormone therapy studied, 108 women (51 percent) had three or more of the metabolic abnormalities described in the modified ATP III definition, and, therefore, met criteria for the metabolic syndrome. Baseline characteristics of women with and without the metabolic syndrome are presented in table 1. Women with the metabolic syndrome were slightly younger than women without the metabolic syndrome (mean age=63.9 vs. 65.8 years, p=0.03). As expected, women with the metabolic syndrome had higher BMI (28.6 kg/m2 compared with 23.8 kg/m2, p<0.001) and had more cardiovascular events during follow-up (63 percent compared with 37.5 percent, p=0.0002). There were no other significant differences between the two groups in mean age of menopause, smoking, physical activity, alcohol consumption, history of oophorectomy, or parental history of MI.
TABLE 1.
Baseline characteristics of women with and without metabolic syndrome.
| Characteristic | Women with metabolic syndrome (n = 108) | Women without metabolic syndrome (n = 104) | p |
|---|---|---|---|
| Mean age, years | 63.9 | 65.8 | 0.03 |
| Mean age at menopause | 47.2 | 47.7 | 0.56 |
| Mean BMI (kg/m2) | 28.6 | 23.8 | <0.001 |
| Smoking, % | |||
| Never | 38.0 | 38.5 | 0.94 |
| Past | 40.7 | 40.4 | 0.96 |
| Current | 21.3 | 21.2 | 0.98 |
| Physical activity, % | |||
| Rarely/never | 57.4 | 47.1 | 0.13 |
| <1-3/wk | 35.2 | 38.5 | 0.62 |
| ≥4/wk | 7.4 | 14.4 | 0.10 |
| Alcohol consumption | |||
| Rarely/never | 56.5 | 51.9 | 0.51 |
| 1-3 /mo | 13.9 | 11.5 | 0.61 |
| > 1 /wk | 29.6 | 36.5 | 0.28 |
| History of oophorectomy, % | 20.4 | 13.5 | 0.18 |
| Parental history of MI, %* | 15.7 | 10.4 | 0.27 |
| Developed CVD during follow-up, % | 63.0 | 37.5 | 0.0002 |
History of MI in either or both parents before age 60
MI = myocardial infarction
CVD= cardiovascular disease
Mean levels of total estradiol, total testosterone, and FAI were higher and SHBG was lower among women with the metabolic syndrome (table 2). Low SHBG and high FAI were associated with all of the individual components of the metabolic syndrome. Higher levels of estradiol were associated with higher BMI and abnormal glucose metabolism, whereas higher levels of testosterone were found among women with low HDL, elevated blood pressure, and elevated BMI.
TABLE 2.
Geometric mean levels of endogenous hormones according to the presence or absence of the metabolic syndrome and each individual component of the metabolic syndrome, adjusted for age and the presence or absence of cardiovascular disease during follow-up. SHBG = sex hormone binding globulin. FAI = free androgen index. TG = triglycerides. HDL = high-density lipoprotein. BP = blood pressure. BMI = body mass index.
| Estradiol (pg/mL) | Testosterone (ng/dL) | SHBG (nmol/L) | FAI | |
|---|---|---|---|---|
| Metabolic Syndrome | ||||
| Yes | 12.2 | 22.7 | 32.6 | 2.5 |
| No | 9.2 | 15.9 | 55.8 | 1.0 |
| p | 0.001 | <0.0001 | <0.0001 | <0.0001 |
| TG ≥150 mg/dL | 11.1 | 20.0 | 34.3 | 2.0 |
| TG < 150 mg/dL | 10.1 | 18.2 | 53.4 | 1.1 |
| p | 0.26 | 0.28 | <0.0001 | <0.0001 |
| HDL < 50 mg/dL | 11.1 | 20.9 | 36.9 | 2.0 |
| HDL ≥50 mg/dL | 9.8 | 16.0 | 55.9 | 1.0 |
| p | 0.19 | 0.003 | <0.0001 | <0.0001 |
| BP ≥130/85 mm Hg | 11.1 | 20.4 | 39.3 | 1.4 |
| BP < 130/85 mm Hg | 9.8 | 17.0 | 48.8 | 1.2 |
| p | 0.17 | 0.05 | 0.006 | 0.002 |
| BMI ≥ 26.7 kg/m2 | 12.8 | 21.4 | 34.0 | 2.2 |
| BMI < 26.7 kg/m2 | 9.0 | 17.1 | 52.4 | 1.1 |
| p | <0.0001 | 0.01 | <0.0001 | <0.0001 |
| Abnormal glucose metabolism | ||||
| Yes | 13.0 | 22.5 | 29.1 | 2.7 |
| No | 10.1 | 18.3 | 46.8 | 1.4 |
| p | 0.02 | 0.07 | <0.0001 | <0.0001 |
| Stratified by BMI | ||||
| BMI <26.7 kg/m2 | ||||
| Metabolic Syndrome | ||||
| Yes | 10.3 | 22.6 | 38.1 | 2.0 |
| No | 8.5 | 15.4 | 60.0 | 0.9 |
| p | 0.22 | 0.01 | <0.0001 | <0.0001 |
| BMI ≥26.7 kg/m2 | ||||
| Metabolic Syndrome | ||||
| Yes | 13.0 | 22.9 | 30.6 | 2.7 |
| No | 12.2 | 18.0 | 44.7 | 1.4 |
| p | 0.57 | 0.06 | 0.002 | 0.0005 |
There were significant trends for geometric mean levels of all sex hormones studied across increasing numbers of ATP III metabolic abnormalities, adjusted for age and the presence or absence of CVD during follow-up. As shown in figure 1, mean levels of estradiol (ptrend = 0.0006), testosterone (ptrend<0.0001), and FAI (ptrend<0.0001) increased according to the number of metabolic syndrome components. Lower mean levels of SHBG (ptrend <0.0001) were strongly associated with increasing numbers of metabolic abnormalities (figure 2).
FIGURE 1.
Mean levels of estradiol (pg/mL), testosterone (ng/dL), and free androgen index according to the number of metabolic syndrome components, adjusted for age and the presence or absence of cardiovascular disease during follow-up.
FIGURE 2.

Mean levels of sex hormone binding globulin (nmol/L) according to the number of metabolic syndrome components, adjusted for age and the presence or absence of cardiovascular disease during follow-up.
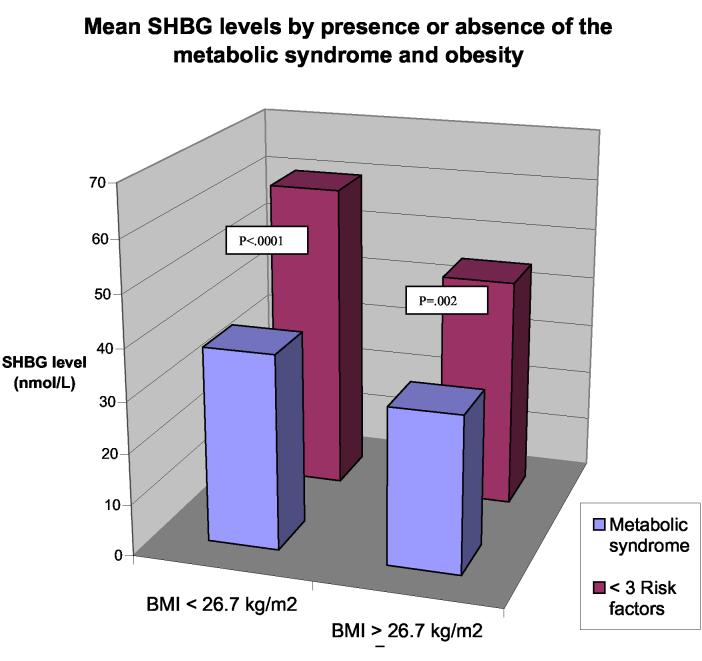
Since BMI is strongly linked to the metabolic syndrome and its components, as well as to high estradiol and low SHBG levels, the presence of obesity in many of the women with metabolic syndrome might partially explain the observed associations. We performed analyses stratified by BMI < 26.7 kg/m2 (our cutpoint for abdominal obesity which corresponded to a waist circumference of 88 cm when measured at year six of follow-up) to determine if relationships persisted among the leaner women (table 2). In this sub-group, FAI and testosterone were higher and SHBG was lower among women with the metabolic syndrome. Estradiol was no longer significantly associated with the metabolic syndrome in either group. Significantly lower SHBG levels were found among women who met criteria for the metabolic syndrome, independent of BMI (figure 3).
FIGURE 3.
Mean sex hormone binding globulin levels (nmol/L) by presence or absence of the metabolic syndrome and elevated body mass index, adjusted for age and presence or absence of cardiovascular disease during follow-up.
In order to control for potential confounders, logistic regression models were performed using the metabolic syndrome as the outcome. Increased crude odds ratios (ORs) for the metabolic syndrome were observed across tertiles of all endogenous hormones studied (table 3). Although women with the metabolic syndrome in this study were significantly younger, adjusting for age had little effect on point estimates (data not shown). Multivariate adjustment for age, smoking, physical activity, alcohol use, and the presence or absence of CVD during follow-up showed that women in the highest tertile of estradiol had an OR for the metabolic syndrome of 4.5 (95 % CI: 2.0, 10.1) compared with women in the lowest tertile. Similarly, women in the highest tertile of testosterone had an OR of 4.3 (95 % CI: 2.0, 9.4). The results for SHBG and FAI were consistently stronger. Women in the lowest tertile of SHBG had an OR of 11.6 (95 % CI: 4.5, 29.8) for the metabolic syndrome. Among those with FAI levels in the highest compared with the lowest tertile, the OR for the metabolic syndrome was 22.9 (95 % CI: 7.3, 72.1).
TABLE 3.
Odds Ratio (OR) and 95% confidence intervals (CI) for the metabolic syndrome by tertiles of endogenous sex hormone levels. Tertiles were based on the distribution in women without the metabolic syndrome.
| Hormone | Number with/without metabolic syndrome | Crude OR | (95% CI) | Multivariate adjusted OR* | (95% CI) | Multivariate adjusted OR +BMI† | (95% CI) | Number with/without metabolic syndrome | Multivariate adjusted OR +BMI†, among women with BMI < 26.7 kg/m2 | (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol | ||||||||||
| Tertile 1 | 16/34 | Referent | Referent | Referent | 8/30 | Referent | ||||
| Tertile 2 | 34/40 | 1.8 | (0.9- 3.8) | 2.3 | (1.0 –5.3) | 1.6 | (0.7 - 3.8) | 14/32 | 1.3 | (0.4- 4.1) |
| Tertile 3 | 56/29 | 4.1 | (1.9- 8.6) | 4.5 | (2.0-10.1) | 2.0 | (0.8 - 4.8) | 5/19 | 0.4 | (0.1- 2.0) |
| Testosterone | ||||||||||
| Tertile 1 | 19/39 | Referent | Referent | Referent | 8/32 | Referent | ||||
| Tertile 2 | 30/34 | 1.8 | (0.9- 3.8) | 1.8 | (0.8- 4.0) | 1.4 | (0.6- 3.3) | 5/26 | 0.6 | (0.1- 2.3) |
| Tertile 3 | 59/31 | 3.9 | (1.9- 7.8) | 4.3 | (2.0- 9.4) | 3.2 | (1.4- 7.3) | 14/24 | 1.6 | (0.5- 5.4) |
| SHBG | ||||||||||
| Tertile 3 | 7/36 | Referent | Referent | Referent | 2/32 | Referent | ||||
| Tertile 2 | 18/34 | 2.7 | (1.0-7.3) | 2.7 | (0.9 -7.5) | 2.5 | (0.8- 7.3) | 7/30 | 3.7 | (0.7-21.3) |
| Tertile 1 | 83/34 | 12.6 | (5.1-31.0) | 11.6 | (4.5-29.8) | 7.3 | (2.7-19.8) | 18/20 | 10.5 | (2.0-56.3) |
| FAI | ||||||||||
| Tertile 1 | 4/36 | Referent | Referent | Referent | 2/31 | Referent | ||||
| Tertile 2 | 18/33 | 4.9 | (1.5-16.0) | 5.9 | (1.7-20.1) | 4.2 | (1.2-14.7) | 6/28 | 2.4 | (0.4- 14.0) |
| Tertile 3 | 86/35 | 22.1 | (7.3-66.8) | 22.9 | (7.3-72.1) | 12.6 | (3.8-41.6) | 19/23 | 7.7 | (1.4-41.4) |
Adjusted for age, smoking, physical activity, alcohol use, and the presence or absence of CVD during follow-up.
Adjusted for age, smoking, physical activity, alcohol use, the presence or absence of CVD during follow-up, and BMI.
CI = confidence interval. SHBG = sex hormone binding globulin. FAI = free androgen index. BMI = body mass index.
In order to evaluate whether the observed associations for sex hormones and the metabolic syndrome could be explained by the known correlations with BMI, we performed analyses additionally adjusted for BMI (table 3). The point estimates for estradiol and testosterone were attenuated, but the results for SHBG and FAI remained significant. To further limit the influence of obesity on the observed associations, we then stratified by the obesity criteria used for the metabolic syndrome (BMI < 26.7 kg/m2). Low SHBG and high FAI continued to be strongly associated with increased odds for the metabolic syndrome. Among those with SHBG levels in the lowest compared with the highest tertile, the multivariate adjusted OR (including additional adjustment for BMI) in women with BMI < 26.7 kg/m2 was 10.5 (95 % CI: 2.0, 56.3). Women with BMI < 26.7 kg/m2 in the highest tertile of FAI had an OR of 7.7 (95 % CI: 1.4, 41.4) for the metabolic syndrome.
DISCUSSION
In this cross-sectional study of postmenopausal women, mean levels of estradiol, testosterone, and FAI were higher, and SHBG was lower among women with the metabolic syndrome. Low SHBG and high FAI were associated with all of the individual components of the metabolic syndrome: obesity, low HDL cholesterol, hypertriglyceridemia, elevated blood pressure, and abnormal glucose metabolism. There were significant trends for mean levels of sex hormones and SHBG across increasing numbers of ATP III metabolic abnormalities. After multivariate adjustment, women in the highest tertile of estradiol, testosterone, and FAI were at least four times more likely to have the metabolic syndrome compared with women in the lowest tertile. The OR for the metabolic syndrome among women with low SBHG was particularly strong; those in the lowest SHBG tertile were more than ten times more likely to have metabolic syndrome compared with those in the highest SHBG tertile. The relationship between low SHBG and metabolic syndrome persisted even after adjustment for BMI.
In our prior analyses of sex hormones and cardiovascular disease in postmenopausal women, those in the lowest SHBG quartile had an age-adjusted two-fold higher risk of cardiovascular disease (22). Controlling for obesity, diabetes, hypertension and elevated lipids, however, eliminated this association, suggesting that the increased cardiovascular risk attributable to low SHBG was mediated by the metabolic syndrome. The increased prevalence of the metabolic syndrome after menopause may be a direct result of ovarian failure or, alternatively, an indirect result of the metabolic consequences of central fat redistribution with estrogen deficiency (29). There is also a higher androgen to estrogen ratio in postmenopausal than premenopausal women (30, 31), which may influence the tendency to develop the metabolic syndrome.
Numerous studies have examined the relationship of endogenous sex hormones, particularly androgens, and cardiovascular risk factors in women (7-16). The current study provides strong evidence of the relationship between low SHBG levels and the metabolic syndrome in its entirety, as well as its components, in postmenopausal women. Low SHBG, considered a marker of hyperandrogenism, has also been postulated to be a marker for insulin resistance (8, 32)—a key feature of the metabolic syndrome. An inverse association between SHBG level and both impaired glucose tolerance and diabetes was found in the Rancho Bernardo cohort (33). Recent data from the SWAN study also found strong associations between low SHBG and high FAI and cardiovascular risk factors (higher insulin, glucose, and hemostatic and inflammatory markers and adverse lipids) (13).
Since SHBG is positively correlated with HDL and negatively correlated with BMI, triglyceride, and insulin levels, it has been suggested that low SHBG levels should be one of the components of the metabolic syndrome in women (34). Few studies, however, have investigated endogenous sex hormones and the clustering of cardiovascular risk factors in women. Premenopausal women with the metabolic syndrome were found to have lower SHBG levels and higher FAI than age-matched controls; no significant difference in total testosterone was observed (17). A recent community-based study of postmenopausal women also found associations between FAI, but not total testosterone, and the metabolic syndrome (SHBG was not reported separately) (18). While it has been reported that high SHBG levels confer risk reduction for the metabolic syndrome in men and pre-menopausal women, a significant relationship was not found in postmenopausal women (19). Our findings may differ due to the high-risk population studied, and, therefore, the increased number of women who met criteria for the metabolic syndrome. Furthermore, this high-risk population may have been particularly suited to demonstrate the association between low SHBG and the metabolic syndrome, given that we used BMI to define the cutpoint for obesity and that almost half of participants exceeded this cutpoint.
Since the complex biological mechanisms that explain the association between low SHBG and the metabolic syndrome are not fully understood, it is unknown whether low SHBG levels are deleterious per se or are simply a marker of metabolic derangement. Total and central obesity are inversely related to SHBG in women (35, 36), and both adipokines and insulin may be direct and indirect negative regulators of SHBG secretion by the liver. SHBG has been hypothesized to impact atherogenesis both directly and indirectly through lipoprotein metabolism or affecting the equilibrium of the estradiol-testosterone balance (37). Testosterone has a higher affinity than estradiol for SHBG, so a small decrease in SHBG results in a relatively more androgenic hormone profile. SHBG levels may also be affected by other metabolic parameters such as elevated local cortisol production in abdominal adipose tissue and/or nutritional factors (19).
Though adiposity may be one mediator of the observed relationships, we did not adjust for BMI in the primary analysis because the presence of abdominal obesity is part of the definition and underlying physiology of the metabolic syndrome. In order to determine, however, whether BMI alone explains these strong associations, additional analyses controlling for BMI were performed. Analyses were also stratified by our cutpoint for obesity, BMI < 26.7 kg/m2. The association of low SHBG and high FAI with the metabolic syndrome remained strong, suggesting that the relationships between low SHBG and high FAI and the metabolic syndrome are relatively independent of obesity. Though these analyses investigated the confounding role of BMI, rather than waist circumference, BMI is a more frequent measure of adiposity in the clinical setting.
The percentage of women in our study with the metabolic syndrome (51 percent) was somewhat higher than other published cohorts since our population was enriched with women who were selected for a prior case-control study based on their development of CVD during follow-up. In the National Health and Nutrition Survey (NHANES), 43.5 percent of women aged 60 through 69 years had the metabolic syndrome (20). Although the high-risk women in this study may not be completely representative of the general population, it is likely that the underlying biologic associations between endogenous hormones and metabolic abnormalities will be similar in other postmenopausal women.
This analysis has several limitations. Hormone measurements were available only at baseline, thereby restricting us to cross-sectional analyses. Therefore, neither causality nor the temporality of the association can be proven. Although only one measure of hormones was available, levels are relatively stable in postmenopausal women (38). Since only estradiol, testosterone, and SHBG were measured, we were unable to investigate whether additional endogenous sex hormones were also associated with the metabolic syndrome. Though we lacked direct measurements of waist circumference, weight, blood pressure, and fasting blood glucose at baseline, this modified definition of the metabolic syndrome was used previously in this cohort and was predictive of cardiovascular disease in a prior study (25).
The use of direct radioimmunoassay (RIA) testosterone assays in women, who usually have testosterone concentrations below the lower limit of the normal adult male range, has been challenged (39, 40). Free levels of testosterone were not available; however, a recent comparison of methods showed that direct free testosterone RIA measurements had unacceptably high systematic bias and random variability (41). Calculated FAI is highly correlated with free testosterone (23, 24, 41), although its accuracy depends on the testosterone and SHBG assays. Rather than absolute accuracy, however, the validity of these positive associations depends on the precision of the assays. A frameshift bias would not affect relationships since the relative ranking of subjects would be similar. Random measurement error would tend to diminish the strength of associations; therefore, the especially strong association between SHBG and the metabolic syndrome remains compelling. Additional studies will be warranted utilizing improved testosterone, free testosterone, and SHBG assays, as they become available.
In summary, we found that levels of endogenous sex hormones were associated with the metabolic syndrome and its individual components. Women with low SHBG levels and high FAI values had a dramatically higher prevalence of metabolic syndrome. The metabolic syndrome was less strongly associated with increased levels of testosterone and estradiol. The association with estradiol was not present among leaner women, suggesting that this association was mediated primarily by obesity. Hyperandrogenism has been previously associated with the metabolic syndrome (42) and its individual components (43) in premenopausal women with PCOS. The current study extends these observations to postmenopausal women, the population with the greatest risk of cardiovascular morbidity and mortality from the metabolic syndrome.
ACKNOWLEDGMENTS
The authors would like to acknowledge the crucial contributions of the entire staff of the WHS, under the leadership of David Gordon, Susan Burt, Mary Breen, Marilyn Chown, Lisa Fields-Johnson, Georgina Friedenberg, Jean MacFadyen, Geneva McNair, Claire Ridge, Laura Pestana, and Harriet Samuelson, as well as Anna Klevak and M.V. Moorthy for their assistance with data analysis. Finally, we are deeply indebted to the dedicated and committed participants of the WHS.
Footnotes
This research was supported by the Doris Duke Charitable trust, as well as National Institute of Health grants CA-47988 and HL-80467.
REFERENCES
- 1.Haffner SM, Valdez RA, Hazuda HP, et al. Prospective analysis of the insulin-resistance syndrome (syndrome X) Diabetes. 1992;41:715–722. doi: 10.2337/diab.41.6.715. [DOI] [PubMed] [Google Scholar]
- 2.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 3.Trevisan M, Liu J, Bahsas FB, et al. Syndrome X and mortality: a population-based study. Am J Epidemiol. 1998;148:958–966. doi: 10.1093/oxfordjournals.aje.a009572. [DOI] [PubMed] [Google Scholar]
- 4.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults: Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2003;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.Talbott E, Guzick D, Clerici A, et al. Coronary heart disease risk factors in women with polycystic ovary syndrome. Arterioscler Thromb Vasc Biol. 1995;15:821–826. doi: 10.1161/01.atv.15.7.821. [DOI] [PubMed] [Google Scholar]
- 6.Hopkinson Z, Sattar N, Fleming R, et al. Polycystic ovarian syndrome: the metabolic syndrome comes to gynaecology. BMJ. 1998;317:329–332. doi: 10.1136/bmj.317.7154.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longcope C, Herbert PN, McKinlay SM, et al. The relationship of total and free estrogens and sex hormone-binding globulin with lipoproteins in women. J Clin Endocrinol Metab. 1990;71:67–72. doi: 10.1210/jcem-71-1-67. [DOI] [PubMed] [Google Scholar]
- 8.Preziosi P, Barrett-Connor E, Papoz L, et al. Interrelation between plasma sex hormone binding globulin and plasma insulin in healthy adult women: the Telecom Study. J Clin Endocrinol Metab. 1993;76:283–287. doi: 10.1210/jcem.76.2.8432770. [DOI] [PubMed] [Google Scholar]
- 9.Phillips GB, Jing T-Y, Laragh JH. Serum sex hormone levels in postmenopausal women with hypertension. J Hum Hypertension. 1997;11:523–526. doi: 10.1038/sj.jhh.1000481. [DOI] [PubMed] [Google Scholar]
- 10.Haffner SM, Newcomb PA, Marcus PM, et al. Relation of sex hormones and dehydroepiandrosterone sulfate (DHEA-SO4) to cardiovascular risk factors in postmenopausal women. Am J Epidemiol. 1995;142:925–934. doi: 10.1093/oxfordjournals.aje.a117740. [DOI] [PubMed] [Google Scholar]
- 11.Kalish GM, Barrett-Connor E, Laughlin GA, et al. Association of endogenous sex hormones and insulin resistance among postmenopausal women: Results from the Postmenopausal Estrogen/Intervention trial. J Clin Endocrinol Metab. 2003;88:1646–1652. doi: 10.1210/jc.2002-021375. [DOI] [PubMed] [Google Scholar]
- 12.Oh J, Barrett-Connor E, Wedick NM, et al. Endogenous sex hormones and the development of Type 2 diabetes in older men and women: the Rancho Bernardo Study. Diabetes Care. 2002;25:55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- 13.Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. Sex-hormone binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 14.Kuller LH, Gutai JP, Meilahn E, et al. Relationship of endogenous sex steroid hormones to lipids and apoproteins in postmenopausal women. Arteriosclerosis. 1990;10:1058–1066. doi: 10.1161/01.atv.10.6.1058. [DOI] [PubMed] [Google Scholar]
- 15.Shelley JM, Green A, Smith AMA, et al. Relationship of endogenous sex hormones to lipids and blood pressure in mid-aged women. Ann Epidemiol. 1998;8:39–45. doi: 10.1016/s1047-2797(97)00123-3. [DOI] [PubMed] [Google Scholar]
- 16.Harrison-Bernard LM, Raij L. Postmenopausal hypertension. Curr Hypertens Rep. 2000;2:202–207. doi: 10.1007/s11906-000-0083-2. [DOI] [PubMed] [Google Scholar]
- 17.Korhonen S, Hippelainen M, Vanhala M, et al. The androgenic sex hormone profile is an essential feature of metabolic syndrome in premenopausal women: a controlled community-based study. Fertil Steril. 2003;79:1327–1334. doi: 10.1016/s0015-0282(03)00347-9. [DOI] [PubMed] [Google Scholar]
- 18.Golden SH, Ding J, Szklo M, et al. Glucose and insulin components of the metabolic syndrome are associated with hyperandrogenism in postmenopausal women. Am J Epidemiol. 2004;160:540–548. doi: 10.1093/aje/kwh250. [DOI] [PubMed] [Google Scholar]
- 19.Hajamor S, Després J, Couillard C, et al. Relationship between sex hormone-binding globulin levels and features of the metabolic syndrome. Metab. 2003;52:724–730. doi: 10.1016/s0026-0495(03)00066-0. [DOI] [PubMed] [Google Scholar]
- 20.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 21.Rexrode KM, Lee IM, Cook NR, et al. Baseline characteristics of participants in the Women's Health Study. Womens Health Gend Based Med. 2000;9:19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 22.Rexrode KM, Manson JE, Lee I, et al. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108:1688–1693. doi: 10.1161/01.CIR.0000091114.36254.F3. [DOI] [PubMed] [Google Scholar]
- 23.Selby C. Sex hormone binding globulin: origin, function and clinical significance. Ann Clin Biochem. 1990;27:532–541. doi: 10.1177/000456329002700603. [DOI] [PubMed] [Google Scholar]
- 24.Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril. 2002;77:660–665. doi: 10.1016/s0015-0282(02)02969-2. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Buring JE, Cook NR, et al. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events An 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 26.Willett WC, Stampfer MJ, Bain C, et al. Cigarette Smoking, relative weight, and menopause. Am J Epidemiol. 1983;117:651–658. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 27.Klag MJ, He J, Mead LA. Validity of physicians' self-reports of cardiovascular disease risk factors. Ann Epidemiol. 1993;3:442–447. doi: 10.1016/1047-2797(93)90074-e. [DOI] [PubMed] [Google Scholar]
- 28.Song Y, Manson JE, Buring JE. A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: the women's health study. Diabetes Care. 2004;27:2108–2115. doi: 10.2337/diacare.27.9.2108. [DOI] [PubMed] [Google Scholar]
- 29.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 30.Longcope C. Hormone dynamics at the menopause. Ann N Y Acad Sci. 1990;592:21–30. doi: 10.1111/j.1749-6632.1990.tb30313.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Ding J, Bush TL, et al. Relative androgen excess and increased cardiovascular risk after menopause: A hypothesized relation. Am J Epidemiol. 2001;154:489–494. doi: 10.1093/aje/154.6.489. [DOI] [PubMed] [Google Scholar]
- 32.Nestler JE. Sex hormone-binding globulin: a marker for hyperinsulinemia and/or insulin resistance (editorial) J Clin Endocrinol Metab. 1993;76:273–274. doi: 10.1210/jcem.76.2.8432767. [DOI] [PubMed] [Google Scholar]
- 33.Goodman-Gruen D, Barrett-Connor E. Sex hormone-binding globulin and glucose tolerance in postmenopausal women: The Rancho Bernardo study. Diabetes Care. 1997;20:645–649. doi: 10.2337/diacare.20.4.645. [DOI] [PubMed] [Google Scholar]
- 34.Hautanen A. Synthesis and regulation of sex-hormone binding globulin in obesity. Int J Obes. 2000;24:S64–S70. doi: 10.1038/sj.ijo.0801281. [DOI] [PubMed] [Google Scholar]
- 35.Haffner SM, Katz MS, Dunn JF. Increased upper body and overall adiposity is associated with decreased sex hormone binding globulin in postmenopausal women. Int J Obes. 1991;15:471–478. [PubMed] [Google Scholar]
- 36.Tchernof A, Toth MJ, Poehlman ET. Sex hormone binding globulin levels in middle-aged premenopausal women Associations with visceral obesity and metabolic profile. Diabetes Care. 1999;22:1875–1881. doi: 10.2337/diacare.22.11.1875. [DOI] [PubMed] [Google Scholar]
- 37.Pugeat M, Moulin P, Cousin P, et al. Interrelations between sex hormone-binding globulin (SHBG), plasma lipoproteins and cardiovascular risk. J Steroid Biochem Molec Biol. 1995;53:567–72. doi: 10.1016/0960-0760(95)00102-6. [DOI] [PubMed] [Google Scholar]
- 38.Hankinson SE, Manson JE, Spiegelman D, et al. Reproducibility of plasma hormone levels in postmenopausal women over a 2-3 year period. Cancer Epidemiol Biomarkers Prev. 1995;4:649–654. [PubMed] [Google Scholar]
- 39.Taieb J, Mathian B, Millot F, et al. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem. 2003;49:1381–1395. doi: 10.1373/49.8.1381. [DOI] [PubMed] [Google Scholar]
- 40.Wang C, Catlin DH, Demers LM, et al. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2004;89:534–543. doi: 10.1210/jc.2003-031287. [DOI] [PubMed] [Google Scholar]
- 41.Miller KM, Rosner W, Lee H, et al. Measurement of free testosterone in normal women and women with androgen deficiency: comparison of methods. J Clin Endocrinol Metab. 2004;89:525–533. doi: 10.1210/jc.2003-030680. [DOI] [PubMed] [Google Scholar]
- 42.Glueck CJ, Papanna R, Wang P, et al. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism. 2003;52:908–915. doi: 10.1016/s0026-0495(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 43.Vrbikova J, Cifkova R, Jirkovska A, et al. Cardiovascular risk factors in young Czech females with polycystic ovarian syndrome. Hum Reprod. 2003;18:980–984. doi: 10.1093/humrep/deg218. [DOI] [PubMed] [Google Scholar]