Abstract
Dynein is a huge multisubunit microtubule (MT)-based motor, whose motor domain resides in the heavy chain. The heavy chain comprises a ring of six AAA (ATPases associated with diverse cellular activities) modules with two slender protruding domains, the tail and stalk. It has been proposed that during the ATP hydrolysis cycle, this tail domain swings against the AAA ring as a lever arm to generate the power stroke. However, there is currently no direct evidence to support the model that the tail swing is tightly linked to dynein motility. To address the question of whether the power stroke of the tail drives MT sliding, we devised an in vitro motility assay using genetically biotinylated cytoplasmic dyneins anchored on a glass surface in the desired orientation with a biotin–streptavidin linkage. Assays on the dyneins with the site-directed biotin tag at eight different locations provided evidence that robust MT sliding is driven by the power stroke of the tail. Furthermore, the assays revealed slow MT sliding independent of dynein orientation on the glass surface, which is mechanically distinct from the sliding driven by the power stroke of the tail.
Keywords: AAA protein, motility, motor protein, power stroke
Cytoplasmic dynein is a large motor complex that uses hydrolysis of ATP to generate force and movement along microtubules (MTs) toward the minus end (1). This directed transport is involved in a wide variety of cellular processes, such as mitosis, Golgi dynamics, and trafficking of various vesicles and organelles (2–6). The dynein complex consists of two identical heavy chains (each >500 kDa) together with a number of smaller associated polypeptides (7, 8). The dynein heavy chain, a member of the AAA+ (ATPases associated with diverse cellular activities) superfamily of mechanoenzymes (9), is responsible for the motile activity of dynein (10, 11) and folds to form three structurally distinct domains, the tail, ring, and stalk. The N-terminal one-third of each heavy chain constitutes an elongated structure known as the tail that contains a dimerization site and binding sites for associated polypeptides and cargoes (7, 12, 13). The C-terminal two-thirds contain six AAA+ modules (AAA1–AAA6) linked in tandem (9) and a seventh non-AAA+ module (14, 15), all of which fold into a ring-shaped structure (AAA ring) (16) that is characteristic of AAA+ proteins (9). Each of the first four AAA+ modules (AAA1–AAA4) is predicted to contain a nucleotide binding/hydrolysis site (17–20), although ATPase activity at the AAA1 module is essential only for the motile activity of dynein (21), and others may regulate the ATPase cycle at AAA1 (21, 22). The MT-binding site is in a small globular tip of a long (≈15 nm) antiparallel coiled-coil protruding from the AAA ring (23, 24). This long coiled-coil, together with the globular tip, is known as the stalk (25).
In general, ATP-driven mechanoenzymes amplify small conformational changes induced by ATP binding and/or hydrolysis into large-scale structural changes to generate force and movement. Recently, using single-particle processing of negatively stained electron microscopic images of axonemal inner-arm dynein c, it has been shown that the dynein tail swings against the AAA ring in a way that depends on the nucleotide state at the ATPase site(s) (26). Based on this observation, the “power stroke” model of the tail has been proposed as the force-generating mechanism of dynein (26). Furthermore, the evidence that tail motions against the AAA ring actually take place during the ATP hydrolysis cycle has been provided by FRET measurements of the recombinant, single-headed dynein motor domain fused with two fluorescent proteins (27).
Although the electron microscopic image analyses and FRET measurements strongly suggest that the dynein tail swings against the AAA ring during the ATP hydrolysis cycle, there is currently no evidence that this tail swing is directly responsible for force generation in dynein. Contrary to the tail swing model, the results of a recent electron microscopic study on cytoplasmic dynein have suggested that the dynein tail does not have any fixed orientation relative to the ring (28). Besides the power stroke of the tail, other models for the force generation mechanism of dynein can be proposed. For example, the stalk may swing against the AAA ring during ATP hydrolysis and have an active role as a lever arm (23, 29, 30). Consistent with this idea, it has been reported that the flexibility of the long stalk changes depending on the nucleotide state of dynein (26). Biased Brownian motion may also play an essential role in dynein motility, as proposed for some kinesin and myosin motors (31–34). In fact, a single molecule of the single-headed axonemal dynein c processively slides on MT, implying that this dynein may be driven by biased Brownian motion (35).
To address the question of whether the tail is a mechanical lever arm responsible for the power stroke or whether other force-generating mechanisms play an essential role in dynein motility, we devised experiments to uncouple the tail swing and the power stroke of the tail. A biotin tag was inserted at particular locations along the tail or in the AAA ring, and then the biotinylated dyneins were anchored on a glass surface by using a biotin-streptavidin linkage. In vitro motility assays using these biotinylated dyneins anchored in various orientations have provided evidence for the power stroke of the tail. Furthermore, the assays have revealed a second mode of slow MT sliding, which is mechanically distinct from the sliding driven by the power stroke of the tail.
Results
Construction of the 380-kDa Dynein Motor Domain with a Biotinylated Site Along the Tail or in the AAA Ring.
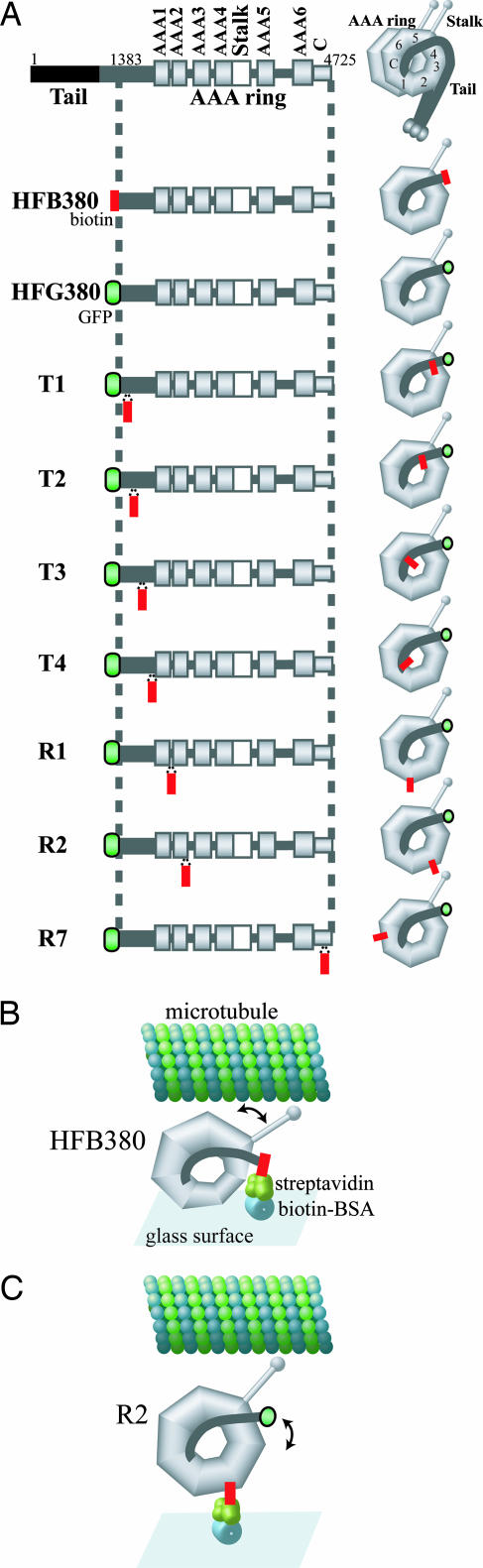
The 380-kDa fragment of the Dictyostelium dynein heavy chain used in the present study is a fully active, single-headed motor domain consisting of the N-terminally truncated tail domain (≈550 aa) and the AAA ring and stalk domains (11, 16). By using the 380-kDa fragment fused with N-terminal His6-FLAG-GFP tandem tags (HFG380) as the starting construct (21, 27), we created seven types of dyneins containing a genetically introduced biotin tag of 72 amino acid residues in different locations along the tail and within the AAA ring (Fig. 1A). The T1, T2, T3, and T4 constructs have the biotin tag inserted along the tail after amino acid residues Thr-1490, Leu-1555, Glu-1732, and Thr-1912, respectively (the N terminus of the truncated tail is Val-1383). The R1, R2, and R7 constructs have the biotin tag inserted between the AAA1 and AAA2 modules, in the AAA2 module, and in the C-terminal seventh module, respectively.
Fig. 1.
Diagrams of dynein constructs. (A) Diagrams of the constructs used in this study. A biotin tag (red square) was inserted into a motor-domain construct of the cytoplasmic dynein heavy chain. (B and C) Schematic drawing of the in vitro motility assay. Streptavidin molecules were coated on a glass surface via biotinamidocaproyl BSA, and then dynein constructs were anchored there through the biotin–streptavidin linkage. (B) The expected orientation of the HFB380 construct on the glass surface is shown. The tail anchored to the glass surface swings to generate a rotational motion of the AAA ring and stalk domains. (C) The expected orientation of the R1 or R2 construct on the glass surface is shown. The tail swing cannot generate any motion of the AAA ring and stalk domains, which are anchored on the glass surface.
We first examined whether insertion of this biotin tag in the dynein motor domain would impair its motor mechanics and ATPase kinetics because of structural changes from the insertion. Motility assays were carried out on biotinylated dyneins whose N-terminal GFP tags were anchored to a glass surface by using a monoclonal anti-GFP antibody. Under the assay conditions, most of the biotinylated dyneins drove robust MT sliding at a velocity similar to that of the control dynein (HFG380) (Fig. 2, open bars), although some of the constructs (T2, for example) showed lower velocities. These findings indicate that insertion of the biotin tag had only a minor inhibitory effect, if any, on the ability of dynein to drive MT sliding. Consistent with these results, the basal and MT-activated ATPase activities of these biotinylated dyneins were similar to or slightly lower than those of the control dynein (Fig. 3). Taken together, it seems that the biotinylation of dynein does not significantly affect motor mechanics and ATPase kinetics.
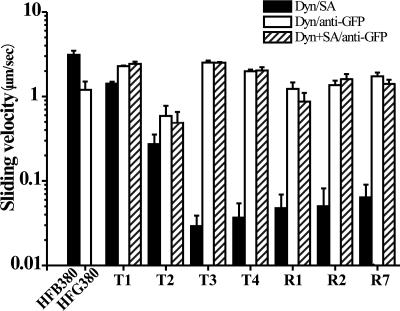
Fig. 2.
MT-sliding velocities are plotted as log-scale histograms. When the dynein (Dyn) constructs were anchored on a glass surface via their biotin moiety, the MT sliding velocity dramatically depends on anchoring position (filled bars, Dyn/SA, with SA being streptavidin). In contrast, when the dyneins were anchored on the glass via the N-terminal GFP moiety, they drove robust MT sliding at a similar velocity, independent of the biotin tag position (open bars, Dyn/anti-GFP), which was not affected by saturating the biotin moiety with streptavidin before measurement (cross-hatched bars, Dyn+SA/anti-GFP). Error bars indicate SD.
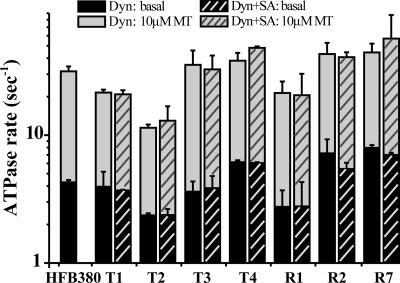
Fig. 3.
ATPase activities of the dynein (Dyn) constructs are shown. ATPase activities were measured with (gray bars) or without (black bars) 10 μM MT. These activities were unaffected by saturating the biotin moiety with streptavidin (SA) before measurement (cross-hatched bars). Error bars indicate SD.
MT-Sliding Velocity Depends on Anchoring Position of Dynein.
We carried out motility assays for the seven biotinylated dynein constructs and the 380-kDa fragment carrying the biotin tag at the N terminus (HFB380) as a control, after anchoring the biotinylated dyneins on a glass surface by biotin–streptavidin linkage (Fig. 1 B and C). The biotin–streptavidin linkage did not exert any inhibitory effect on motor mechanics or ATPase kinetics, as judged from the observation that an excess amount of free streptavidin in the assay medium affected neither ATPase activity (Fig. 3, cross-hatched bars) nor MT sliding over biotinylated dyneins whose N termini were anchored on the glass surface with anti-GFP antibodies (Fig. 2, cross-hatched bars).
In motility assays on a glass surface coated with streptavidin, all of the dynein constructs drove one-directional MT sliding. However, the sliding velocity dramatically varied depending on the position of tag insertion within the dynein molecule (Fig. 2, filled bars). HFB380, whose biotin tag was located at the N terminus, drove MT sliding at a velocity of 3.2 ± 0.4 μm/s. For dyneins carrying the biotin tag within the truncated tail (T1–T4), as the biotin tag was inserted closer to the tail-AAA ring junction, the velocity was slower, down to 0.03 ± 0.01 and 0.04 ± 0.02 μm/s for T3 and T4, respectively. The observation that MT-sliding velocity was highly sensitive to the location of the anchoring point on the dynein tail is consistent with the power stroke model, in which the tail is proposed to work as a lever arm to generate rotation of the AAA ring and stalk domains (see Discussion) (26). Dynein molecules carrying the biotin tag within the AAA ring (R1, R2, and R7) also drove slow one-directional MT sliding (0.04 ± 0.02, 0.05 ± 0.03, and 0.06 ± 0.03 μm/s, respectively) (see Movie 1, which is published as supporting information on the PNAS web site).
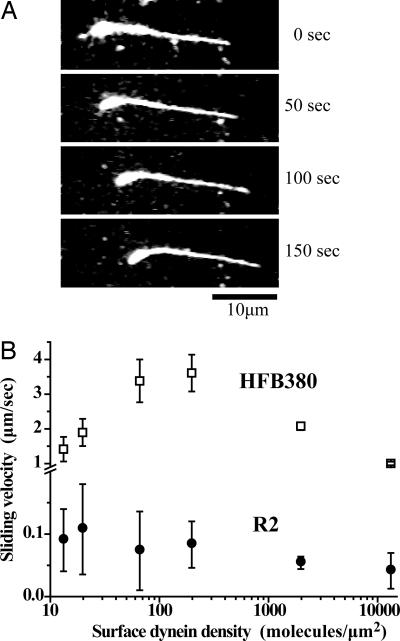
The slow sliding was driven by minus end-directed motors, as shown by the observation that most polarity-labeled MT filaments (33 among 34 moving filaments) slid pointing their plus end forward (Fig. 4A).
Fig. 4.
Slow MT sliding driven by dyneins anchored on a glass surface at their AAA ring is shown. (A) Sequential images of a polarity-marked MT driven by the R2 construct. The thick curved end marks the minus end. (B) Velocity-density profiles for the HFB380 and R2 constructs. Error bars indicate SD.
Dynein Exhibits Two Modes of MT Sliding.
When the AAA ring of dynein is anchored on a glass surface by biotin–streptavidin linkage, the tail swing cannot contribute to the slow MT sliding because the tail can freely swing without moving the AAA ring and stalk domains as illustrated in Fig. 1C. Therefore, the slow, one-directional sliding may indicate the presence of a second sliding mechanism that does not require the tail swing. However, there remains another possibility: a small number of dynein molecules binding nonspecifically to the glass surface, not the majority of biotinylated dyneins anchored through the specific biotin–streptavidin linkage, drove this slow sliding. To exclude this possibility, we examined the relationship between sliding velocity and surface dynein density. As previously reported (36) and confirmed here, dynein constructs (HFB380) anchored on a glass surface through N-terminal biotin drive robust MT sliding (≈3 μm/s) at high dynein densities, but the sliding velocity becomes slower at a lower density (<65 molecules per μm2) where the number of dynein molecules accessible to a single MT filament approaches the minimum number of dynein molecules required for continuous MT sliding (Fig. 4B).
Similar velocity measurements were performed on one of the dynein constructs with the biotin tag inserted in the AAA ring (R2). The measurements showed that most of the MT filaments on the glass surface slid slowly even at the lowest dynein density examined here. The velocity–density profile in Fig. 4B shows that the velocity remained at the same level over a wide range of dynein densities, down to 15 molecules per μm2, at which concentration only two or three dynein molecules were estimated to be accessible to a single MT filament (see Materials and Methods). The flat velocity–density profile of the R2 construct, however, does not necessarily mean that it is a highly processive motor with high duty ratio (the ratio of stroke time to the total ATPase cycle time), because a large variation in velocity measurements caused by the slow MT sliding does not allow for such a quantitative argument.
We then roughly estimated the ratio of dynein molecules anchored through the specific biotin–streptavidin linkages relative to those attaching nonspecifically on the glass surface by simply counting the number of MTs with and without pretreating the streptavidin-coated glass surface with 5 mM d-biotin to block all of the biotin-binding sites. When the glass surface was not treated with biotin before covering it with biotinylated dynein at a density of 15 molecules per μm2, the total number of filaments observed in 15 randomly selected microscope fields was 148. With biotin pretreatment, however, no filaments were observed under the same conditions. Similar control experiments using a nonbiotinylated dynein construct, HFG380, confirmed that the biotinylated construct was very selectively anchored on the glass surface through the biotin–streptavidin linkage; under the same conditions as above, the total number of filaments observed in 15 randomly selected microscope fields was only two with biotin pretreatment and no MT filaments were observed without biotin pretreatment. These results suggest that the ratio of biotin-specific to nonspecific attachment of dynein molecules on the glass surface would be at least ≈150:≈2. Because a single MT filament sliding slowly in one direction is estimated to interact with only two or three dynein molecules at this dynein density (15 molecules per μm2), we concluded that this slow MT sliding was driven by dynein molecules anchored through the specific biotin–streptavidin linkage.
Discussion
Here we have shown that cytoplasmic dynein can exhibit two modes of MT sliding activity. One is responsible for robust MT sliding and is likely to be driven by the power stroke of the tail. The other is responsible for much slower one-directional sliding and may be driven by biased Brownian motion, as proposed to other molecular motors (31–34), or by active tilting of the stalk against the AAA ring (23, 29, 30). Even though the robust and slow sliding motions of MT are driven by two distinct mechanisms, they exhibit the same directionality.
Because robust sliding of MT requires the contribution of the distal end of the truncated tail, the active stalk tilting against the AAA ring (23, 29, 30) or biased Brownian motion (31) can be excluded as driving mechanisms, at least for the robust MT sliding, although they may contribute to the slow sliding as discussed below. Thus, the robust sliding observed here is most likely to be driven by the power stroke mechanism wherein the tail swings against the AAA ring to generate rotation of the ring that accompanies the stalk swing against the bound MT (26). Consistent with this notion, it is reported that MT sliding on recombinant yeast dynein also requires the contribution of the tail (37).
When the tail was anchored on the glass surface at a location closer to the tail-ring junction, the MT sliding velocity became slower, as observed for the HFB380, T1, T2, and T3 constructs. This strong dependence of the sliding velocity on the anchoring location implies that a distal part of the tail works as a lever arm for the power stroke. In the framework of the power stroke model, the sliding velocity depends on ATPase rate, duty ratio, and step size of dynein (38). Thus, our finding implies further that the step size of the biotinylated dynein construct may change depending on the anchoring position along the tail, because ATPase kinetics of dynein (ATPase rate and duty ratio) would be unaffected by shifting the position. The biotinylated dynein anchored at the N-terminal GFP tag may exert an 8-nm step for the robust sliding, as expected from recent reports on the step size of dimeric cytoplasmic dynein on MT (37, 39), whereas the same dynein construct anchored at the biotin tag would exert a much shorter step for the slower sliding.
A similar strong dependence of the motile activity of myosin and kinesin on their anchoring position has been reported. When the rod-like IQ domain of myosin was truncated or elongated, in vitro motility assays for myosins anchored at the end of the IQ domain showed that the velocity of actin filament sliding was linearly related to the length of the IQ domain, which supports the model whereby the IQ domain works as a mechanical lever for the power stroke (40–43). Truncation or elongation of the kinesin neck region, which is thought to be the kinesin lever arm, has revealed that kinesin motile activity depends on the length of the neck region (44–46). The result has confirmed that the kinesin neck also acts as a lever arm for the power stroke. Thus, all three types of molecular motors, myosin, kinesin, and dynein, seem to share a similar motility mechanism, the power stroke of the lever arm, even though dynein has a very different evolutionary history and molecular architecture from those of myosin and kinesin.
Anchoring the dynein molecules on a glass surface in the desired orientation made it possible to uncouple the tail swing and the ring rotation against the glass surface by holding the AAA ring via the biotin–streptavidin linkage. Under these conditions, the power stroke of the tail was suppressed and the second mode of MT sliding became apparent, because the tail was allowed to swing freely without rotation of the AAA ring. All three constructs with biotin tags in the AAA ring (R1, R2, and R7) drove slow MT sliding at similar velocities. The T3 and T4 constructs with the biotin tag in the tail proximal to the tail-ring junction showed similar slow MT sliding. The sliding velocity vs. surface dynein density profile for the R2 construct showed that the dynein molecules anchored by the specific biotin–streptavidin linkage were responsible for this slow sliding (Fig. 4B), which is also likely true for other dynein constructs exhibiting slow MT sliding.
The slow MT sliding of the biotinylated dynein anchored at the AAA ring (R1, R2, R7) or at the tail close to the tail-ring junction (T3, T4) would be caused by changes in motor mechanics, not from changes in ATPase kinetics such as the ATPase rate or duty ratio, because the same dynein drove robust MT sliding when anchored at the N-terminal GFP tag. The shift of anchoring position from the N terminus to the AAA ring, for example, may affect the step size, but not ATPase kinetics as discussed above for the T1–T3 constructs. It would also be unlikely that the slow velocity resulted from steric hindrance to the MT sliding. This notion was judged from the spatial locations of the tail end and the biotin tag in the R1 or R2 construct. From electron microscopic observations, the biotin tag in these constructs is expected to be located at the edge of the AAA ring and on the opposite side of the stalk, whereas the N-terminal tail end is close to the stalk base (S. Burgess, personal communication). Therefore, steric hindrance to MT sliding, if any, would be more severe for HFB380 than for the R1 or R2 construct, because the anchoring site of HFB380 is very close to the stalk on which the MT filament slides. Therefore, at least for the R1 and R2 constructs, the slow MT sliding is an intrinsic property detected when the tail swing is uncoupled from robust MT sliding.
When molecules of KIF1A, a member of the kinesin superfamily, were anchored on a glass surface by biotin–streptavidin linkage either at their N or C terminus, MT filaments slid at similar velocities independently of the anchoring orientation of these KIF1A molecules, suggesting that this MT sliding is driven by biased Brownian motion (31). In the present study, biotinylated dyneins anchored in various orientations exhibited similar orientation-independent, one-directional MT sliding, leading us to speculate that this slow MT sliding may be driven by biased Brownian motion as in the case of KIF1A (31) or dynein c (35), although we cannot exclude the possibility that the active tilting of the stalk against the AAA ring may drive the orientation-independent MT sliding. Thus, the mechanism for this second mode of MT sliding remains to be identified.
Robust MT sliding, which is driven by the power stroke of the tail of dynein molecules, must be physiologically relevant for intracellular cargo transport along MT tracks given that the velocity of cargo transport (≈1 μm/s) (47, 48) is in the range observed here (≈3 μm/s). Although the contribution of the second mode of slow MT sliding to dynein function remains to be clarified, cytoplasmic dynein may use this mode under certain conditions to supplement the power stroke of the tail.
Materials and Methods
Construction, Expression, and Purification.
Expression plasmids for the C-terminal 380-kDa fragment (V1383-I4725) of the Dictyostelium discoideum cytoplasmic dynein heavy chain fused at its N terminus with His6-FLAG-GFP (HFG380) or His6-FLAG-biotin (HFB380) tandem tags have been described (21, 27, 36). As the biotin tag, we used the C-terminal 72 residues (G524–A595) of the Klebsiella pneumoniae oxalacetate decarbxylase α-subunit (49) as reported (36). To produce the seven kinds of biotinylated HFG380, the biotin tag was PCR-amplified and inserted between two codons within the HFG380 gene, corresponding to T1490–E1491, L1555–E1556, E1732–K1733, T1912–Q1913, A2172–E2173, S2471–S2472, or S4450–S4451 of the dynein heavy chain. The resulting constructs were designated T1, T2, T3, T4, R1, R2, and R7, respectively. Each of the constructs was subcloned into the MB38 vector for tetracycline-regulated expression in Dictyostelium (50).
Plasmids carrying each of the expression constructs were introduced by electroporation into Dictyostelium cells. The transformed cells were selected and harvested as described (11, 36). The expressed dynein heavy chains were purified by nickel affinity chromatography and then MT affinity (11). The heavy chains with biotin tag were almost fully biotinylated in vivo as shown (36). Tubulin was purified from porcine brains (51). The protein concentrations were determined by the Bradford method (52) using BSA as a standard.
In Vitro Motility Assay.
We carried out an in vitro motility assay by fixing the purified recombinant dynein constructs on a glass surface via streptavidin or anti-GFP antibody. These assays were performed at 25°C in assay buffer (10 mM Pipes-KOH/50 mM potassium acetate/4 mM MgSO4/1 mM EGTA/10 μM paclitaxel/1 mM DTT, pH 7.0).
For motility assays using biotin–streptavidin linkage, assay chambers were sequentially coated with 2 mg/ml biotinamidocaproyl BSA (Sigma-Aldrich, St. Louis, MO), 1 mg/ml streptavidin (Wako, Osaka, Japan), and ≈2 mg/ml α-casein (Merck, Darmstadt, Germany) (36). A purified dynein-containing solution (including 70 nM dynein construct, 1 mM ATP, and 0.1 mg/ml casein) was then perfused into the chambers twice, with a 5-min interval.
For assays using anti-GFP antibody, assay chambers were sequentially coated with 1 mg/ml streptavidin, 1 mg/ml protein G-biotin (Sigma-Aldrich), 125 μg/ml anti-GFP antibody (3E6; Qbiogene, Irvine, CA), and ≈2 mg/ml α-casein (11, 21). Purified dynein (230 nM) was then perfused into the assay chambers twice, with a 5-min interval.
Finally, these chambers were filled with assay buffer containing ≈0.3 μM paclitaxel-stabilized MT and then 1 mM ATP solution containing 0.05% methylcellulose. MT sliding was observed under a BX-51 dark-field microscope (Olympus, Tokyo, Japan) with a ×40 objective lens. The images recorded were digitized and analyzed.
The polarity-marked MT was prepared as described (53). Surface dynein density was estimated by using quantitative Western blotting (36). The surface area over which dynein interacted with an MT filament was expected to be of the order of the product of the MT length and twice the reach of the dynein constructs (average, ≈0.15 μm2). Thus, when surface dynein density was 15 molecules per μm2, the number of dynein molecules interacting with one MT filament was estimated to be two or three (15 molecules per μm2 × 0.15 μm2).
Measurements of Basal and MT-Activated ATPase Activities.
Just before ATPase measurements were carried out, the amount of ATP in the purified dynein construct solution was depleted to 0.1 mM by using an NAP-5 desalting column (GE Healthcare Bio-Sciences, Piscataway, NJ). Basal and MT-activated ATPase activities were measured as described (11, 21) using an EnzChek phosphate assay kit (Invitrogen, Carlsbad, CA).
Supplementary Material
Acknowledgments
We thank Dr. Y. Y. Toyoshima (University of Tokyo) for providing Tetrahymena cilia used for preparing polarity-marked MTs. This work was supported by a grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; a grant-in-aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and a grant from the Core Research for Evolutional Science and Technology (CREST) program from the Japan Science and Technology Agency (to K.S.).
Abbreviations
- AAA
ATPase associated with diverse cellular activities
- MT
microtubule.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
See Commentary on page 17587.
References
- 1.Paschal BM, Vallee RB. Nature. 1987;330:181–183. doi: 10.1038/330181a0. [DOI] [PubMed] [Google Scholar]
- 2.Holzbaur EL, Vallee RB. Annu Rev Cell Biol. 1994;10:339–372. doi: 10.1146/annurev.cb.10.110194.002011. [DOI] [PubMed] [Google Scholar]
- 3.Vallee RB, Sheetz MP. Science. 1996;271:1539–1544. doi: 10.1126/science.271.5255.1539. [DOI] [PubMed] [Google Scholar]
- 4.Hirokawa N. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- 5.Karki S, Holzbaur EL. Curr Opin Cell Biol. 1999;11:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- 6.Vale RD. Cell. 2003;112:467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 7.King SM. Biochim Biophys Acta. 2000;1496:60–75. doi: 10.1016/s0167-4889(00)00009-4. [DOI] [PubMed] [Google Scholar]
- 8.King SJ, Bonilla M, Rodgers ME, Schroer TA. Protein Sci. 2002;11:1239–1250. doi: 10.1110/ps.2520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuwald AF, Aravind L, Spouge JL, Koonin EV. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- 10.Koonce MP, Samso M. Mol Biol Cell. 1996;7:935–948. doi: 10.1091/mbc.7.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishiura M, Kon T, Shiroguchi K, Ohkura R, Shima T, Toyoshima YY, Sutoh K. J Biol Chem. 2004;279:22799–22802. doi: 10.1074/jbc.M313362200. [DOI] [PubMed] [Google Scholar]
- 12.Habura A, Tikhonenko I, Chisholm RL, Koonce MP. J Biol Chem. 1999;274:15447–15453. doi: 10.1074/jbc.274.22.15447. [DOI] [PubMed] [Google Scholar]
- 13.Tynan SH, Gee MA, Vallee RB. J Biol Chem. 2000;275:32769–32774. doi: 10.1074/jbc.M001537200. [DOI] [PubMed] [Google Scholar]
- 14.King SM. J Cell Sci. 2000;113:2521–2526. doi: 10.1242/jcs.113.14.2521. [DOI] [PubMed] [Google Scholar]
- 15.Burgess SA, Walker ML, Sakakibara H, Oiwa K, Knight PJ. J Struct Biol. 2004;146:205–216. doi: 10.1016/j.jsb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Samso M, Radermacher M, Frank J, Koonce MP. J Mol Biol. 1998;276:927–937. doi: 10.1006/jmbi.1997.1584. [DOI] [PubMed] [Google Scholar]
- 17.Gibbons IR, Gibbons BH, Mocz G, Asai DJ. Nature. 1991;352:640–643. doi: 10.1038/352640a0. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa K. Nature. 1991;352:643–645. doi: 10.1038/352643a0. [DOI] [PubMed] [Google Scholar]
- 19.Koonce MP, Grissom PM, McIntosh JR. J Cell Biol. 1992;119:1597–1604. doi: 10.1083/jcb.119.6.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mocz G, Gibbons IR. Biochemistry. 1996;35:9204–9211. doi: 10.1021/bi960662u. [DOI] [PubMed] [Google Scholar]
- 21.Kon T, Nishiura M, Ohkura R, Toyoshima YY, Sutoh K. Biochemistry. 2004;43:11266–11274. doi: 10.1021/bi048985a. [DOI] [PubMed] [Google Scholar]
- 22.Silvanovich A, Li MG, Serr M, Mische S, Hays TS. Mol Biol Cell. 2003;14:1355–1365. doi: 10.1091/mbc.E02-10-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gee MA, Heuser JE, Vallee RB. Nature. 1997;390:636–639. doi: 10.1038/37663. [DOI] [PubMed] [Google Scholar]
- 24.Koonce MP. J Biol Chem. 1997;272:19714–19718. doi: 10.1074/jbc.272.32.19714. [DOI] [PubMed] [Google Scholar]
- 25.Goodenough U, Heuser J. J Mol Biol. 1984;180:1083–1118. doi: 10.1016/0022-2836(84)90272-9. [DOI] [PubMed] [Google Scholar]
- 26.Burgess SA, Walker ML, Sakakibara H, Knight PJ, Oiwa K. Nature. 2003;421:715–718. doi: 10.1038/nature01377. [DOI] [PubMed] [Google Scholar]
- 27.Kon T, Mogami T, Ohkura R, Nishiura M, Sutoh K. Nat Struct Mol Biol. 2005;12:513–519. doi: 10.1038/nsmb930. [DOI] [PubMed] [Google Scholar]
- 28.Meng X, Samso M, Koonce MP. J Mol Biol. 2006;357:701–706. doi: 10.1016/j.jmb.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Goodenough UW, Heuser JE. J Cell Biol. 1982;95:798–815. doi: 10.1083/jcb.95.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgess SA. J Mol Biol. 1995;250:52–63. doi: 10.1006/jmbi.1995.0357. [DOI] [PubMed] [Google Scholar]
- 31.Okada Y, Higuchi H, Hirokawa N. Nature. 2003;424:574–577. doi: 10.1038/nature01804. [DOI] [PubMed] [Google Scholar]
- 32.Kitamura K, Tokunaga M, Iwane AH, Yanagida T. Nature. 1999;397:129–134. doi: 10.1038/16403. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka H, Homma K, Iwane AH, Katayama E, Ikebe R, Saito J, Yanagida T, Ikebe M. Nature. 2002;415:192–195. doi: 10.1038/415192a. [DOI] [PubMed] [Google Scholar]
- 34.Gebhardt JC, Clemen AE, Jaud J, Rief M. Proc Natl Acad Sci USA. 2006;103:8680–8685. doi: 10.1073/pnas.0510191103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakakibara H, Kojima H, Sakai Y, Katayama E, Oiwa K. Nature. 1999;400:586–590. doi: 10.1038/23066. [DOI] [PubMed] [Google Scholar]
- 36.Shima T, Imamula K, Kon T, Ohkura R, Sutoh K. J Struct Biol. 2006 doi: 10.1016/j.jsb.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Reck-Peterson SL, Yildiz A, Carter AP, Gennerich A, Zhang N, Vale RD. Cell. 2006;126:335–348. doi: 10.1016/j.cell.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howard J. Mechanics of Motor Proteins and the Cytoskeleton. Sunderland, MA: Sinauer; 2001. pp. 213–227. [Google Scholar]
- 39.Toba S, Watanabe TM, Yamaguchi-Okimoto L, Toyoshima YY, Higuchi H. Proc Natl Acad Sci USA. 2006;103:5741–5745. doi: 10.1073/pnas.0508511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uyeda TQ, Abramson PD, Spudich JA. Proc Natl Acad Sci USA. 1996;93:4459–4464. doi: 10.1073/pnas.93.9.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warshaw DM, Guilford WH, Freyzon Y, Krementsova E, Palmiter KA, Tyska MJ, Baker JE, Trybus KM. J Biol Chem. 2000;275:37167–371672. doi: 10.1074/jbc.M006438200. [DOI] [PubMed] [Google Scholar]
- 42.Ruff C, Furch M, Brenner B, Manstein DJ, Meyhofer E. Nat Struct Biol. 2001;8:226–229. doi: 10.1038/84962. [DOI] [PubMed] [Google Scholar]
- 43.Sakamoto T, Wang F, Schmitz S, Xu Y, Xu Q, Molloy JE, Veigel C, Sellers JR. J Biol Chem. 2003;278:29201–29207. doi: 10.1074/jbc.M303662200. [DOI] [PubMed] [Google Scholar]
- 44.Endres NF, Yoshioka C, Milligan RA, Vale RD. Nature. 2006;439:875–878. doi: 10.1038/nature04320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yun M, Bronner CE, Park CG, Cha SS, Park HW, Endow SA. EMBO J. 2003;22:5382–5389. doi: 10.1093/emboj/cdg531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart RJ, Thaler JP, Goldstein LS. Proc Natl Acad Sci USA. 1993;90:5209–5213. doi: 10.1073/pnas.90.11.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma S, Chisholm RL. J Cell Sci. 2002;115:1453–1460. doi: 10.1242/jcs.115.7.1453. [DOI] [PubMed] [Google Scholar]
- 48.Kural C, Kim H, Syed S, Goshima G, Gelfand VI, Selvin PR. Science. 2005;308:1469–1472. doi: 10.1126/science.1108408. [DOI] [PubMed] [Google Scholar]
- 49.Schwarz E, Oesterhelt D, Reinke H, Beyreuther K, Dimroth P. J Biol Chem. 1988;263:9640–9645. [PubMed] [Google Scholar]
- 50.Blaauw M, Linskens MH, van Haastert PJ. Gene. 2000;252:71–82. doi: 10.1016/s0378-1119(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 51.Sloboda RD, Rosenbaum JL. Methods Enzymol. 1982;85:409–416. doi: 10.1016/0076-6879(82)85041-6. [DOI] [PubMed] [Google Scholar]
- 52.Bradford MM. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 53.Vale RD, Toyoshima YY. Cell. 1988;52:459–469. doi: 10.1016/s0092-8674(88)80038-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.