Abstract
Paclitaxel-loaded mixed polymeric micelles consisting of poly(ethylene glycol)-distearoyl phosphoethanolamine conjugates (PEG-PE), solid triglycerides (ST), and cationic Lipofectin® lipids (LL) have been prepared. Micelles with the optimized composition (PEG-PE/ST/LL/paclitaxel = 12/12/2/1 by weight) had an average micelle size of about 100 nm, and zeta-potential of about 26 mV. Micelles were stable and did not release paclitaxel when stored at 4°C in the darkness (just 2.9% of paclitaxel have been lost after 4 months with the particle size remaining unchanged). The release of paclitaxel from such micelles at room temperature was also insignificant. However, at 37°C, approx. 16% of paclitaxel was released from PEG-PE/ST/LL/paclitaxel micelles in 72 h, probably, because of phase transition in the ST-containing micelle core. In vitro anticancer effects of PEG-PE/ST/LL/paclitaxel and control micelles were evaluated using human mammary adenocarcinoma (BT-20) and human ovarian carcinoma (A2780) cell lines. Paclitaxel in PEG-PE/ST/LL micelles demonstrated the maximum anti-cancer activity. Cellular uptake of fluorescently-labeled paclitaxel-containing micelles by BT-20 cells was investigated using a fluorescence microscopy. It seems that PEG-PE/ST/LL micelles, unlike micelles without the LL component, could escape from endosomes and enter the cytoplasm of BT-20 cancer cells thus increasing the anticancer efficiency of the micellar paclitaxel.
Keywords: Polymeric micelles, mixed micelles, PEG-PE, cationic lipids, paclitaxel, in vitro anticancer effect
Introduction
Paclitaxel (Taxol), a diterpenoid derived from the needles and bark of the Pacific Yew tree (Taxus brevifolia), is an anticancer agent for the treatment of various cancers including ovarian and breast cancers (Baselga et al. 1998, Ling et al. 1998). The anticancer mechanism of paclitaxel as a potent inhibitor of cancer cell replication is related to its ability to block cancer cells in the late G2-mitotic phase of the cell cycle by stimulating microtubule polymerization and suppressing their dynamics (Horwitz, 1994, Jordan, 2002). Paclitaxel has very low water solubility, and clinically it is used as a solution in Cremophor EL/ethanol (1/1, w/w) (Fjallskog et al. 1993). However, Cremophor (polyethoxylated castor oil) can provoke a number of side effects, such as hypersensitivity, nephrotoxicity and neurotoxicity (Nassberger et al. 1991, Windebank et al. 1994, He et al. 2003). Although the incidence of serious hypersensitivity reactions has been reduced by a premedication regimen with corticosteroids and antihistamine agents, side effects have still been found to occur in 5-30% of treated patients (Weiss et al. 1990). High toxicity of paclitaxel itself represents an additional issue, and the maintenance of therapeutically significant systemic concentration of the drug was reported to cause severe reactions (Rowinsky et al. 1993, Sarosy and Reed, 1993).
To overcome these problems and increase paclitaxel bioavailability, many types of drug delivery systems, such as nanoparticles (Damascelli et al. 2003, Mitra and Lin, 2003, Potineni et al. 2003), liposomes (Kunstfeld et al. 2003), emulsions (Kan et al. 1999, Constantinides et al. 2000, Rodrigues et al. 2002) and various micelles (Liggins and Burt, 2002 Krishnadas et al. 2003 Lukyanov et al. 2003a), have been tried as pharmaceutical carriers for paclitaxel. Recently published data suggest that polymeric micelles may be of particular interest for delivery of sparingly soluble drugs including anticancer drugs. Micelles are spherical nanoparticles of a colloidal size, into which many amphiphilic molecules self-assemble. In water, hydrophilic parts of such molecules form the micelle corona, while hydrophobic fragments form the core of a micelle that may serve as a cargo space for poorly soluble pharmaceuticals (Muranishi 1990, Lasic 1992). Because of their small size (approx. 5-50 nm), micelles are able to spontaneously accumulate in pathological areas with the damaged (“leaky”) vasculature, such as infarcts (Palmer et al. 1984) and tumors (Gabizon 1995, Yuan et al. 1995), via the enhanced permeability and retention (EPR) effect (Maeda et al. 2000; 2001).
Polymeric micelles formed by amphiphilic synthetic copolymers demonstrate a whole set of attractive properties as drug carriers (Kwon and Kataoka 1995, Jones and Leroux 1999, Torchilin 2001). Because of their low critical micelle concentration, polymeric micelles are stable in vitro. In addition, the micelle corona formed by hydrophilic polymer blocks provides longevity to micelles in vitro by preventing their opsonization and capture by the cells of the reticuloendothelial system (Torchilin and Trubetskoy 1995). Micelles made from conjugates of poly(ethylene glycol) (PEG) and diacyllipids, such as phosphatidylethanolamine (PE), are of particular interest (Trubetskoy and Torchilin 1995) because the use of lipid moieties as hydrophobic blocks allows for an efficient incorporation (solubilization) of poorly soluble drugs and provides additional stability to the micelles, since the existence of two hydrocarbon chains in the lipid moiety strongly increases the hydrophobic interactions in the micelle’s core. We have reported some data on stable and long-circulating polymeric micelles formed by PEG-PE conjugates (Trubetskoy and Torchilin 1996). Such micelles can be loaded with a variety of poorly soluble drugs including paclitaxel (Weissig et al. 1998a, Gao et al. 2002) and are capable of delivering their load even into poorly permeable tumors in mice with a higher efficiency than other long-circulating carriers (Weissig et al. 1998b, Lukyanov et al. 2002).
There may be several ways to still further enhance the drug delivery potential of polymeric micelles, PEG-PE-based micelles among them. Thus, targeting ligands, including antibodies, could be attached to the micelle surface to increase the accumulation of micelles and micelle-incorporated drugs in appropriate targets including tumors (Torchilin 2001). We have recently described the preparation of PEG-PE-based immunomicelles modified with monoclonal 2C5 antibody with nucleosome-restricted specificity [2C5; reactive towards a variety of different cancer cells (Iakoubov and Torchilin 1997)], and have demonstrated that paclitaxel-loaded 2C5-immunomicelles specifically recognize various cancer cells, possess increased cytotoxicity in vitro, deliver increased quantities of the drug into experimental tumors, and provide improved tumor growth inhibition in vitro (Gao et al. 2003, Torchilin et al. 2003).
On the other hand, it is known that the net positive charge usually enhances the endocytosis of various nanoparticles, and positively charged lipid mixtures, such as Lipofectin® (an equimolar mixture of N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride, DOTMA, and dioleoyl phosphatidylethanolamine, DOPE) noticeably improves the endocytosismediated intracellular delivery of various drugs and DNA entrapped within liposomes and other lipid constructs made of these compositions (Chawla and Amiji, 2002, Ota et al. 2002, Kaiser and Toborek, 2003, Almofti et al. 2003). PEG-PE micelles have been found to carry a net negative charge (Lukyanov et al. 2003b), which might hinder their internalization by cells. The alteration of this negative charge by the addition of positively charged lipids to PEG-PE could improve the uptake of paclitaxel-loaded mixed PEG-PE/positively charged lipid micelles by cancer cells thus increasing the efficiency of this drug delivery system. With this in mind, we attempted to increase intracellular delivery and, thus, the anticancer activity of the micellar paclitaxel by preparing paclitaxel-containing micelles from the mixture of PEG-PE and Lipofectin® lipids. In addition, since it was shown that triglycerides could form paclitaxel-loaded emulsions (Lundberg 1997, Kan et al. 1999, Constantinides et al. 2000, Rodrigues et al. 2002), we introduced solid triglycerides into the micelle core to provide a higher load of paclitaxel and to minimize its release in the circulation. Here, we present the results of our in vitro studies with these systems.
Materials and methods
Materials
Paclitaxel (Taxol) was purchased from Sigma Chem., Inc. (St. Louis, MO). (1,2-diacyl-SN-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (PEG-PE) and phosphatidylethanolamine lissamine rhodamine B (Rh-PE) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). The mixture of positively charged lipids (Lipofectin®) was obtained from Invitrogene™ (Carlsbad, CA). All other reagents and components of buffer solutions were analytical grade preparations.
Preparation of solid triglyceride
Solid triglyceride (ST, melting point of 31-37°C) was extracted from margarine (Land OȁLakes, Inc., MN) as described in Kallio et al. (1989). Briefly, 5 g of margarine was dissolved in 100 ml of hexane, shaken, and let stand in the dark for 12 h. A clear solution was obtained after a small amount of insoluble materials settled down. The supernatant was paper filtered three times. The filtrate was washed with 60% ethanol (1/1, v/v) by shaking for 10 min and then centrifugating for 10 min at 2000 rpm. The hexane layer was separated and dried. The filtrate was washed in triplicate as above. The residual ST was weighed and dissolved in chloroform for the following experiments.
Preparation of paclitaxel-loaded micelles
To prepare paclitaxel-loaded micelles, various quantities of PEG-PE, ST, Lipofectin lipids (LL), and paclitaxel were dissolved in 30 ml of chloroform. To fluorescently label the micelles, 1 wt% of Rh-PE (λex568/λem590) was added to the composition for micelle preparation. Via its hydrophobic fragment, PE, Rh-PE firmly anchors into the micelle core and remains associated with the micelle as long as the micelle exists. Chloroform was evaporated using N2 gas, and the film formed was additionally dried in a vacuum. A required volume of 0.9% NaCl to make a final paclitaxel concentration of 1 mg/ml was added to the dried film and the mixture was incubated in a water bath at 37°C and shaken 5 min. Then, the mixture was placed in the ice water, sonicated for 10 min, and subsequently filtered three times through each of 0.45, 0.2, and 0.1 mm polycarbonate membrane (Millipore Co., Bedford, MA). The filtrate was divided into ampoules and sealed under N2.
Efficiency of paclitaxel incorporation
To find the quantity of the micelle-incorporated paclitaxel, each paclitaxel-containing micelle preparation was filtered through a 200 nm polycarbonate filter (Millipore Co., Bedford, MA), and the percentage of the paclitaxel in the filtrate (micellized paclitaxel) was determined using the HPLC method as described previously (Willey et al. 1993). The value obtained was named the micellization efficiency (calculated from calibration curves). The D-7000 HPLC system was used equipped with a diode array and fluorescence detector (Hitachi, Japan) and a 150 × 6.0 mm YMC-Pack ODS column (YMC Co., Ltd., Japan). The mobile phase was acetonitrile/water (60/40, v/v), and the flow rate was 1.0 ml/min. Paclitaxel was detected at 227 nm.
Micelle size measurement
The particle size measurement and size distribution analysis were performed using a Coulter® N4-Plus Submicron Particle Sizer (Coulter Corporation, Miami, FL). Forty microlitres of mixed micelle dispersion was diluted with the deionized distilled water until a concentration providing a light scattering intensity of 5 × 104-1 × 106 counts per second was achieved. The particle size distribution of each sample was measured in triplicate.
Zeta-potential measurement
Micelle surface charge analysis was performed using a Zeta Phase Analysis Light Scattering (PALS) UltraSensitive Zeta Potential Analyzer instrument (Brookhaven Instruments, Holtsville, NY). Each sample of micelle suspension was diluted with deionized distilled water to have the signal intensity within the limits required by the instrument. The zeta-potential of each sample was determined from five-to-eight independent measurements.
In vitro paclitaxel release
The in vitro paclitaxel release was investigated at 4, 25, and 37°C. At each temperature, 1 ml of drug-loaded micelles was placed into a SpectraPor® molecular porous regenerated cellulose dialysis membrane with a molecular weight cut off size of 3500 Da, and dialyzed against 4 L of PBS (pH 7.4). After 12 h, the dialysis medium was replaced with 4 L of fresh medium, and 10 μl of micelle suspension was taken out and diluted 40 times with methanol/chloroform (1/2, v/v) for determination of paclitaxel using HPLC method (Willey et al. 1993). The concentration of paclitaxel in each sample was calculated using a calibration curve.
Storage stability
Drug-loaded micelles were stored in a dark place at 4°C for 6 months. The stability was monitored by the changes in particle size and drug concentration during the storage period. The particle size distribution and paclitaxel concentration were determined as above.
Cell cultures
Human mammary adenocarcinoma (BT-20) and human ovarian carcinoma (A2780) cell lines were purchased from the American Type Culture Collection (Manassas, VA). BT-20 and A2780 cells were maintained in RPMI 1640 and EMEM cell culture medium, respectively. Cell culture media were supplemented with FBS to 10%, Na pyruvate to 1mm, l-glutamine to 1 mM and penicillin and streptomycin to 50 U/ml and 50 mg/ml, respectively.
In vitro anticancer effects
The in vitro anticancer effects of drug-loaded micelles were evaluated using the MTT [3-(4,5-dimethylthia-zol-2-yl)-2,5-diphenyl-tetrazolium bromide] method (Ni et al. 1998). Briefly, cells were plated at 2 104 cells per well density in 96-well plates (Corning, Inc., Corning, NY). After 24 h incubation at 37°C, 5% CO2, the medium was replaced with a medium containing different paclitaxel formulations. After an additional 24 h incubation at 37°C, 5% CO2, the media were replaced with PBS containing 0.1 mg/ml MTT, and the cells were incubated for 3 h at 37°C, 5% CO2. The method is based on the fact that living cells reduce MTT to formazan. The cytotoxicity was measured following the absorbance of the degraded MTT (formazan) at 492 nm using a MCC/340 ELIZA Reader (Lab Systems, Finland). The IC50 values, or the concentrations of various preparations at which the cell growth inhibition was 50% compared to untreated control cells, were estimated (or extrapolated) from the dose-response curves.
Cellular uptake of micelles by BT-20 cancer cells
The cellular uptake of various micelles was studied using micelles labeled with 1% Rh-PE (w/w). Adherent BT-20 cells were grown on glass cover slips placed into six-well tissue culture plates. When the cells reached a confluency of 60-70%, the cells were washed twice with Hank’s buffer and treated with a 1% solution of bovine serum albumin (Hank’s/BSA) in EMEM medium. After 1 h incubation at 37°C, 5% CO2, the BSA-containing EMEM was replaced with Rh-PE labeled micelles in the medium to a PEG-PE concentration of 3.5 × 10-3 mg/ml. After additional incubation for 0.5, 2, and 4 h, at 37°C, 5% CO2, the cover slips were washed three times with cold saline, and mounted individually cell-side down on clean glass slides using a fluorescence-free glycerol-based Trevigen® mounting medium (Trevigen, Gaitherburg, MD). Mounted slides were studied with a Nikon Eclipse E400 microscope under fluorescence using a Rh/TRITC filter.
Results and discussion
Formulation study
To find a ST/paclitaxel optimum ratio, which allows for the best paclitaxel solubilization, a series of paclitaxel-loaded mixed micelles were prepared with different ST/paclitaxel weight ratios (34.0, 17.0, 8.5, 4.25, and 2.125) at the same quantity of PEG-PE and LL, and the paclitaxel micellization efficiency by each of the micelle dispersions was determined. The effect of the ST on paclitaxel micellization efficiency is shown in Figure 1, which demonstrates that to acquire a paclitaxel micellization of 100% when PEG-PE/LL/paclitaxel ratio was fixed at 12/2/1 (w/w/w), the ST/paclitaxel weight ratio should be greater than 8.5.
Figure 1.
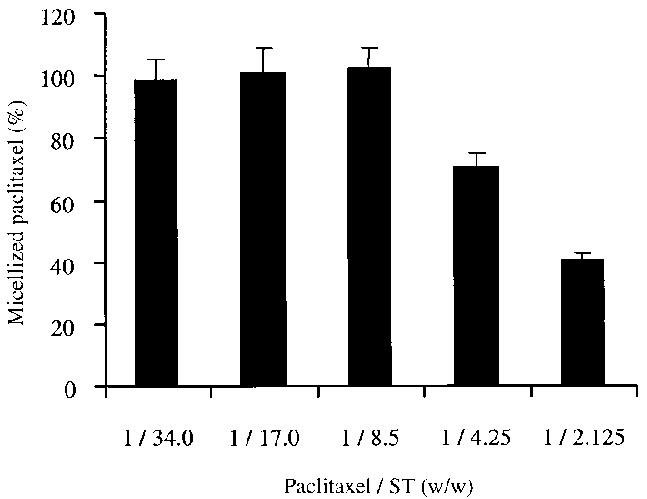
The effect of the ST content in mixed micelles on paclitaxel micellization efficiency.
The effects of the addition of ST, LL and paclitaxel on the size and zeta-potential of the initial PEG-PE micelles are shown in Table I. The particle size of the plain PEG-PE micelles was 12.8 ± 2.6nm. When additionally loaded with 1:1 weight ratio of ST, their average size was enlarged to 66.8 ± 6.6 nm due to the efficient solubilization of ST by the micelle core. Further addition of LL to the final PEG-PE/ST/LL weight ratio of 12/12/2 resulted in an additional increase in the micelle size to 95.7 ± 3.5 nm. When paclitaxel was also loaded into the micelles yielding the final preparation with PEG-PE/ST/LL/paclitaxel weight ratio of 12/12/2/1, the micelle size remained virtually unchanged at 98.2 ± 7.2 nm. The plain PEG-PE micelles were negatively charged with a zeta-potential of -31.1 ± 1.7 mV. The addition of the uncharged ST to PEG-PE micelles did not noticeably change the micelle zeta-potential. A slight increase in the zeta-potential of the resulting micelles to -27.9 ± 4.5 mV might be explained by the increase in the micelle size because of ST solubilization in the micelle core (Table I) and resulting drop in the micelle surface charge density. When positively charged Lipofectin® lipids were added to PEG-PE micelles with coresolubilized ST, the zeta-potential of such micelles was dramatically increased to -8.0 ± 0.4mV. Additional loading of paclitaxel into mixed micelles that yielded the final PEG-PE/ST/LL/paclitaxel micelles did not cause any further change in micelle zeta-potential (it was -6.2 ± 0.7 mV).
Table I.
Effect of solid triglyceride (ST), Lipofectin® lipids (LL), and paclitaxel on the particle size and zeta-potential of the micelles.
| Micelle components (w/w) | Average diameter (nm)* | Zeta-potential (mV)† |
|---|---|---|
| PEG-PE | 12.8 ± 2.6 | -31.1 ± 1.7 |
| PEG-PE/ST (12/12) | 66.8 ± 6.6 | -27.9 ± 4.5 |
| PEG-PE/ST/paclitaxel (12/12/1) | 71.2 ± 7.8 | -27.0 ± 4.8 |
| PEG-PE/ST/LL (12/12/2) | 95.7 ± 3.5 | -8.0 ± 0.4 |
| PEG-PE/ST/LL/paclitaxel (12/12/2/1) | 98.2 ± 7.2 | -6.2 ± 0.7 |
Average diameter is presented as mean±SD (n = 3).
Zeta-potential is presented as mean ±SD (n = 5 ∼ 8).
Thus, our final optimized preparation of paclitaxelloaded micelles had a composition as PEG-PE/ST/LL/paclitaxel of 12/12/2/1 by weight, average micelle size of slightly under 100 nm, and zeta-potential of about 26 mV. The content of paclitaxel in the preparation was about 4 wt%, and paclitaxel concentration was approx. 1 mg/ml.
The in vitro release of paclitaxel from these mixed micelles in PBS, pH = 7.4, at different temperatures is presented in Figure 2, which shows that after the incubation for 72 h at 4°C, the release of paclitaxel was 2.3 ± 0.2%, and only slightly higher (4.1 ± 0.3%) after the same incubation time at room temperature. However, when kept for 72 h at 37°C, paclitaxel release was 16.3 ± 1.2%. Such a significant difference in the release rate between room temperature and 37°C may be explained by the fact that in the micelles prepared, paclitaxel was loaded in the hydrophobic micelle core with high ST content. The triglyceride is in its solid form at room temperature and below, while at a temperature around 37°C it undergoes a phase transition into its liquid form thus facilitating paclitaxel release.
Figure 2.

The in vitro release of paclitaxel from mixed PEG-PE/ST/LL/paclitaxel (12/12/2/1 by weight) micelles in PBS, pH =7.4 at different temperatures.
The preparation of paclitaxel in mixed micelles, after storage for 4 months at 4°C in the dark, showed a loss of only 2.9% of paclitaxel, probably because of the partial paclitaxel decomposition. The micelle size remains practically unchanged (98.2 ± 7.2 nm vs 96.8 ± 16.4 nm before and after storage, respectively). The long-term stability of paclitaxel-containing mixed micelles is currently being evaluated.
In vitro anticancer effects
The in vitro anticancer effects of paclitaxel-containing micelles are shown in Figures 3 and 4. Clearly, the addition of LL facilitates the intracellular uptake of paclitaxel-containing micelles with the compensated negative charge, since the anticancer effect of paclitaxel in PEG-PE/ST/LL micelles was significantly greater than that of free paclitaxel and of paclitaxel in PEG-PE/ST micelles in both cancer cell lines. In A2780 cancer cells, the IC50 values of free paclitaxel, paclitaxel in PEG-PE/ST micelles, and paclitaxel in PEG-PE/ST/LL micelles were 12.2, 3.9, and 0.7 μM, respectively (Figure 3). In BT-20 cancer cells, the IC50 values of the same preparations were 13.0, 8.5 and 4.7 μM, respectively (Figure 4).
Figure 3.

The in vitro viability of A2780 cancer cells after the incubation with different paclitaxel preparations. See “Materials and methods section” for details.
Figure 4.
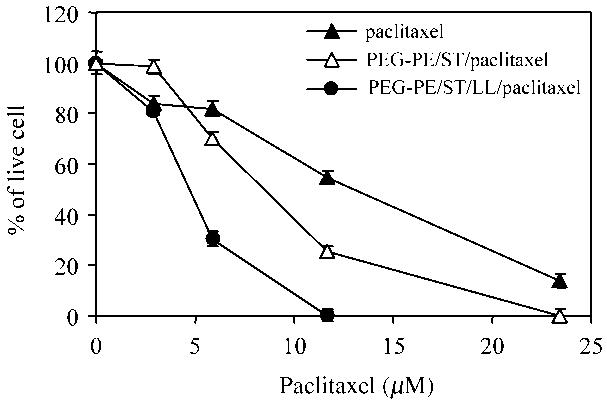
The in vitro viability of BT-20 cancer cells after the incubation with different paclitaxel preparations. See “Materials and methods section” for details.
It is now believed that the endocytosis of the DNA-loaded Lipofectin® particles is the major mechanism of cellular delivery of DNA by the lipofection (Legendre and Szoka, 1992, Felgner et al. 1994, Zabner et al. 1995, Sakurai et al. 2000). After endocytosis, the DNA-loaded particles can escape from the endosomes and enter the cytoplasm of most cells because of the interaction between cationic lipid and endosome membranes (Hafez et al. 2001). Therefore, it is possible that after the enhanced endocytosis, paclitaxel-loaded mixed PEG-PE/ST/LL micelles escaped from the endosomes and entered the cytoplasm of cancer cells, where paclitaxel could slowly release from the micelles and kill cancer cells with higher efficiency than free drug or drug in strongly negatively charged PEG-PE micelles. (This hypothesis was further verified by the set of experiments described in the next paragraph.)
In addition, it is known that Lipofectin® particles with high positive charge could induce a significant non-specific toxicity towards cells as was shown with Lipofectin-based gene delivery systems (Filion and Phillips, 1997). At the same time, mixed PEG-PE/positively charged lipid micelles with the net charge close to neutral were not toxic (paclitaxel-free control micelles did not affect the normal morphology and attachment of experimental cells), while they still increased the anticancer efficiency of the micelle-incorporated paclitaxel.
Micelle interaction with BT-20 cells by fluorescence microscopy
The interaction with cells and the intracellular fate of paclitaxel-containing PEG-PE/ST/LL micelles and similar micelles prepared without the addition of the LL was investigated by fluorescence microscopy using the BT-20 cell culture. As follows from the data presented in Figure 5, both PEG-PE/ST and PEG-PE/ST/LL micelles underwent endocytosis by BT-20 cells, which was confirmed by the presence of fluorescent endosomes in cells after co-incubation with fluorescently-labeled micelles. When the co-incubation time was 30 min, only LL-containing micelles were found in endosomes of some BT-20 cells. In 2 h, both LL-free and LL-containing micelles were found to be endocytosed by BT-20 cells. In the case of PEG-PE/ST/LL micelles, however, endosomes look partially degraded, which may serve as evidence of a destabilizing effect of the LL component on the endosomal membranes. This hypothesis is still further confirmed by the pattern observed after 4 h: if one can clearly see late (fused) fluorescent endosomes in the case of LL-free PEG-PE/ST micelles, cells with PEG-PE/ST/LL micelles demonstrate the presence of small fluorescent structures in the cytoplasm, but no “normal” endosomes could be seen. This observation supports the possibility of the enhanced cytoplasmic delivery of drugs, when endosome-destabilizing PEG-PE/ST/LL micelles are used as drug carriers.
Figure 5.

Light (left images in each pair) and fluorescent (right images in each pair) microscopy of BT-20 cells incubated with Rh-PE-labeled PEG-PE/ST/paclitaxel micelles and PEG-PE/ST/LL/paclitaxel micelles for 0.5, 2, and 4 h. Arrows on fluorescent microscopy images show: fluorescent endosomes in cells incubated with PEG-PEG-PE/ST/LL/paclitaxel micelles for 0.5 h and with PEG-PE/ST/paclitaxel micelles for 2 h; partially degraded endosomes in cells incubated with PEG-PE/ST/LL/paclitaxel micelles for 2 h; fused late endosomes in cells incubated with PEG-PE/ST/paclitaxel micelles for 4 h; small fluorescent structures in cells incubated with PEG-PE/ST/LL/paclitaxel micelles for 4 h. See “Materials and methods section” for details.
In conclusion, the in vitro anticancer effects of paclitaxel were significantly improved when the drug was carried by mixed micelles made of PEG-PE and positively charged lipids. Such micelles may become a promising intracellular delivery system for clinical applications of paclitaxel.
Acknowledgements
This work was supported by the NIH grant EB01961 to V.P.T.
References
- Almofti MR, Harashima H, Shinohara Y, Almofti A, Baba Y, Kiwada H. Cationic liposome-mediated gene delivery: biophysical study and mechanism of internalization. Arch Biochem Biophys. 2003;410:246–253. doi: 10.1016/s0003-9861(02)00725-7. [DOI] [PubMed] [Google Scholar]
- Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xeno-grafts. Cancer Res. 1998;58:2825–2831. [PubMed] [Google Scholar]
- Chawla JS, Amiji MM. Biodegradable poly(epsilon-capro-lactone) nanoparticles for tumor-targeted delivery of tamoxifen. Int J Pharm. 2002;249:127–138. doi: 10.1016/s0378-5173(02)00483-0. [DOI] [PubMed] [Google Scholar]
- Constantinides PP, Lambert KJ, Tustian AK, Schneider B, Lalji S, Ma W, Wentzel B, Kessler D, Worah D, Quay SC. Formulation development and antitumor activity of a filtersterilizable emulsion of paclitaxel. Pharm Res. 2000;17:175–182. doi: 10.1023/a:1007565230130. [DOI] [PubMed] [Google Scholar]
- Damascelli B, Patelli GL, Lanocita R, Di Tolla G, Frigerio LF, Marchiano A, Garbagnati F, Spreafico C, Ticha V, Gladin CR, Palazzi M, Crippa F, Oldini C, Calo S, Bonaccorsi A, Mattavelli F, Costa L, Mariani L, Cantu G. A novel intraarterial chemotherapy using paclitaxel in albumin nanoparticles to treat advanced squamous cell carcinoma of the tongue: preliminary findings. Am J Roentgenol. 2003;181:253–260. doi: 10.2214/ajr.181.1.1810253. [DOI] [PubMed] [Google Scholar]
- Felgner JH, Kumar R, Sridhar CN, Wheeler CJ, Tsai YJ, Border R, Ramsey P, Martin M, Felgner PL. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem. 1994;269:2550–2561. [PubMed] [Google Scholar]
- Filion MC, Phillips NC. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. Biochim Biophys Acta. 1997;1329:345–356. doi: 10.1016/s0005-2736(97)00126-0. [DOI] [PubMed] [Google Scholar]
- Fjallskog ML, Frii L, Bergh J. Is Cremophor EL, solvent for paclitaxel, cytotoxic? Lancet. 1993;342:8873–8875. doi: 10.1016/0140-6736(93)92735-c. [DOI] [PubMed] [Google Scholar]
- Gabizon AA. Liposome circulation time and tumor targeting—implications for cancer-chemotherpy. Adv Drug Deliv Rev. 1995;16:285–294. [Google Scholar]
- Gao Z, Lukyanov AN, Singhal A, Torchilin VP. Diacyllipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett. 2002;2:979–982. [Google Scholar]
- Gao Z, Lukyanov AN, Chakilam AR, Torchilin VP. PEG-PE/phosphatidylcholine mixed immunomicelles specifically deliver encapsulated taxol to tumor cells of different origin and promote their efficient killing. J Drug Targ. 2003;11:87–92. doi: 10.1080/1061186031000138623. [DOI] [PubMed] [Google Scholar]
- Hafez IM, Maurer N, Cullis PR. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 2001;8:1188–1196. doi: 10.1038/sj.gt.3301506. [DOI] [PubMed] [Google Scholar]
- He L, Wang GL, Zhang Q. An alternative paclitaxel microemulsion formulation: hypersensitivity evaluation and pharmacokinetic profile. Int J Pharm. 2003;250:45–50. doi: 10.1016/s0378-5173(02)00478-7. [DOI] [PubMed] [Google Scholar]
- Horwitz SB. Taxol (paclitaxel): mechanisms of action. Ann Oncol. 1994;5(Suppl 6):S3–S6. [PubMed] [Google Scholar]
- Iakoubov LZ, Torchilin VP. A novel class of antitumor antibodies: nucleosome-restricted antinuclear autoantibodies (ANA) from healthy aged nonautoimmune mice. Oncol Res. 1997;9:439–446. [PubMed] [Google Scholar]
- Jones M, Leroux J. Polymeric micelles—a new generation of colloidal drug carriers. Eur J Pharmacol Biopharmacol. 1999;48:101–111. doi: 10.1016/s0939-6411(99)00039-9. [DOI] [PubMed] [Google Scholar]
- Jordan MA. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem AntiCanc Agents. 2002;2:1–17. doi: 10.2174/1568011023354290. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Toborek M. High-efficiency transfection of human endothelial cells mediated by cationic lipids. J Vasc Res. 2003;38:133–143. doi: 10.1159/000051040. [DOI] [PubMed] [Google Scholar]
- Kallio H, Laakso P, Huopalahti R, Linko RR, Oksman P. Analysis of butter fat triacylglycerols by supercritical fluid chromatography/electron impact mass spectrometry. Anal Chem. 1989;61:698–700. doi: 10.1021/ac00182a012. [DOI] [PubMed] [Google Scholar]
- Kan P, Chen ZB, Lee CJ, Chu IM. Development of nonionic surfactant/phospholipid o/w emulsion as a paclitaxel delivery system. J Control Release. 1999;58:271–278. doi: 10.1016/s0168-3659(98)00164-3. [DOI] [PubMed] [Google Scholar]
- Krishnadas A, Rubinstein I, Onyuksel H. Sterically stabilized phospholipid mixed micelles: in vitro evaluation as a novel carrier for water-insoluble drugs. Pharm Res. 2003;20:297–302. doi: 10.1023/a:1022243709003. [DOI] [PubMed] [Google Scholar]
- Kunstfeld R, Wickenhauser G, Michaelis U, Teifel M, Umek W, Naujoks K, Wolff K, Petzelbauer P. Paclitaxel encapsulated in cationic liposomes diminishes tumor angiogenesis and melanoma growth in a “humanized” SCID mouse model. J Invest Dermatol. 2003;120:476–482. doi: 10.1046/j.1523-1747.2003.12057.x. [DOI] [PubMed] [Google Scholar]
- Kwon GS, Kataoka K. Block-copolymer micelles as longcirculating drug vehicles. Adv Drug Deliv Rev. 1995;16:295–309. [Google Scholar]
- Lasic DD. Mixed micelles in drug delivery. Nature. 1992;355:279–280. doi: 10.1038/355279a0. [DOI] [PubMed] [Google Scholar]
- Legendre JY, Szoka FC., Jr. Delivery of plasmid DNA into mammalian cell lines using pH-sensitive liposomes: comparison with cationic liposomes. Pharm Res. 1992;9:1235–1242. doi: 10.1023/a:1015836829670. [DOI] [PubMed] [Google Scholar]
- Liggins RT, Burt HM. Polyether-polyester diblock copolymers for the preparation of paclitaxel loaded polymeric micelle formulations. Adv Drug Deliv Rev. 2002;54:191–202. doi: 10.1016/s0169-409x(02)00016-9. [DOI] [PubMed] [Google Scholar]
- Ling YH, Yang Y, Tornos C, Singh B, Perez-Soler R. Paclitaxel-induced apoptosis is associated with expression and activation of c-Mos gene product in human ovarian carcinoma SKOV3 cells. Cancer Res. 1998;58:3633–3640. [PubMed] [Google Scholar]
- Lukyanov AN, Gao Z, Mazzola L, Torchilin VP. Polyethylene glycol-diacyllipid micelles demonstrate increased acculumation in subcutaneous tumors in mice. Pharm Res. 2002;19:1424–1429. doi: 10.1023/a:1020488012264. [DOI] [PubMed] [Google Scholar]
- Lukyanov AN, Gao Z, Torchilin VP. Micelles from polyethylene glycol/phosphatidylethanolamine conjugates for tumor drug delivery. J Control Release. 2003a;91:97–102. doi: 10.1016/s0168-3659(03)00217-7. [DOI] [PubMed] [Google Scholar]
- Lukyanov AN, Hartner WC, Torchilin VP. Increased accumulation of PEG-PE micelles in the area of experimental myocardial infarction in rabbits. J Control Release. 2003b doi: 10.1016/j.jconrel.2003.10.008. in press. [DOI] [PubMed] [Google Scholar]
- Lundberg BB. A submicron lipid emulsion coated with amphipathic polyethylene glycol for parenteral administration of paclitaxel (Taxol) J Pharm Pharmacol. 1997;49:16–21. doi: 10.1111/j.2042-7158.1997.tb06744.x. [DOI] [PubMed] [Google Scholar]
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release. 2001;74:47–61. doi: 10.1016/s0168-3659(01)00309-1. [DOI] [PubMed] [Google Scholar]
- Mitra A, Lin S. Effect of surfactant on fabrication and characterization of paclitaxel-loaded polybutylcyanoacrylate nanoparticulate delivery systems. J Pharm Pharmacol. 2003;55:895–902. doi: 10.1211/0022357021341. [DOI] [PubMed] [Google Scholar]
- Muranishi S. Absorption enhancers. Crit Rev Ther Drug Carrier Syst. 1990;7:1–33. [PubMed] [Google Scholar]
- Nassberger L, Bergstrand A, DePierre JW. An electron and fluorescence microscopic study of LLC-PK1 cells, a kidney epithelial cell line: normal morphology and cyclosporin A- and cremophor-induced alterations. Int, J Exp Pathol. 1991;72:365–378. [PMC free article] [PubMed] [Google Scholar]
- Ni R, Nishikawa Y, Carr BI. Cell growth inhibition by a novel vitamin K is associated with induction of protein tyrosine phosphorylation. J Biol Chem. 1998;273:9906–9911. doi: 10.1074/jbc.273.16.9906. [DOI] [PubMed] [Google Scholar]
- Ota T, Maeda M, Tatsuka M. Cationic liposomes with plasmid DNA influence cancer metastatic capability. Anticancer Res. 2002;22:4049–4052. [PubMed] [Google Scholar]
- Palmer TN, Caride VJ, Caldecourt MA, Twickler J, Abdullah V. The mechanism of liposome accumulation in infarction. Biochim Biophys Acta. 1984;797:363–368. doi: 10.1016/0304-4165(84)90258-7. [DOI] [PubMed] [Google Scholar]
- Potineni A, Lynn DM, Langer R, Amiji MM. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive biodegradable system for paclitaxel delivery. J Control Release. 2003;86:223–234. doi: 10.1016/s0168-3659(02)00374-7. [DOI] [PubMed] [Google Scholar]
- Rodrigues DG, Covolan CC, Coradi ST, Barboza R, Maranhao RC. Use of a cholesterol-rich emulsion that binds to low-density lipoprotein receptors as a vehicle for paclitaxel. J Pharm Pharmacol. 2002;54:765–772. doi: 10.1211/0022357021779104. [DOI] [PubMed] [Google Scholar]
- Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol) Semin Oncol. 1993;20(4 Suppl 3):1–15. [PubMed] [Google Scholar]
- Sakurai F, Inoue R, Nishino Y, Okuda A, Matsumoto O, Taga T, Yamashita F, Takakura Y, Hashida M. Effect of DNA/liposome mixing ratio on the physicochemical characteristics, cellular uptake and intracellular trafficking of plasmid DNA/cationic liposome complexes and subsequent gene expression. J Control Release. 2000;66:255–269. doi: 10.1016/s0168-3659(99)00280-1. [DOI] [PubMed] [Google Scholar]
- Sarosy G, Reed E. Taxol dose intensification and its clinical implications. J Natl Med Assoc. 1993;85:427–431. [PMC free article] [PubMed] [Google Scholar]
- Torchilin VP. Structure and design of polymeric surfactantbased drug delivery systems. J Control Release. 2001;73:137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- Torchilin VP, Trubetskoy VS. Which polymers can make nanoparticulate drug carriers long-circulating. Adv Drug Deliv Rev. 1995;16:141–155. [Google Scholar]
- Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proc Natl Acad Sci USA. 2003;100:6039–6044. doi: 10.1073/pnas.0931428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trubetskoy VS, Torchilin VP. Use of polyoxyethylene-lipid conjugates as long-circulating carriers for delivery of therapeutic and diagnostic agents. Adv Drug Deliv Rev. 1995;16:311–320. [Google Scholar]
- Trubetskoy VS, Torchilin VP. Polyethylene glycol based micelles as carriers of therapeutic and diagnostic agents. STP Pharma Sci. 1996;6:79–86. [Google Scholar]
- Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, Baker JR, Jr., Van Echo DA, Von Hoff DD, Leyland-Jones B. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- Weissig V, Lizano C, Torchilin VP. Micellar delivery system for dequalinium—A lipophilic cationic drug with anticarcinoma activity. J Liposome Res. 1998a;8:391–400. [Google Scholar]
- Weissig V, Whiteman KR, Torchilin VP. Accumulation of protein-loaded long-circulating micelles and liposomes in subcutaneous Lewis lung carcinoma in mice. Pharm Res. 1998b;15:1552–1556. doi: 10.1023/a:1011951016118. [DOI] [PubMed] [Google Scholar]
- Willey TA, Bekos EJ, Gaver RC, Duncan GF, Tay LK, Beijnen JH, Farmen RH. High-performance liquid chromatographic procedure for the quantitative determination of paclitaxel (Taxol) in human plasma. J Chromatogr. 1993;621:231–238. doi: 10.1016/0378-4347(93)80100-i. [DOI] [PubMed] [Google Scholar]
- Windebank AJ, Blexrud MD, de Groen PC. Potential neurotoxicity of the solvent vehicle for cyclosporine. J Pharmacol Exp Ther. 1994;268:1051–1056. [PubMed] [Google Scholar]
- Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- Zabner J, Fasbender AJ, Moninger T, Poellinger KA, Welsh MJ. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem. 1995;270:18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]


