Abstract
Poorly soluble photodynamic therapy (PDT) agent, meso-tetratphenylporphine (TPP), was effectively solubilized using non-targeted and tumor-targeted polymeric micelles prepared of polyethylene glycol/phosphatidyl ethanolamine conjugate (PEG-PE). Encapsulation of TPP into PEG-PE-based micelles and immunomicelles (bearing an anti-cancer monoclonal 2C5 antibody) resulted in significantly improved anticancer effects of the drug at PDT conditions against murine (LLC, B16) and human (MCF-7, BT20) cancer cells in vitro. For this purpose, the cells were incubated for 6 or 18 h with the TPP or TPP-loaded PEG-PE micelles/immunomicelles and then light-irradiated for 30 min. The phototoxic effect depended on the TPP concentration and specific targeting by immunomicelles. An increased level of apoptosis was shown in the PDT-treated cultures. The attachment of the anti-cancer 2C5 antibodies to TPP-loaded micelles provided the maximum level of cell killing at a given time. The results of this study showed that TPP-containing PEG-PE micelles may represent a useful formulation of the photosensitizer for practical PDT.
Keywords: Photodynamic therapy, meso-Tetraphenylporphine, Micelles, Cancer cells, Cytotoxicity, Apoptosis
1. Introduction
Photodynamic therapy (PDT), a promising and evolving treatment modality was shown to be highly effective in the curative and palliative treatment of malignant tumors and other diseases [1-4]. PDT relies on the accumulation of a photosensitizing drug in the neoplastic tissue and subsequent formation of cytotoxic products by irradiating the drug-containing tumor with a light of a suitable wavelength. The effect of PDT on tumor cells involves a complex combination of events, where the highly reactive singlet oxygen (1O2) originated by the photodynamic action plays an essential role in cell killing processes [5-8]. The basic objective in PDT research is the search for new and more efficient photosensitizers, which must show both minimal dark toxicity and secondary effects and high photodynamic yield upon irradiation with the red light.
Porphyrins are well known photosensitizing agents, which induce photodamage to malignant tumors by producing 1O2via the energy transfer from the first triplet state of porphyrin to the ground triplet state of molecular oxygen [9-12]. The most important limitation of their use for clinical application is their low water solubility, such as for example for meso-tetraphenylporphine (TPP) (Fig. 1). In addition, individual molecules of PDT agents do not specifically accumulate in tumors. To overcome these problems, TPP and similar drugs could be incorporated into certain long-circulating drug delivery systems, which can increase drug solubility and bioavailability; additionally, such drug carrier systems can target tumors via the passive accumulation (enhanced permeability and retention, EPR, effect) [13,14] or with the help of carrier-attached tumorspecific ligands (antibodies) [15].
Fig. 1.
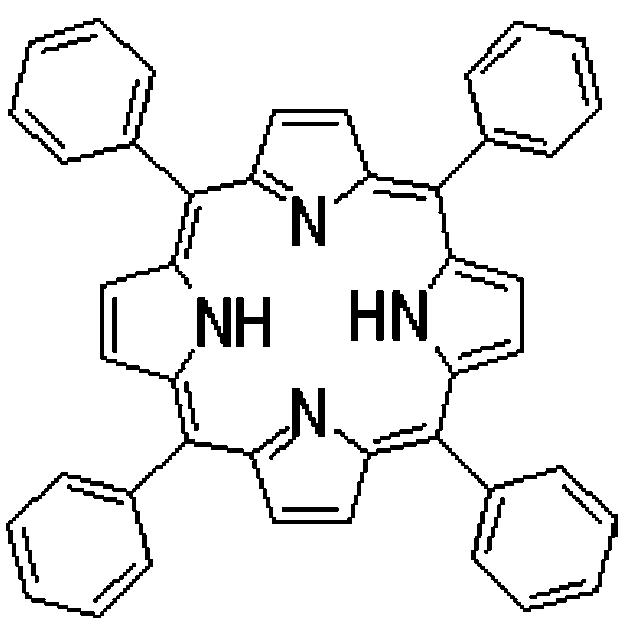
Chemical structure of meso-tetraphenylporphine.
Polymeric micelles are popular pharmaceutical carriers for poorly soluble drugs [16,17], which can be solubilized by the micelle hydrophobic core. Because of their characteristic small size (between 5 and 50 nm), good solubilization efficiency, and extremely high stability (low CMC values), the micelles, especially PEG-PE-based micelles [18], may represent an ideal EPR effect-mediated delivery system for poorly soluble PDT drugs and could also be made targeted by attaching tumorspecific ligands, such as anti-tumor antibodies, to their surface.
Here, we describe the preparation and characterization of TPP-containing PEG-PE micelles as well as tumor-targeted TPP-containing PEG-PE-based immunomicelles modified with an anti-cancer antibody, and demonstrate their high cytotoxicity against cancer cells in vitro under the conditions of PDT (upon light irradiation).
2. Materials and methods
Materials.
PEG-PE and PE-(lissamine-rhodamine B) (Rh-PE) were from Avanti Polar Lipids, Inc. (Alabaster, AL) and used without further purification. p-Nitrophenylcarbonyl (pNP)-PEG-PE was synthesized in our lab following the procedure described in [19,20]. Meso-tetraphenylporphine (TPP) was purchased from Frontier Scientific. Eagle’s Minimum Essential Medium (MEM), Dulbecco’s Modified Eagle medium (DMEM), trypsin and serum-free medium were from Mediatech, Inc. (Herndon, VA). Heat-inactivated fetal bovine serum (FBS) was from Atlanta Biologicals (Lawrenceville, Ga). Dimethyl sulfoxide (DMSO), 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI) was from Sigma Chemical Co. (Milwaukee, WI). Cancer-specific antinucleo-some monoclonal 2C5 antibody (mAb 2C5) was prepared by Harlan Bioproducts for Science (Indianapolis, IN) using the cell line provided by our laboratory. 3-(4,5-dimethylthia-zol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was from Promega (Madison, WI). Cancer cell lines were purchased from the American Type Culture Collection (Rockville, MD). All other reagents and buffer solution components were analytical grade preparations.
2.1. Methods
2.1.1. Preparation of micelles
Long-circulating TPP-containing micelles were prepared from PEG2000-PE. Ten mg/ml of the polymers were solubilized in chloroform and TPP (0.5 mg in chloroform) was added. After the removal of the organic solvent on a rotary evaporator, micelles were formed by resuspending the film obtained in the HEPES-buffered saline, pH 7.4. Crystals of the non-solubilized drug were removed by filtration. To make immunomicelles, lipid film was prepared with the addition of 5 mol% of pNP-PEG-PE. To form micelles, the film was rehydrated at 50°C in a 5 mM Na citrate-buffered saline, pH 5.0, and vortexed for 5 min.
To attach mAb 2C5 to the micelles for obtaining immunomicelles [15,19], 0.5 ml of a 13 μM solution of mAb 2C5 in Tris buffer, pH 8.0, was added to 0.5 ml of pNP-PEG-PE-containing micelles with a PEG-PE concentration of 3.6 mM. The mixture was incubated for 3 h at room temperature and dialyzed against 10 mM Hepes-buffered saline, pH 7.4 (HBS), using cellulose ester membranes with a cut-off size of 300 kDa (Spectrum Medical Industries). In some cases, 0.5% mol of the fluorescent probe, Rh-PE, was added to the micelle composition.
2.2. Characterization of TPP-loaded micelles
Loading efficiency.
The loading efficiency of TPP was expressed as the percentage of TPP associated with the micelles. Concentrations of constituents were determined by the F-2000 Fluorescence Spectrophotometer (Hitachi, Ltd. Tokyo, Japan). TPP were detected at 432 and 655 nm.
Micelle size measurement.
The micelle size (hydrodynamic diameter) was measured by the dynamic light scattering (DLS) using a N4 Plus Submicron Particle System (Coulter Corporation, Miami, FL). The micelle suspensions under investigation were diluted with a HEPES buffer until a concentration providing a light scattering intensity of 5×104to 1×106counts/s was achieved. The particle size distributions of all samples were measured in triplicate.
Micelle stability in the presence of blood serum components.
To test the stability of micelles in the presence of a blood protein, TPP-loaded micelles at the PEG-PE concentration of 3.5 mM were incubated with 0.2% bovine serum albumin (BSA) at room temperature and at 37°C for 72 h. The samples obtained were diluted in HEPES buffer (pH 7.4) by 1000-fold and analyzed for the presence of micelles and their size by the DLS.
Micelle storage stability.
To test the storage stability, TPP-loaded micelles were stored in the dark both at room temperature and at 4°C for 3 months. The stability of the micelles was monitored by the changes in particle size and drug content in the samples of TPP-loaded samples during the storage period. The samples were diluted in HEPES buffer (pH 7.4) by 1000-fold and analyzed for the presence of micelles and their size by the DLS.
Cell cultures.
The LLC (murine Lewis lung carcinoma), B16 (murine melanoma), MCF-7 (human breast adenocarcinoma), and BT20 (human breast adenocarcinoma) cell lines obtained from the ATCC (Rockville, MD), were maintained in DMEM and EMEM media supplemented with 10% of the FBS, 50 U/ml of penicillin, and 50 mg/ml of streptomycin at 37°Cin a humidified 5% CO2incubator.
Cytotoxicity assay.
Cells (2×104cells/well) were inoculated into a 96-well microplate (Coring, Inc., Coring, NY). After being grown overnight, cells were incubated at 37°C, in a humidified 5% CO2incubator with various concentrations of the free TPP dissolved in dimethyl sulfoxide (DMSO) or TPP in PEG-PE plain micelles or 2C5-PEG-PE-immunomicelles in the dark for 6 and 18 h. After the incubation, the cells were washed for removing unbound drug and then plates were photo-irradiated (except the dark controls) by the red light from the 630 nm light source at 12 mm spot (Lumacare® Products, CA) for 30 min. Then the cells were incubated at 37°C, 5% CO2-air atmosphere for another 24 h in a drug-free medium. The cell survival was then measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay. The absorbance of the degraded MTT at 492 nm was measured using an ELISA reader (Lab system Multiskan MCC/340, Finland).
Interaction of 2C5-immunomicelles with cancer cells in vitro.
LLC, B16, MCF-7, and BT20 cells were grown on cover slips placed in 6-well tissue culture plates. After the cells reached a confluence of 60-70%, the plates were washed with Hank’s buffer, treated with 1% BSA in a serum free media (2 ml per well) and incubated for 1 h at 37°C, 5% CO2in a humidified atmosphere. To these cells, Ph-PE-labeled 2C5-immunomicelles and plain micelles were added to a final concentration of PEG-PE of 0.10 mg/ml and incubated for 2 h at 37°C, 5% CO2.After the incubation, the cells were washed with Hank’s buffer, and the cover slips were mounted cell-side down on glass slides by using fluorescence-free glycerol-based mounting medium (Fluor mounting medium, Trevigen). Microscopic detection was performed with the Olympus BX61WI (Nashua, NH).
TPP-induced apoptosis.
The cells (60-70% confluence) were detached using 0.25% trypsin-2 mM EDTA in HBSS (Hankes' Balanced Salt Solution). Cells were seeded at (2×104cells/well) into 96-well tissue culture plates and allowed to reattach overnight. After 24 h incubation at 37°C, 5% CO2, the medium was replaced with various concentrations of TPP-loaded PEG-PE plain micelles (control) or TPP-loaded 2C5-PEG-PE-immunomicelles in the dark for an additional 6 and 18 h. Then cell were washed, photo-irradiated and re-incubated as described in the previous paragraph. After that, the cells were trypsinised and were pelleted by centrifugation at 700 g for 5 min. The cells were fixed with methanol:acetic acid (3:1), transferred onto a glass slide, and mounted for the microscopic study. The level of apoptosis was estimated following cell shrinkage, membrane blebing, formation of apoptotic bodies and nuclear fragmentation by fluorescence microscopy after PDT-treated cells (LLC cells in this particular experiment) were stained with 4′, 6′-diamidino-2-phenylindole hydrochloride (DAPI), a fluorescent dye specific for nucleus and allowing to see the nuclear de-fragmentation resulting from the apoptosis.
3. Results and discussion
3.1. Characterization of TPP-loaded PEG-PE micelles
The efficiency of the TPP incorporation into PEG-PE micelles was 26±2.5%, which corresponded to approximately 12 μg of TPP per 1 mg of micelle-forming PEG-PE. The average size of TPP-loaded PEG-PE micelles was around 30 nm (31±8.3). The stability of micelles were followed for 12 weeks both at 25 and at 4°C and no changes in micelle size or size distribution were found during this time period— micelles maintain the same size of about 30 nm and did not release the incorporated drug. Micelle incubation in the presence of BSA for 72 h at room temperature and at 37°C also did not provoke any changes in the micelle size (32±2.6 nm) or size distribution. Micelles maintain the same size and did not show any TPP leakage, which permits to hope that these micelles will be sufficiently stable in the blood within the time intervals allowing for their accumulation in the tumor target.
Antibody attachment to TPP-loaded PEG-PE micelles was performed by using a simple and reproducible protocol utilizing pNP-PEG-PE reagent earlier developed by us for the attachment of various amino group-containing ligands to long-circulating PEGylated liposomes [15,19,20].
3.2. In vitro phototoxicity assay
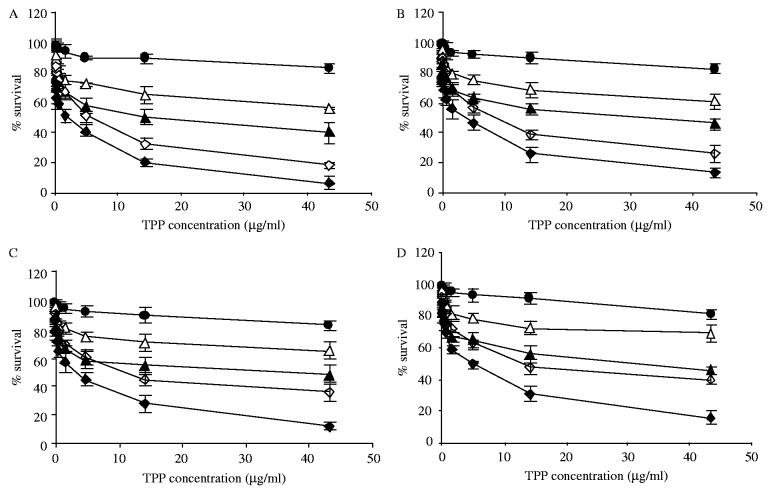
The viability of LLC, B16, MCF-7, and BT20 cells upon photoirradiation in the presence of the free TPP or TPP encapsulated in PEG-PE micelles and 2C5-immunomicelles were evaluated by the MTT assay as a function of preparation concentration and light irradiation exposure time. In this assay, TPP or TPP-loaded PEG-PE-based micelles were incubated with each of four listed cell lines for a definite period of time (6 or 18 h), and then cells were thoroughly washed with the PBS to remove a non-cell surface-associated photosensitizer preparation prior to photoirradiation. Then photoirradiation was performed, and cell viability was determined after the specified time period. A representative cytotoxicity graphs for 6 and 18 hrs incubation in varying concentrations of TPP or TPP encapsulated into PEG-PE-micelles or 2C5-PEG-PE-immunomicelles after the light treatment are shown in Fig. 2A-D for all four different cell lines (LLC, B16, MCF-7 and BT20). The results indicated that the used micellar preparations of TPP provided a significant photodynamic effect in vitro.
Fig. 2.
Phototoxicity of free TPP and TPP encapsulated in PEG-PE micelles and 2C5-PEG-PE-immunomicelles after light irradiation towards LLC (A), B16 (B), MCF-7 (C) and BT20 cells (D). •—free TPP, preincubation for 18 h; △—TPP in PEG-PE micelles, preincubation for 6 h; ▲—TPP in 2C5-PEG-PE-immunomicelles, preincubation for 6 h, ◇—TPP in PEG-PE micelles, preincubation for 18 h; ◆—TPP in 2C5-PEG-PE-immunomicelles, preincubation for 18 h.
Low ‘dark toxicity’ is one of the important criteria for assessing the usefulness of photosensitizers, since the major side effects in clinical PDT result from the dark toxicity of photosensitizers towards normal tissues. TPP-loaded PEG-PE micelles were basically non-toxic (low dark toxicity) showing the level of cell viability of more than 95% even at the maximal used TPP concentration and incubation times. Evidently, PEG corona of the micelles prevents cells from the contact with the micelle lipid core-solubilized TPP, being itself completely biocompatible and non-toxic [21,22]. TPP-loaded PEG-PE immunomicelles in the absence of light also allowed for more than 95% cell survival even at highest concentrations of TPP.
Light irradiation changes the situation completely. In all cases, although the free TPP produced some post-light irradiation cytotoxicity (killing of up to 20% cells upon cell preincubation with the drug for 18 h), a dramatic increase in the post-light-irradiation cytotoxicity was observed for TPP-containing PEG-PE-micelles and immunomicelles compared to TPP alone. At maximum TPP concentration (43.3 μg/ml) and maximum pre-irradiation incubation time (18 h), TPP in PEG-PE micelles demonstrated the killing of 80% and more cancer cells, while TPP in 2C5-PEG-PE-immunotargeted micelles demonstrated the killing of 95% of cancer cells. The increase in the efficacy of TPP in immunomicelles could, most be explained by the fact that immunomicelles are better internalized by cancer cells and thus keep the drug intracellularly allowing for more effective cell damage similarly to the way it was observed with drug-loaded anti-her2 immunoliposomes [23]. This might be especially important in case of in vivo application of such tumor-targeted micelles, which, after accumulating in the tumor via the EPR effect and disintegrating there, should not allow the drug to escape back into the circulation.
Naturally, the efficiency of cell killing was TPP concentration-dependent within the studied dose interval (TPP or TPP encapsulated in PEG-PE micelles were ranging from 0.02 to 43.3 μg/ml as free drug).
3.3. Binding of 2C5-immunomicelles to cancer cells
The hypothesis that the enhanced PDT toxicity of TPP in mAb 2C5-PEG-PE-immunomicelles was additionally confirmed by direct visualization of cancer cell targeting with drug-loaded 2C5-immunomicelles. Earlier, we have shown that certain non-pathogenic monoclonal antinuclear autoantibodies with nucleosome-restricted specificity, mAb 2C5 being among them, recognize the surface of numerous tumors, but not normal, cells via the tumor cell surface-bound nucleosomes released from the apoptotically dying tumor cells and attaching to the surface of surrounding live tumor cells [24-26]. These antibodies may serve as specific ligands for the delivery of drugs and drug carriers to various tumors [15].
Similar to earlier findings with immunomicelles [15], the results presented in Fig. 3 clearly show that Rh-PE labeled TPP-loaded 2C5-immunomicelles effectively and specifically bind to the surface of various unrelated tumor cell lines: murine LLC and B16 cells and human breast adenocarcinoma BT20 and MCF-7 cells. The incubation of the same cells with antibody-free TPP-loaded PEG-PE micelles resulted in only marginal micelle-to-cell association (virtually no cell-associated fluorescence could be seen). The differences in the fluorescence intensity observed between different cell lines in case of 2C5-immunolipo-somes could be explained by the varying density of nucleosome binding sites on these cells.
Fig. 3.
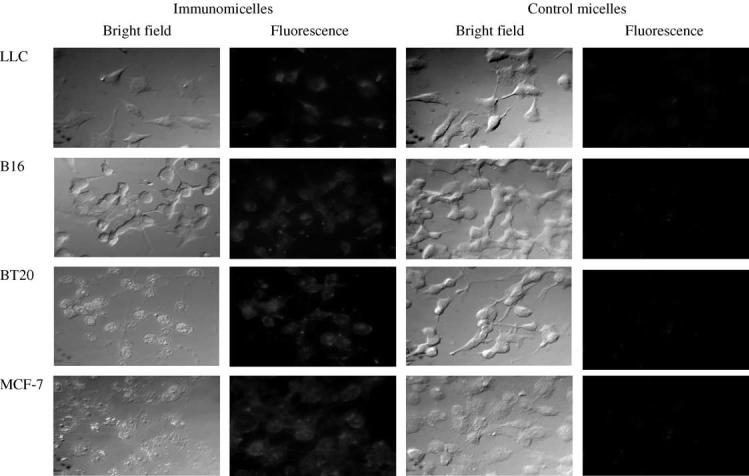
Fluorescence microscopy of the binding of Rh-PE-labeled TPP-loaded PEG-PE-based micelles and 2C5-immunomicelles to murine LLC (Lewis lung carcinoma) and B16 (melanoma) cells; and human BT-20 (mammary adenocarcinoma) and MCF-7 (breast adenocarcinoma) cells. Left images—phase contrast; right images—fluorescence.
3.4. TPP-induced apoptosis of LLC cells
In our study, PDT resulted in cell eradication within 24 h post-irradiation without evidence of inflammation, suggesting that the mechanism of cytotoxicity is apoptosis. Apoptotic cell death can be generally identified by a series of morphological, biochemical and molecular changes in the cells. Thus, as a result of the apoptosis, cellular DNA breaks into nuclear fragments [27]. In addition, PDT is known to inactivate growth factors and chemoattractants, also explaining the observed lack of inflammation [28-30]. To confirm, that the PDT with TPP-loaded PEG-PE micelles induces the apoptotic cell death, we have performed the fluorescence microscopy of PDT-treated LLC cells stained with DAPI, a fluorescent dye allowing visualizing the nuclear de-fragmentation resulting from the apoptosis.
Our observation (partially presented in Fig. 4) showed that PDT-subjected LLC cells demonstrated a significant level of apoptosis at both observation times with ∼40% of the cells being apoptotic after 6 h incubation with TPP-loaded micelles, and ∼80% cells being apoptotic after 18 h. Cells treated with TPP-loaded immunomicelles showed more DNA fragments at 6 h when compared to cells treated with TPP-loaded plain micelles. Apoptotic DNA fragments were not seen in the control group. Thus, as expected, TPP-loaded PEG-PE micelles and immunomicelles enhance PDT-provoked apoptotic death of cancer cells.
Fig. 4.
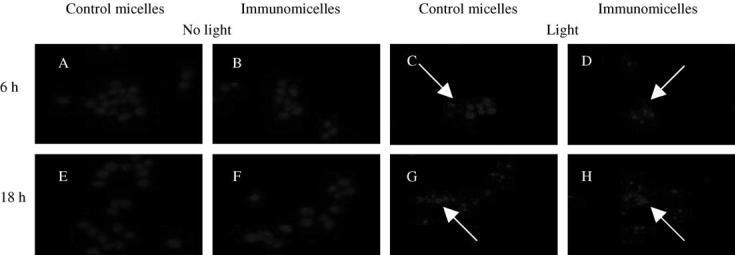
Nuclear fragmentation following the PDT of LLC cells with TPP-loaded PEG-PE micelles. (A) cells treated with control TPP-loaded plain micelles and no light at 6 h, (B) immunomicelles at 6 h (C) control micelles with light at 6 h (D) immunomicelles with light 6 h (E) control micelles 18 h no light (F) immunomicelles 18 h no light (G) control micelles 18 h with light (H) immunomicelles 18 h with light. An apoptotic cell is indicated by an arrow. Cells were DAPI stained for visualization of fragmented nuclei by fluorescence microscopy.
Conclusion
The solubility problem of TPP could be solved by incorporating the drug into PEG-PE micelles. TPP-containing PEG-PE-micelles demonstrate strong cytotoxicity under the PDT conditions (activation with red light). This cytotoxicity may be still further enhanced by attaching cancer-specific monoclonal 2C5 antibody to TPP-loaded PEG-PE micelles, i.e. by using drug-containing immunomicelles, due to specific binding of immunomicelles to cancer cells. TPP-containing PEG-PE micelles may represent a useful formulation of the photosensitizer for practical PDT. One can think that PEG-PE micelles can also be used for encapsulation of other photosensitizers, especially phthalocyanine derivatives and second-generation photosensitizers. In vivo PDT with micellesolubilized phototoxic agents is the subject of our current research.
Acknowledgements
This work was supported by the NIH research grant R01 EB001961 to Vladimir Torchilin.
References
- [1].Dougherty TJ, Gomer CJ, Henderson BW, Jori G, kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McCaughan JS., Jr Photodynamic therapy. Drugs Aging. 1999;15:49–68. doi: 10.2165/00002512-199915010-00005. (a Review) [DOI] [PubMed] [Google Scholar]
- [3].Morgan J, Oseroff AR. Mitochondria-based photodynamic anti-cancer therapy. Adv. Drug Delivery Rev. 2001;49:71–86. doi: 10.1016/s0169-409x(01)00126-0. [DOI] [PubMed] [Google Scholar]
- [4].Schmidt-Erfurth U, Hasan T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv. Ophthalmol. 2000;45:195–213. doi: 10.1016/s0039-6257(00)00158-2. [DOI] [PubMed] [Google Scholar]
- [5].Sibata MN, Tedesco AC, Marchetti JM. Photophysicals and photochemicals studies of zinc (II) phthalocyanine in long time circulation micelles for photodynamic therapy use. Eur. J. Pharm. Sci. 2004;23:131–138. doi: 10.1016/j.ejps.2004.06.004. [DOI] [PubMed] [Google Scholar]
- [6].Niedre M, Patterson MS, Wilson BC. Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochem. Photobiol. 2002;75:382–391. doi: 10.1562/0031-8655(2002)075<0382:DNILDO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- [7].Ito T. Cellular nd subcellular mechanism of photodynamic action: the 1O2 hypothesis as a driving force in recent research. Photochem. Photobiol. 1978;28:493–508. doi: 10.1111/j.1751-1097.1978.tb06957.x. [DOI] [PubMed] [Google Scholar]
- [8].Weishaupt K, Gomer CJ, Dougherty TJ. Identification of singlet oxygen as the cytotoxic agent I photo-inactivation of a murine tumor. Cancer Res. 1976;36:2326–2329. [PubMed] [Google Scholar]
- [9].Banfi S, Caruso E, Caprioli S, Mazzagatti L, Canti G, Ravizza R, Gariboldi M, Monti E. Photodynamic effects of porphyrin and chlorin photosensitizers in human colon adenocarcinoma cells. Bioorg. Med. Chem. 2004;15:4853–4860. doi: 10.1016/j.bmc.2004.07.011. [DOI] [PubMed] [Google Scholar]
- [10].Hadjur C, Wangnieres G, Ihringer F, Monnier P, van den Bergh H. Production of the free radicals O2.- and.OH by irradiation of the photosensitizer zinc (II) phthalocyanine. Photochem. Photobiol B: Biology. 1997;38:196–202. doi: 10.1016/s1011-1344(96)07440-4. [DOI] [PubMed] [Google Scholar]
- [11].Keene JP, Kessel D, Land EJ, Redmond RW, Truscott TG. Direct detection of singlet oxygen sensitized by haematoporphyrin and related compounds. Photochem. Photobiol. 1986;43:117–120. doi: 10.1111/j.1751-1097.1986.tb09501.x. [DOI] [PubMed] [Google Scholar]
- [12].Seret A, Gandin E, van de Vorst E,A. Singlet oxygen production by UV and visible photoexcitied porphyrins under different states of aggregation. Photobiochem. Photobiophys. 1986;12:259–266. [Google Scholar]
- [13].Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics. J. Control. Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. (a review) [DOI] [PubMed] [Google Scholar]
- [14].Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C, Martin FJ. Sterically stabilized liposomes: improvements in pharmaco-kinetics and antitumor therapeutic efficacy. Proc. Natl. Acad. Sci. USA. 1991;88:11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proc. Natl. Acad. Sci. USA. 2003;100:6039–6044. doi: 10.1073/pnas.0931428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Torchilin VP. Structure and design of polymeric surfactant based drug delivery systems. J. Control. Release. 2001;73:137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- [17].Jones M, Leroux J. Polymeric micelles-a new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 1999;48:101–111. doi: 10.1016/s0939-6411(99)00039-9. Review. [DOI] [PubMed] [Google Scholar]
- [18].Lukyanov AN, Torchilin VP. Micelles from lipid derivatives of watersoluble polymers as delivery systems for poorly soluble drugs. Adv. Drug Deliv. Rev. 2004;56:1273–1289. doi: 10.1016/j.addr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- [19].Torchilin VP, Levchenko TS, Lukyanov AN, Khaw BA, Klibanov AL, Rammohan R, Samokhin GP, Whiteman KR. p-Nitrophenylcarbonyl-PEG-PE-liposomes: fast and simple attachment of specific ligands, including monoclonal antibodies, to distal ends of PEG chains via p-nitrophenylcarbonyl group. Biochim. Biophys. Acta. 2001;1511:397–411. doi: 10.1016/s0005-2728(01)00165-7. [DOI] [PubMed] [Google Scholar]
- [20].Torchilin VP, Rammohan R, Weissig V, Levchenko TS. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc. Natl. Acad. Sci. USA. 2001;98:8786–91. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Working PK, Newman MS, Johnson J, Cornacoff JB. safety of poly(ethylene glycol) and poly(ethylene glycol) derivatives. ACS Symp. Ser. 1997;680:45–60. [Google Scholar]
- [22].Working PK, Newman MS, Johnson J, Cornacoff JB. Safety of poly(ethylene glycol) and poly(ethylene glycol) derivatives. ACS Symp. Ser. 1997;680:45–60. [Google Scholar]
- [23].Park JW, Kirpotin DB, Hong K, Shalaby R, Shao Y, Nielsen UB, Marks JD, Papahadjopoulos D, Benz CC. Tumor targeting using anti-her2 immunoliposomes. J. Control. Release. 2001;74:95–113. doi: 10.1016/s0168-3659(01)00315-7. [DOI] [PubMed] [Google Scholar]
- [24].Iakoubov LZ, Torchilin VP. A novel class of antitumor antibodies; nucleosome-restricted antinuclear auoantibodies (ANA) from healthy aged nonautoimmune mice. Oncol. Res. 1997;9:439–446. [PubMed] [Google Scholar]
- [25].Iakoubov L, Rokhlin O, Torchilin VP. Anti-nuclear autoantibodies of the aged reactive against the surface of tumor but not normal cells. Immunol. Lett. 1995;47:147–149. doi: 10.1016/0165-2478(95)00066-e. [DOI] [PubMed] [Google Scholar]
- [26].Iakoubov LZ, Torchilin VP. Nucleosome-releasing treatment makes surviving tumor cells better targets for nucleosome-specific anticancer antibodies. Cancer Detect. Prev. 1998;22:470–475. doi: 10.1046/j.1525-1500.1998.00055.x. [DOI] [PubMed] [Google Scholar]
- [27].Bochaton-Piallat ML, Gabbiani F, Redard M, Desmouliere A, Gabbiani G. Apoptosis participates in cellularity regulation during rat aortic intimal thickening. Am. J. Pathol. 1998;146:1059–1064. [PMC free article] [PubMed] [Google Scholar]
- [28].Statius van Eps RG, Mark LL, Schiereck J, LaMuraglia GM. Photodynamic therapy inhibits the injury-induced fibrotic response of vascular smooth muscle cells. Eur. J. Vasc. Endovasc. Surg. 1999;18:417–423. doi: 10.1053/ejvs.1999.0911. [DOI] [PubMed] [Google Scholar]
- [29].Statius van Eps RG, LaMuraglia GM. Photodynamic therapy inhibits transforming growth factor b activity associated with vascular smooth muscle cell injury. J. Vasc. Surg. 1997;25:1044–1053. doi: 10.1016/s0741-5214(97)70128-9. [DOI] [PubMed] [Google Scholar]
- [30].Statius van Eps RG, Adili F, LaMuraglia GM. Photodynamic therapy inactivates cell-associated basic fibroblast growth factor: a silent way of vascular smooth muscle cell eradication. Cardiovasc. Res. 1997;35:334–340. doi: 10.1016/s0008-6363(97)00120-x. [DOI] [PubMed] [Google Scholar]