Abstract
Spontaneous development of lupus-like disease in MRL-lpr mice is accompanied by a constellation of behavioral deficits, including blunted responsiveness to sucrose. Although autoimmunity-induced damage of limbic areas is proposed to underlie this deficit, the systemic nature of the disease precludes inference of a causal relationship between CNS damage and functional loss. Based on the stimulatory effects of d-amphetamine sulfate (AMPH) on sucrose intake, the present study pharmacologically probes the functional status of central dopaminergic circuits involved in control of behavioral reward. The response rates were compared between diseased MRL-lpr mice and congenic MRL +/+ controls tested in the sucrose preference paradigm. Neuronal loss was assessed by Fluoro Jade B (FJB) staining of nucleus accumbens and the CA2/CA3 region. While control mice significantly increased intake of sucrose solutions 60 min after administration of AMPH (i.p., 0.5 mg/kg), the intake in drugged MRL-lpr mice was comparable to those given saline injection. Increased FJB staining was detected in the nucleus accumbens and hippocampus of diseased mice, and AMPH treatment neither altered this nor other measures of organ pathology. The results obtained are consistent with previously observed changes in the mesolimbic dopamine system of MRL-lpr mice and suggest that the lesion in the nucleus accumbens and deficits in dopamine release underlie impaired responsiveness to palatable stimulation during the progress of systemic autoimmune disease. As such, they point to a neurotransmitter-specific regional brain damage which may account for depressive behaviors in neuropsychiatric lupus erythematosus.
Keywords: Lupus, Autoimmunity, Limbic system, Nucleus accumbens, Amphetamine, Motivated behavior, Depression, Sucrose intake, MRL model
1. Introduction
Systemic lupus erythematosus (SLE) is a chronic, multisystemic autoimmune disease that is characterized by the damage of many vital organs, including the brain [15]. In addition to serological manifestations (e.g. imbalanced cytokine network and increased production of autoreactive antibodies), significant number of SLE patients develop various neurologic and psychiatric (NP) symptoms, ranging from seizures and strokes to depression, anxiety, and psychosis [16]. The most frequent psychiatric symptoms include depression and anxiety [71], of which lower mood often heralds clinical manifestations of the disease [67].
Similar to humans, the MRL/MpJ-Faslpr/J (MRL-lpr) mice spontaneously develop an accelerated form of lupus-like disease accompanied by CNS involvement. In comparison to age-matched congenic MRL/MpJ (MRL +/+) controls, a substantial proportion of MRL-lpr mice develop deficits reflective of goal-directed and motivated behavior. They include blunted responsiveness to sucrose and saccharine solutions [6,43], impaired exploration of novel environments [50,52], impaired isolation-induced aggressiveness [44], and increased immobility (floating) in the forced swim test [53]. The constellation of behavioral deficiencies has been operationally termed “autoimmunity-associated behavioral syndrome” (AABS) and it coincides with a profound divergence in the immune statuses of the two MRL substrains around 7–8 weeks of age [52,54]. Increased neurodegeneration (as revealed by Fluoro Jade B, FJB staining), reduced dendritic complexity and density of pyramidal neurons are common observations in brains of diseased MRL-lpr mice [5,46,49]. Brain growth appears retarded [49] and ventricles increase in size along an early and accelerated development of autoimmune manifestations [17]. Taken together, there is considerable evidence that systemic inflammation and autoimmunity induce degeneration of central neurons, thus likely forming the structural basis of behavioral deficits in the MRL-lpr substrain.
Although the contribution of peripheral disease manifestations on behavioral performance could not be excluded, blunted responsiveness to palatable stimulation pointed to the mesolimbic dopamine system as one of the targets of the systemic autoimmune/inflammatory disease. It is well-documented that this system plays a significant role in goal-directed and reward-mediated behavior [74], and that the nucleus accumbens (NAc) is one of the primary reward centers, receiving dopaminergic inputs from ventral tegmental area, VTA [66]. Not surprisingly, the VTA of diseased MRL-lpr mice brains shows increased number of FJB-positive neurons and reduced staining for tyrosine-hydroxylase, TH [4]. Post-mortem analysis of brains from MRL-lpr mice by HPLC reveals significant imbalances in dopamine and 5-HT contents in the paraventricular nucleus [47]. These changes coincide with aberrant performance on the sucrose preference test, thus suggesting that lesions in the mesolimbic dopamine system contribute to impaired motivated behavior.
We presently use the appetitive response to sucrose and central psychostimulant to pharmacologically probe the functional status of the NAc dopamine system. In particular, sucrose licking was shown to be associated with release of DA from NAc and pharmacological blockade of DA turnover augmented the licking response [22]. Diseased MRL-lpr mice were challenged with the d-amphetamine sulfate (AMPH), known to be effective in activating central dopaminergic circuits within major compartments of the brain reward system [38], including the NAc [26]. The rationale for using d-amphetamine sulfate was that it has the capacity to significantly increase sugar intake in rodents [11,19]. In addition, in rats that are high responders, sugar consumption correlates significantly with AMPH-stimulated accumbens-dopamine overflow [60]. These studies also suggested that the responsiveness to sucrose reflects the NAc dopaminergic response to the AMPH treatment. Our overall expectation was that similar stimulation with AMPH will produce an attenuated effect on sucrose consumption in diseased MRL-lpr mice due to dysfunctional and/or damaged mesolimbic dopamine pathways. The results obtained contribute to the nature of the sucrose preference deficit by documenting a centrally-mediated mechanism and neurodegenerative process in the nucleus accumbens of autoimmune MRL-lpr mice. They also suggest that spontaneous progress of SLE-like disease is detrimental to the reward system function, as revealed by the lack of responsiveness to amphetamine challenge. As such, present observations are consistent with the hypothesis that lupus-like disease compromises dopaminergic neurotransmission in the CNS.
2. Methods
2.1. Animals
Four- to five-month-old MRL-lpr and age-matched MRL +/+ mice were obtained from the breeding colony at McMaster University, with the original stock purchased from The Jackson Laboratories (Bar Harbor, ME). Mice were housed singly with 8:00 a.m.–8:00 p.m. light schedule and ad lib access to food and water. The temperature of the colony room was kept at 25 ± 2 °C with a relative humidity of 45 ± 5%. In Experiment 1, 10 age-matched females of each substrain were used, while the groups of males in Experiment 2 were as follows: MRL-lpr AMPH (n = 10), MRL-lpr saline (n = 8), MRL +/+ AMPH (n = 10) and MRL +/+ saline (n = 10). The experimental protocols were approved by the McMaster Animal Care Committee and carried out in accordance with rules and regulations of the Canadian Council of Animal Care.
2.2. Drug administration
d-Amphetamine sulfate (AMPH, Sigma–Aldrich Canada, Oakville, Ont.) was dissolved in 0.9% saline and 0.5 mg/kg was injected i.p. using a 26.5 gauge needle (5 ml/kg of body weight; e.g., 0.2 ml/40 g mouse). This dose was chosen based on a previously reported effect [11] and our pilot study where 0.5 and 1.5 mg/kg doses were compared in a small cohort of mice exposed to the sucrose preference test [41].
2.3. Sucrose preference test
Reduced preference for sweet solution (chocolate) was first noted in one of the pioneering studies on behavior of MRL-lpr mice [21]. This phenomenon was further explored using the sucrose preference paradigm (proposed to measure sensitivity to reward), and as such a reduction in sucrose intake was taken to have face validity to anhedonia (loss of interest or pleasure) in human depression [73]. More extensive analysis of the dose-dependent performance, post-ingestive factors, and taste responsiveness has been performed by our group in previous studies [6,43,48]. Based on an established methodology, mice were presently trained to drink 3 ml of a 4% sucrose solution from a graduated syringe fastened to the cage lid with a 2.5 in. paper clip. They had 24 h access to sucrose over 3 days, and free access to food and water. The solution was then removed for 24 h to allow sucrose to clear from circulation. Two cohorts of mice were used to examine whether substrain-specific responsiveness to AMPH is general, or depends on gender, time between AMPH administration and exposure to sucrose, and/or order of sucrose concentrations. In Experiment 1, mice were first injected with 0.9% saline 60 min (~19:30 h) before the 1 h sucrose preference test (20:30–21:30 h) was given over four consecutive nights. Each night syringes were filled with one of four sucrose solutions, presented in ascending order (i.e. 1, 2, 4, or 8%). Upon the assessment of “baseline” performance, mice were given for 3 days tap water only. Following this break, animals were injected with AMPH 60 min prior to testing and the sucrose test was conducted in the same manner as described above. In Experiment 2, separate cohorts of mice (as described above) were used to control for a possible gender-specific difference [14], circulating levels of AMPH, and the “carry-over” effect noted when mice were exposed for prolonged periods of time to sucrose solutions. Namely, as in other healthy mice, the MRL +/+ mice tend to increase sucrose intake over a 10-day period [51]. In summary, this design differed from Experiment 1 such that males were injected with AMPH 12 h before the test, “baseline” performance was not measured, and sucrose solutions were given in random order to minimize “carry-over” and “learning” effects. In addition, a 0.5% dose of sucrose was included to better estimate responsiveness to low concentrations. During all testings, mice had uninterrupted access to bottles filled with fresh tap water.
2.4. Tissue preparation
Upon completion of the sucrose preference test (16–23 weeks of age), mice from Experiment 2 were sacrificed for the purpose of brain and spleen collection and weighing. They were anaesthetized with Somnotol (60 mg/kg) and transcar-dially perfused with 40 ml of 0.9% saline. Extracted brains were immersed into 4% paraformaldehyde (PFA) for fixation at 4 °C for 2 days, transferred to 30% sucrose (in PBS) for 3 days, and frozen by immersion in isopentane (cooled in liquid nitrogen) before cutting. Horizontal sections (~3.16 mm interaural and −6.84 mm Bregma) were cut with a Jung Frigocut 2800 E cryostat to concurrently obtain workable fields of the NAc and CA2/CA3 region. They were subsequently placed on APTEX-coated glass microscope slides, and left to dry at room temperature for 24 h before processing.
2.5. FJB staining procedure
The Fluoro Jade B (FJB) stain has an affinity for the entire degenerating neuron regardless of the type of cell death [29,56]. Despite incomplete knowledge of the staining mechanisms, the FJB method shows high reliability in the detection of dying neurons [75].
Brain sections were processed according to the previously published protocol [5]. The staining solution was a 0.001% FJB in 0.1% acetic acid (prepared from a 0.01% stock solution, Histo-Chem Inc., Jefferson, AR). Slides were processed in three 2 min xylene washes before being coverslipped with DPX (Sigma Chem. Co., St. Louis, MO). The FJB reactivity in the mesolimbic system was quantified using a Zeiss Laser Scanning Confocal Microscope (LSM 510, Carl Zeiss Inc.) argon laser (wavelength 488 nm). Confocal micrographs were obtained using a Fluar 20×/0.75 objective in combination with a 1024 × 1024 pixel resolution, and saved in the TIFF format. FJB-positive neurons were counted manually from TIFF files by an unbiased observer using standard imaging software (Adobe Photoshop 7).
2.6. Statistical analysis
In Experiment 1, the data were analyzed using an ANOVA with substrain as a main factor and treatment and concentration as repeated measures. In Experiment 2, in addition to substrain, treatment was considered as a main factor in an ANOVA with repeated measures (concentration). Significance level was set at p < 0.05 and all computations (including Pearson’s correlation) were performed using the SPSS 13 statistical package. One mouse in each AMPH-treated group died prematurely thus reducing the sample size for neuropathological analysis to N = 36. Graphs show means ± S.E.M.
3. Results
As expected [43], MRL-lpr females (injected with Sal) showed a lower intake of sucrose in comparison to the MRL +/+ group (substrain, F(1, 18) = 9.104, p < .01; Fig. 1A). However, this difference was exacerbated when Sal was replaced with AMPH (substrain by treatment, F(1, 18) = 8.561, p < .01; Fig. 1B). Since substrain by treatment by concentration interaction was not significant (F(3, 54) = 0.753), the assumption was that when 0.5 mg/kg of AMPH was injected 60 min before the preference test, MRL +/+ mice increased sucrose intake comparably at all concentrations. In contrast, the MRL-lpr group failed to show enhanced response to AMPH.
Fig. 1.
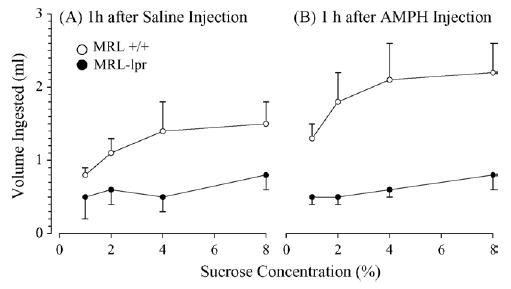
Consumption of sucrose in the brief sucrose preference test 60 min after i.p. injections. (A) Diseased MRL-lpr females injected with saline (Sal) showed a lower intake of sucrose in comparison to age-matched MRL +/+ controls. (B) This substrain difference was exacerbated when the same group of animals was injected with d-amphetamine (AMPH, 0.5 mg/kg b.w.), as MRL +/+ mice significantly increased their performance and MRL-lpr mice showed intake comparable to performance after Sal injections.
In Experiment 2, a similar phenomenon was observed without exposing mice to sucrose beforehand (the “baseline” assessment), despite the fact that AMPH was injected 12 h before the preference test, and that sucrose concentrations were randomized (substrain by treatment, p < .02; Fig. 2). The intake of sucrose solutions was generally higher in the second experiment, likely reflecting higher demands for liquids in bigger males [48]. Increased spleen weight (substrain: F(1, 33) = 18.822, p < .001; Fig. 3A) and lower brain weight in diseased MRL-lpr mice (substrain: F(1, 33) = 59.855, p < .001; Fig. 3B) confirmed the autoimmune status and brain atrophy in this substrain. As observed earlier [7], the hippocampal CA2/CA3 region showed an increased number of FJB+ neurons in MRL-lpr brains (sub-strain: F(1, 31) = 39.034, p < .001) and this was not altered by the AMPH treatment (Fig. 4A). A similar drug-independent neurodegenerative process was observed in the NAc (substrain: F(1, 31) = 5.674, p < .05; Fig. 4B) and correlated with the reduced brain mass in MRL-lpr mice (r15 = −.0488, p < .05). The staining method and horizontal sectioning used in the present study did not allow us to clearly delineate the shell and core regions of the NAc. Consequently, a whole count in the NAc region was taken and a representative image of brightly lit FJB+ neurons is shown in Fig. 5.
Fig. 2.
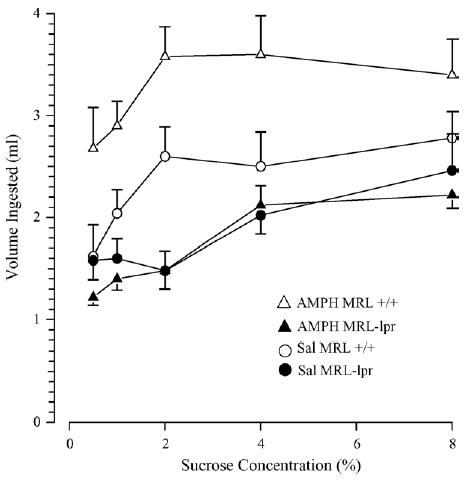
Sucrose intake in diseased MRL-lpr and age-matched MRL +/+ males. As expected, Sal-treated MRL +/+ controls had higher consumption than diseased, Sal-treated MRL-lpr mice. Although administration of AMPH further increased the intake in the MRL +/+ substrain, it had no effects in the AMPH-treated MRL-lpr group tested at different sucrose concentrations.
Fig. 3.
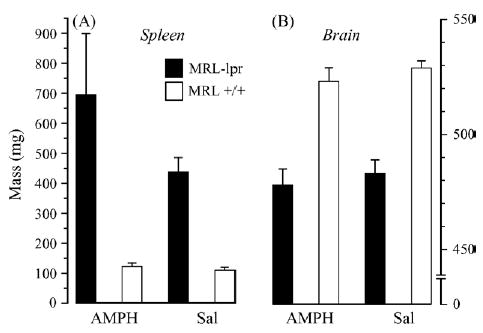
Substrain-related differences in spleen and brain weights. (A) Increased spleen weight and (B) lower brain weight confirmed autoimmune status and brain atrophy in diseased MRL-lpr mice.
Fig. 4.
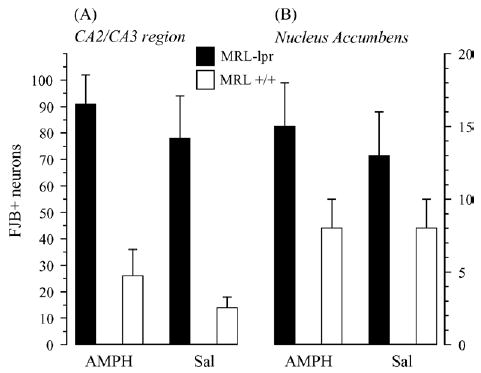
Significant increase in the density of FJB+ neurons was observed in the hippocampus CA2/CA3 region and nucleus accumbens of the MRL-lpr group, suggesting an enhanced neurodegenerative process in limbic structures during a more severe development of lupus-like disease.
Fig. 5.
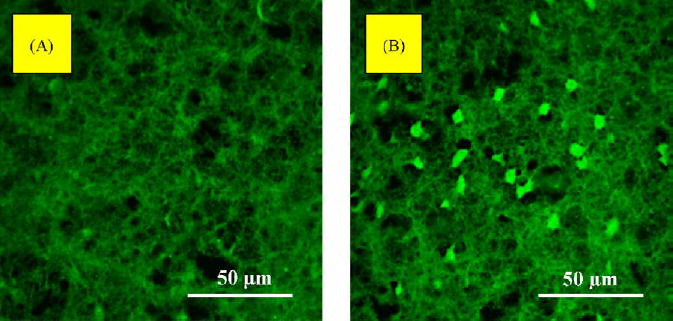
Representative images (obtained by a confocal microscope) of the horizontal sections of the nucleus accumbens (NAc) in autoimmune mice (A) and control mice (B). Numerous brightly stained FJB-positive neurons confirmed a degenerative process in MRL-lpr brains in comparison to asymptomatic MRL +/+ controls.
4. Discussion
Amphetamine increases release of catecholaminergic neurotransmitters [28] and blocks their reuptake by presynaptic axonal terminals [27]. Although the pharmacological effects of l-AMPH and d-AMPH involve both norepinephrine and dopamine release, behavioral effects of d-AMPH are largely mediated via the central dopamine system [42]. Amphetamine easily crosses the blood–brain barrier [42] and exerts stimulatory effects in the CNS, including limbic structures [37]. In the current experiment we used d-AMPH sulfate to probe the central dopaminergic system and further test the hypothesis that dysfunctional mesolimbic dopamine pathways mediate impaired motivational behavior of lupus-prone animals. Indeed, the results obtained reveal that diseased MRL-lpr mice fail to increase sucrose intake in response to systemic injection of d-AMPH sulfate repeatedly administered in two different experimental designs. This was consistent with evidence that D1 receptor-deficient mice [18] and mice that cannot synthesize DA demonstrate deficits in goal-directed response to sucrose [12]. Increased FJB staining in the NAc of MRL-lpr animals suggests neuronal degeneration in this region, known to have a significant role in control of the neural reward circuitry [74]. Viewed from a more general perspective, obtained results imply that at least some deficits in behavioral performance of diseased MRL-lpr mice are not an epiphenomenon due to peripheral symptomatology.
The midbrain has a significant role in goal-directed and reward-mediated behavior [57], and dopaminergic inputs from the ventral tegmental area (VTA) are important in activity of the NAc [74]. Consumption of food increases the release of dopamine in the NAc of rodents [8] and this release is mediated by the VTA [62]. Similarly, consumption of sucrose has been shown to increase the release of DA in the NAc [22–24]. In the present study neurodegenerative changes in the NAc may provide a structural basis of impaired response to sucrose in autoimmune MRL-lpr animals. Signs of neurodegeneration of dopaminergic neurons in the VTA has been found in diseased animals in the past [4], potentially impairing activity of the NAc and behavioral performance in MRL-lpr animals. Neural degeneration in the CA2/CA3 region was not affected by repeated administration of d-AMP and the severity of damage is consistent with previous findings [7]. The NAc receives glutamatergic inputs from a variety of sources, including the limbic neocortex, the ventral subiculum and hippocampal formation, the amygdala and the dorsal medial thalamus [40]. Hippocampal input appears to play a critical role in modulating resting membrane potential of medium spiny neurons, the output neurons of the NAc [39]. Our previous studies revealed that neuronal complexity and spine density of hippocampal neurons is profoundly reduced by the onset of systemic autoimmunity and inflammation in MRL-lpr mice [46,49]. Taken together, degeneration in the NAc, VTA, and hippocampus may jointly contribute to the behavioral impairment of MRL-lpr animals in the sucrose preference paradigm. Conversely, increased sucrose intake in AMPH-treated MRL +/+ controls might be associated with increased spine density in the hippocampus, as shown in the CA1 region after AMPH self-administration and sucrose-reward experience [13].
The progress of lupus-like disease in the MRL-lpr substrain is accompanied by elevated serum levels of pro-inflammatory cytokines, such as IL-1, IL-6 and TNF-alpha [63,65]. Given their well-documented capacity to alter motivated behavior and emotional reactivity [2], one can assume that circulating cytokines contribute to impaired performance in the sucrose preference test. Indeed, we show this relationship in our previous studies [6,45,48,51], which did not involve assessments of neurodegeneration. However, the precise mechanisms by which cytokines can impair motivated behavior in the MRL model is far from being understood because of their multiple effects on system organs and the complexity of lupus-like disease. Namely, it is known that exogenous administration or increased endogenous synthesis of interleukin (IL)-1, IL-6, TNF-alpha and interferons can activate major endocrine pathways, alter metabolism of precursor molecules and affects metabolism of central neurotransmitters [3], including the dopamine system [36,58,76]. In addition to hypothalamus and hippocampus, dopamine metabolism in the nucleus accumbens can be significantly affected by systemic administration of IL-6 [61] or lipopolysaccharide, a non-specific activator of pro-inflammatory cytokine release [10]. In additional to “functional damage”, several lines of evidence suggest that cytokines promote neurodegenerative process when the brain is injured [1,20,25,34,68]. Given that the blood–brain barrier is breached at an early age [59,69] and that the HPA axis is dysfunctional in diseased MRL-lpr mice [30,32,33], it is presently difficult to dissociate central from peripheral effects, or functional impairment from structural damage produced by neuroactive cytokines.
Secretion of the pituitary hormone prolactin is under the control of dopamine. Given that elevated secretion of prolactin is common in SLE [31], one may wonder whether this endocrine imbalance is a consequence of impaired dopamine regulation in the CNS. Although neuronal damage has been recently reported [64], there is no evidence on whether dopaminergic neurons die excessively in neuropsychiatric SLE (NP-SLE). Consistent with this notion, the dopamine agonist bromocriptine suppresses secretion of prolactin and ameliorates affect in SLE patients [70] and disease activity in autoimmune mice [35]. If the loss of dopaminergic neurons occurs in human and animal forms of SLE, an immediate question would be whether endocrine, immune, or other factors induce neurodegeneration. Our previous studies suggest that dopamine accumulates in the paraventricular nucleus and median eminence/arcuate nucleus areas [47]. Even if increased intracellular levels of dopamine are neurotoxic [9], the dilemma about factors that lead to dopamine accumulation would still remain. One possibility is that the enzymatic system is affected by the autoimmune process (such as autoantibodies binding to and inactivating different kinases, transferases, proteases, etc.). Another mechanism may include sustained binding of corticosteroids [33,72] and increased vulnerability of neurons to various metabolic insults [55]. However, at this stage of knowledge conclusive statements about an etiologically and clinically complex condition such as NP-SLE would be premature.
In summary, the present study reveals profound differences in response to palatable stimulation between MRL-lpr and MRL +/+ mice when their dopamine system is pharmacologically probed. More importantly, significant differences in the number of dying neurons are observed in the NAc, a neural area known to be involved in reward modulation. These results are consistent with the hypothesis that the progression of autoimmune disease impairs motivated behavior by producing a lesion in the dopaminergic reward system. However, there are other neuronal systems that innervate and originate from the NAc and are able to modulate responsiveness to palatable stimulation [11]. Whether they are also affected by the progress of spontaneous systemic autoimmunity and inflammation needs to be examined in future studies.
Acknowledgments
This work was supported by funds to B. Sakic from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (1R21 AR49163-01), Canadian Institutes of Health Research (CIHR), and to H. Szechtman from Natural Sciences and Engineering Research Council of Canada (RGPIN 544-01). D. Ballok is a Ph.D. student fellow of the CIHR and B. Sakic is a recipient of the Father Sean O’Sullivan Research Centre career development award.
References
- 1.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–44. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 2.Anisman H, Merali Z. Cytokines, stress and depressive illness: brain–immune interactions. Ann Med. 2003;35:2–11. doi: 10.1080/07853890310004075. [DOI] [PubMed] [Google Scholar]
- 3.Anisman H, Merali Z, Poulter MO, Hayley S. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11:963–72. doi: 10.2174/1381612053381701. [DOI] [PubMed] [Google Scholar]
- 4.Ballok DA, Earls AM, Krasnik C, Hoffman SA, Sakic B. Autoimmune-induced damage of the midbrain dopaminergic system in lupus-prone mice. J Neuroimmunol. 2004;152:83–97. doi: 10.1016/j.jneuroim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Ballok DA, Millward JM, Sakic B. Neurodegeneration in autoimmune MRL-lpr mice as revealed by Fluoro Jade B staining. Brain Res. 2003;964:200–10. doi: 10.1016/s0006-8993(02)03980-x. [DOI] [PubMed] [Google Scholar]
- 6.Ballok DA, Szechtman H, Sakic B. Taste responsiveness and diet preference in autoimmune MRL mice. Behav Brain Res. 2003;140:119–30. doi: 10.1016/s0166-4328(02)00276-0. [DOI] [PubMed] [Google Scholar]
- 7.Ballok DA, Woulfe J, Sur M, Cyr M, Sakic B. Hippocampal damage in mouse and human forms of systemic autoimmune disease. Hippocampus. 2004;14:649–61. doi: 10.1002/hipo.10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassareo V, DiChiara G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J Neurosci. 1997;17:851–61. doi: 10.1523/JNEUROSCI.17-02-00851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol. 2001;65:135–72. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 10.Borowski T, Kokkinidis L, Merali Z, Anisman H. Lipopolysaccharide, central in vivo biogenic amine variations, and anhedonia. Neuroreport. 1998;9:3797–802. doi: 10.1097/00001756-199812010-00006. [DOI] [PubMed] [Google Scholar]
- 11.Brennan K, Roberts DC, Anisman H, Merali Z. Individual differences in sucrose consumption in the rat: motivational and neurochemical correlates of hedonia. Psychopharmacology (Berlin) 2001;157:269–76. doi: 10.1007/s002130100805. [DOI] [PubMed] [Google Scholar]
- 12.Cannon CM, Palmiter RD. Reward without dopamine. J Neurosci. 2003;23:10827–31. doi: 10.1523/JNEUROSCI.23-34-10827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex. 2005;15:341–8. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- 14.Curtis KS, Davis LM, Johnson AL, Therrien KL, Contreras RJ. Sex differences in behavioral taste responses to and ingestion of sucrose and NaCl solutions by rats. Physiol Behav. 2004;80:657–64. doi: 10.1016/j.physbeh.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Dall’Era M, Davis JC. Systemic lupus erythematosus. How to manage, when to refer. Postgrad Med. 2003;114:31–37. 40. doi: 10.3810/pgm.2003.11.1528. [DOI] [PubMed] [Google Scholar]
- 16.Denburg SD, Denburg JA. Cognitive dysfunction and antiphospholipid antibodies in systemic lupus erythematosus. Lupus. 2003;12:883–90. doi: 10.1191/0961203303lu497oa. [DOI] [PubMed] [Google Scholar]
- 17.Denenberg VH, Sherman GF, Rosen GD, Morrison L, Behan PO, Galaburda AM. A behavior profile of the MRL/Mp lpr/lpr mouse and its association with hydrocephalus. Brain Behav Immun. 1992;6:40–9. doi: 10.1016/0889-1591(92)90058-v. [DOI] [PubMed] [Google Scholar]
- 18.El-Ghundi M, O’Dowd BF, Erclik M, George SR. Attenuation of sucrose reinforcement in dopamine D1 receptor deficient mice. Eur J Neurosci. 2003;17:851–62. doi: 10.1046/j.1460-9568.2003.02496.x. [DOI] [PubMed] [Google Scholar]
- 19.Evans KR, Vaccarino FJ. Effects of d- and l-amphetamine on food intake: evidence for a dopaminergic substrate. Pharmacol Biochem Behav. 1987;27:649–52. doi: 10.1016/0091-3057(87)90189-4. [DOI] [PubMed] [Google Scholar]
- 20.Gibson RM, Rothwell NJ, Le Feuvre RA. CNS injury: the role of the cytokine IL-1. Vet J. 2004;168:230–7. doi: 10.1016/j.tvjl.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Grota LJ, Ader R, Cohen N. Taste aversion learning in autoimmune Mrl-lpr/lpr and Mrl +/+ mice. Brain Behav Immun. 1987;1:238–50. doi: 10.1016/0889-1591(87)90026-2. [DOI] [PubMed] [Google Scholar]
- 22.Hajnal A, Norgren R. Accumbens dopamine mechanisms in sucrose intake. Brain Res. 2001;904:76–84. doi: 10.1016/s0006-8993(01)02451-9. [DOI] [PubMed] [Google Scholar]
- 23.Hajnal A, Norgren R. Repeated access to sucrose augments dopamine turnover in the nucleus accumbens. Neuroreport. 2002;13:2213–6. doi: 10.1097/00001756-200212030-00010. [DOI] [PubMed] [Google Scholar]
- 24.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–7. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 25.Hayley S, Anisman H. Multiple mechanisms of cytokine action in neurodegenerative and psychiatric states: neurochemical and molecular substrates. Curr Pharm Des. 2005;11:947–62. doi: 10.2174/1381612053381611. [DOI] [PubMed] [Google Scholar]
- 26.Hedou G, Homberg J, Feldon J, Heidbreder CA. Amphetamine microinfusion in the dorso-ventral axis of the prefrontal cortex differentially modulates dopamine neurotransmission in the shell-core subterritories of the nucleus accumbens. Ann NY Acad Sci. 1999;877:823–7. doi: 10.1111/j.1749-6632.1999.tb09331.x. [DOI] [PubMed] [Google Scholar]
- 27.Heikkila RE, Orlansky H, Cohen G. Studies on the distinction between uptake inhibition and release of (3H)dopamine in rat brain tissue slices. Biochem Pharmacol. 1975;24:847–52. doi: 10.1016/0006-2952(75)90152-5. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman BB. Catecholamines, sympathomimetic drugs and adrenergic receptor antagonists. In: Goodman LS, Gilman AG, Hardman JG, et al., editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill Professional; 2001. pp. 215–68. [Google Scholar]
- 29.Hopkins KJ, Wang G, Schmued LC. Temporal progression of kainic acid induced neuronal and myelin degeneration in the rat forebrain. Brain Res. 2000;864:69–80. doi: 10.1016/s0006-8993(00)02137-5. [DOI] [PubMed] [Google Scholar]
- 30.Hu Y, Dietrich H, Herold M, Heinrich PC, Wick G. Disturbed immunoendocrine communication via the hypothalamo-pituitary-adrenal axis in autoimmune disease. Int Arch Allergy Immunol. 1993;102:232–41. doi: 10.1159/000236531. [DOI] [PubMed] [Google Scholar]
- 31.Jara LJ, Vera-Lastra O, Miranda JM, Alcala M, Varez-Nemegyei J. Prolactin in human systemic lupus erythematosus. Lupus. 2001;10:748–56. doi: 10.1191/096120301717164994. [DOI] [PubMed] [Google Scholar]
- 32.Lechner O, Dietrich H, Oliveira dos SA, Wiegers GJ, Schwarz S, Harbutz M, et al. Altered circadian rhythms of the stress hormone and melatonin response in lupus-prone MRL/MP-fas(Ipr) mice. J Autoimmun. 2000;14:325–33. doi: 10.1006/jaut.2000.0375. [DOI] [PubMed] [Google Scholar]
- 33.Lechner O, Hu Y, Jafarian-Tehrani M, Dietrich H, Schwarz S, Herold M, et al. Disturbed immunoendocrine communication via the hypothalamo-pituitary-adrenal axis in murine lupus. Brain Behav Immun. 1996;10:337–50. doi: 10.1006/brbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 34.Licinio J. Central nervous system cytokines and their relevance for neurotoxicity and apoptosis. J Neural Transm Suppl. 1997;49:169–75. doi: 10.1007/978-3-7091-6844-8_18. [DOI] [PubMed] [Google Scholar]
- 35.McMurray R, Keisler D, Kanuckel K, Izui S, Walker SE. Prolactin influences autoimmune disease activity in the female B/W mouse. J Immunol. 1991;147:3780–7. [PubMed] [Google Scholar]
- 36.MohanKumar PS, Thyagarajan S, Quadri SK. Interleukin-1 stimulates the release of dopamine and dihydroxyphenylacetic acid from the hypothalamus in vivo. Life Sci. 1991;48:925–30. doi: 10.1016/0024-3205(91)90040-i. [DOI] [PubMed] [Google Scholar]
- 37.Mycek MJ, Harvey RA, Champe PC. Pharmacology. 2nd ed. Philadelphia: Lippincott-Raven Publishers; 1997. CNS stimulants; pp. 99–106. [Google Scholar]
- 38.Naranjo CA, Tremblay LK, Busto UE. The role of the brain reward system in depression. Prog Neuropsychopharmacol Biol Psychiat. 2001;25:781–823. doi: 10.1016/s0278-5846(01)00156-7. [DOI] [PubMed] [Google Scholar]
- 39.O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–39. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–61. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 41.Prasad N. McMaster University; 1999. Anhedonia in autoimmune MRL mice (MSc Thesis) [Google Scholar]
- 42.Rang HP, Dale MM, Ritter JM, et al. Pharmacology. 5th ed. Edinburgh: Churchill Livingstone; 2003. Noradrenergic transmission. [Google Scholar]
- 43.Sakic B, Denburg JA, Denburg SD, Szechtman H. Blunted sensitivity to sucrose in autoimmune MRL-lpr mice: a curve-shift study. Brain Res Bull. 1996;41:305–11. doi: 10.1016/s0361-9230(96)00190-6. [DOI] [PubMed] [Google Scholar]
- 44.Sakic B, Gurunlian L, Denburg SD. Reduced aggressiveness and low testosterone levels in autoimmune MRL-lpr males. Physiol Behav. 1998;63:305–9. doi: 10.1016/s0031-9384(97)00422-8. [DOI] [PubMed] [Google Scholar]
- 45.Sakic B, Hanna SE, Millward JM. Behavioral heterogeneity in an animal model of neuropsychiatric lupus. Biol Psychiat. 2005;57:679–87. doi: 10.1016/j.biopsych.2004.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakic B, Kolb B, Whishaw IQ, Gorny G, Szechtman H, Denburg JA. Immunosuppression prevents neuronal atrophy in lupus-prone mice: evidence for brain damage induced by autoimmune disease? J Neuroimmunol. 2000;111:93–101. doi: 10.1016/s0165-5728(00)00364-7. [DOI] [PubMed] [Google Scholar]
- 47.Sakic B, Lacosta S, Denburg J, Szechtman H. Altered neurotransmission in brains of autoimmune mice: pharmacological and neurochemical evidence. J Neuroimmunol. 2002;129:84–96. doi: 10.1016/s0165-5728(02)00171-6. [DOI] [PubMed] [Google Scholar]
- 48.Sakic B, Szechtman H, Braciak TA, Richards CD, Gauldie J, Denburg JA. Reduced preference for sucrose in autoimmune mice: a possible role of interleukin-6. Brain Res Bull. 1997;44:155–65. doi: 10.1016/s0361-9230(97)00107-x. [DOI] [PubMed] [Google Scholar]
- 49.Sakic B, Szechtman H, Denburg JA, Gorny G, Kolb B, Whishaw IQ. Progressive atrophy of pyramidal neuron dendrites in autoimmune MRL-lpr mice. J Neuroimmunol. 1998;87:162–70. doi: 10.1016/s0165-5728(98)00085-x. [DOI] [PubMed] [Google Scholar]
- 50.Sakic B, Szechtman H, Denburg SD, Denburg JA. Immunosuppressive treatment prevents behavioral deficit in autoimmune MRL-lpr mice. Physiol Behav. 1995;58:797–802. doi: 10.1016/0031-9384(95)00135-6. [DOI] [PubMed] [Google Scholar]
- 51.Sakic B, Szechtman H, Gauldie J, Denburg JA. Behavioral effects of infection with IL-6 adenovector. Brain Behav Immun. 2001;15:25–42. doi: 10.1006/brbi.1999.0576. [DOI] [PubMed] [Google Scholar]
- 52.Sakic B, Szechtman H, Keffer M, Talangbayan H, Stead R, Denburg JA. A behavioral profile of autoimmune lupus-prone MRL mice. Brain Behav Immun. 1992;6:265–85. doi: 10.1016/0889-1591(92)90048-s. [DOI] [PubMed] [Google Scholar]
- 53.Sakic B, Szechtman H, Talangbayan H, Denburg SD, Carbotte RM, Denburg JA. Disturbed emotionality in autoimmune MRL-lpr mice. Physiol Behav. 1994;56:609–17. doi: 10.1016/0031-9384(94)90309-3. [DOI] [PubMed] [Google Scholar]
- 54.Sakic B, Szechtman H, Talangbayan H, Denburg SD, Carbotte RM, Denburg JA. Behaviour and immune status of MRL mice in the postweaning period. Brain Behav Immun. 1994;8:1–13. doi: 10.1006/brbi.1994.1001. [DOI] [PubMed] [Google Scholar]
- 55.Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiat. 2000;48:755–65. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- 56.Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–30. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 57.Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–7. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 58.Shintani F, Kanba S, Nakaki T, Nibuya M, Kinoshita N, Suzuki E, et al. Interleukin-1 beta augments release of norepinephrine, dopamine, and serotonin in the rat anterior hypothalamus. J Neurosci. 1993;13:3574–81. doi: 10.1523/JNEUROSCI.13-08-03574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sidor M, Sakic B, Malinowski P, Ballok DA, Oleschuk CJ, Macri J. Elevated immunoglobulin levels in the cerebrospinal fluid from lupus-prone mice. J Neuroimmunol. 2005;165:104–13. doi: 10.1016/j.jneuroim.2005.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sills TL, Crawley JN. Individual differences in sugar consumption predict amphetamine-induced dopamine overflow in nucleus accumbens. Eur J Pharmacol. 1996;303:177–81. doi: 10.1016/0014-2999(96)00161-6. [DOI] [PubMed] [Google Scholar]
- 61.Song C, Merali Z, Anisman H. Variations of nucleus accumbens dopamine and serotonin following systemic interleukin-1, interleukin-2 or interleukin-6 treatment. Neuroscience. 1999;88:823–36. doi: 10.1016/s0306-4522(98)00271-1. [DOI] [PubMed] [Google Scholar]
- 62.Taber MT, Fibiger HC. Feeding-evoked dopamine release in the nucleus, accumbens: regulation by glutamatergic mechanisms. Neuroscience. 1997;76:1105–12. doi: 10.1016/s0306-4522(96)00450-2. [DOI] [PubMed] [Google Scholar]
- 63.Tang B, Matsuda T, Akira S, Nagata N, Ikehara S, Hirano T, et al. Age-associated increase in interleukin 6 in MRL/lpr mice. Int Immunol. 1991;3:273–8. doi: 10.1093/intimm/3.3.273. [DOI] [PubMed] [Google Scholar]
- 64.Trysberg E, Nylen K, Rosengren LE, Tarkowski A. Neuronal and astrocytic damage in systemic lupus erythematosus patients with central nervous system involvement. Arthritis Rheum. 2003;48:2881–7. doi: 10.1002/art.11279. [DOI] [PubMed] [Google Scholar]
- 65.Tsai CY, Wu TH, Huang SF, Sun KH, Hsieh SC, Han SH, et al. Abnormal splenic and thymic IL-4 and TNF-alpha expression in MRL-lpr/lpr mice. Scand J Immunol. 1995;41:157–63. doi: 10.1111/j.1365-3083.1995.tb03548.x. [DOI] [PubMed] [Google Scholar]
- 66.Tzschentke TM, Schmidt WJ. Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward. Crit Rev Neurobiol. 2000;14:131–42. [PubMed] [Google Scholar]
- 67.van Dam AP, Wekking EM, Callewaert JAC, Schipperijn AJM, Oomen HAPC, Dejong J, et al. Psychiatric symptoms before systemic lupus erythematosus is diagnosed. Rheumatol Int. 1994;14:57–62. doi: 10.1007/BF00300248. [DOI] [PubMed] [Google Scholar]
- 68.Viviani B, Bartesaghi S, Corsini E, Galli CL, Marinovich M. Cytokines role in neurodegenerative events. Toxicol Lett. 2004;149:85–9. doi: 10.1016/j.toxlet.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 69.Vogelweid CM, Johnson GC, Besch-Williford CL, Basler J, Walker SE. Inflammatory central nervous system disease in lupus-prone MRL/lpr mice: comparative histologic and immunohistochemical findings. J Neuroimmunol. 1991;35:89–99. doi: 10.1016/0165-5728(91)90164-3. [DOI] [PubMed] [Google Scholar]
- 70.Walker SE, Smarr KL, Parker JC, Weidensaul DN, Nelson W, McMurray RW. Mood states and disease activity in patients with systemic lupus erythematosus treated with bromocriptine. Lupus. 2000;9:527–33. doi: 10.1177/096120330000900709. [DOI] [PubMed] [Google Scholar]
- 71.Wekking EM. Psychiatric symptoms in systemic lupus erythematosus—an update. Psychosom Med. 1993;55:219–28. doi: 10.1097/00006842-199303000-00011. [DOI] [PubMed] [Google Scholar]
- 72.Wick G, Hu Y, Schwarz S, Kroemer G. Immunoendocrine communication via the hypothalamo-pituitary-adrenal axis in autoimmune diseases. Endocrinol Rev. 1993;14:539–63. doi: 10.1210/edrv-14-5-539. [DOI] [PubMed] [Google Scholar]
- 73.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–34. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 74.Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–40. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 75.Ye X, Carp RI, Schmued LC, Scallet AC. Fluoro-Jade and silver methods: application to the neuropathology of scrapie, a transmissible spongi-form encephalopathy. Brain Res Protoc. 2001;8:104–12. doi: 10.1016/s1385-299x(01)00086-1. [DOI] [PubMed] [Google Scholar]
- 76.Zalcman S, Greenjohnson JM, Murray L, Nance DM, Dyck D, Anisman H, et al. Cytokine-specific central monoamine alterations induced by interleukin-1, -2 and -6. Brain Res. 1994;643:40–9. doi: 10.1016/0006-8993(94)90006-x. [DOI] [PubMed] [Google Scholar]


