Abstract
Many cuckoo species lay eggs that match those of their hosts, which can significantly reduce rejection of their eggs by the host species. However, egg mimicry is problematic for generalist cuckoos that parasitize several host species with different egg types. Some generalist cuckoos have overcome this problem by evolving several host-specific races (gentes), each with its own, host-specific egg type. It is unknown how generalist cuckoos lacking gentes are able to avoid egg rejection by hosts. Here we use reflectance spectrophotometry (300–700 nm) on museum egg collections to test for host-specific egg types in an Australian generalist cuckoo reported to have a single egg type. We show that the colour of pallid cuckoo (Cuculus pallidus) eggs differed between four host species, and that their eggs closely mimicked the eggs of the host they parasitized. These results reveal that pallid cuckoos have host-specific egg types that have not been detected by human observation, and indicate that gentes could be more common than previously realized.
Keywords: brood parasitism, coevolution, gentes, egg mimicry, spectrophotometry
1. Introduction
One of the most effective defences against parasitism by cuckoos is rejection of odd eggs that appear in the nest. Unlike other defences, such as nest desertion (e.g. Hosoi & Rothstein 2000) and cuckoo chick desertion (e.g. Langmore et al. 2003), this defence eliminates the parasite without loss of the current breeding attempt. Not surprisingly then, egg rejection is widespread among cuckoo hosts (reviewed in Rothstein & Robinson 1998). However, in response many cuckoo species have evolved eggs that match those of their host (e.g. Higuchi 1998; Davies & Brooke 1988; Arias-de-Reyna 1998; Nakamura et al. 1998). Laying a mimetic egg can significantly improve the chances of acceptance by the host (e.g. Davies & Brooke 1988; Higuchi 1989).
Many cuckoos are specialists and parasitize one or few host species. For example, black-eared cuckoos Chrysococcyx osculans mainly parasitize Pyrrholaemus sagittatus and Pyrrholaemus brunneus, and they lay chocolate brown eggs that closely resemble those of their hosts (Brooker & Brooker 1989). However, some cuckoos are generalists and parasitize a range of host species. Egg mimicry may be problematic for these species, if their various hosts lay eggs of differing colours and patterns. The most studied generalist cuckoo, the European cuckoo Cuculus canorus, has resolved this problem by evolving a number of host-specific races (gentes), each with a distinctive egg type to match its host (Brooke & Davies 1988; Gibbs et al. 2000). Each individual female cuckoo always lays its eggs in the nests of its favoured host species (Nakamura & Miyazawa 1997; Honza et al. 2002; Avilés & Møller 2004), probably the host that reared it, and its eggs closely match those of that particular host (Davies 2000; Avilés & Møller 2004).
Polymorphic, host-specific egg types have been reported in many other generalist cuckoos, including several other Cuculus species (C. saturatus, Higuchi & Sato 1984; C. poliocephalus and C. sparverioides, Baker 1923; C. solitarius, Cherry & Bennett 2001), several Chrysococcyx cuckoos (C. Caprius, Reed 1968; Lawes & Kirkman 1996; C. cupreus and C. klaas, Tarboton 2001), the brush cuckoo Cacomantis variolosus (Beruldsen 2003), and the Jacobin cuckoo Clamator jacobonus (Tarboton 2001). It is unknown how other generalist cuckoos avoid egg rejection by their hosts. If hosts have similar egg types, cuckoos might lay a ‘compromise’ egg type that is intermediate in colour and pattern between those of its primary hosts (Edvardsen et al. 2001). Another possibility is that host-specific egg types are universal among generalist cuckoos, but they have not always been detected.
Assessing egg mimicry is not always straightforward. The great spotted cuckoo Clamator glandarius appears to have eggs that mimic those of a host, the magpie Pica pica. Closer inspection using spectrophotometric methods suggested that the eggs were not significantly related to the appearance of host eggs (Soler et al. 2003). Conversely, in the red-chested cuckoo Cuculus solitarius, each gens lays a different egg type, some of which appear to mimic the eggs of their hosts, while others appear non-mimetic. Spectrophotometry has shown that the cuckoo eggs actually match the eggs of their hosts most closely at wavelengths that cannot be perceived by the human eye (Cherry & Bennett 2001). These studies emphasize the inadequacy of human comparisons applied to the coloration of bird eggs, and the importance of techniques such as spectrophotometry to measure colour objectively.
Here we use reflectance spectrophotometry to assess the extent of egg mimicry in an Australian generalist parasite, the pallid cuckoo (Cuculus pallidus). Pallid cuckoos occur throughout Australia and parasitize a range of species that build cup-shaped nests, including honeyeaters (Lichenostomus spp., Anthochaera spp., Manorina spp., Melithreptus spp.), robins (Melanodryas spp.), whistlers (Pachycephala spp.), woodswallows (Artamus spp.) and willie wagtails (Rhipidura leucophrys; Brooker & Brooker 1989). Thirty-two species have been identified as biological hosts, and host preference varies geographically (Brooker & Brooker 1989). Although pallid cuckoos parasitize a wide variety of hosts, existing descriptions of egg types do not refer to any variation in colour or pattern in relation to host species (Brooker & Brooker 1989; Higgins 1999). Their eggs are described as oval in shape (23.9×17.4 mm) and fleshy pink, sometimes with a few dots of a deeper hue (figure 1; Higgins 1999). They are generally thought to resemble the eggs of two common host species, Lichenostomus melanops and Lichenostomus virescens (Ramsay 1865; McGilp 1941, cited in Brooker & Brooker 1989).
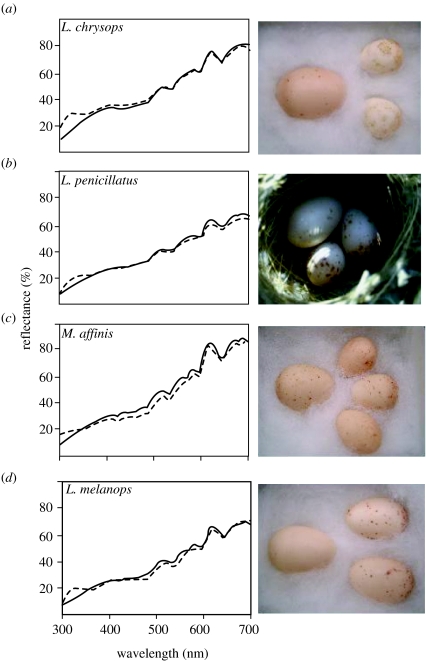
Figure 1.
Mean reflectance spectra of host eggs (solid line) and the pallid cuckoo eggs laid in their respective nests (broken line). (a) Lichenostomus chrysops, (b) L. penicillatus, (c) Melithreptus affinis, (d) L. melanops. Sample photographs of parasitized clutches from each species are shown beside spectra, with the cuckoo egg on the left.
2. Material and methods
(a) Host species
Host species were selected for colour measurement on the basis of available parasitized clutches (clutches containing both host and cuckoo eggs) at the Australian National Wildlife Collection, CSIRO, Canberra, Australia. We measured eggs from all hosts for which there were at least five parasitized clutches. Sample sizes were subsequently boosted by additional measurements at the Australian Museum, Sydney. Host species included in the analysis were four species of melaphagid honeyeaters: Lichenostomus penicillatus (N=5), Lichenostomus chrysops (N=5), L. melanops (N=6) and Melithreptus affinis (N=13). Lichenostomus penicillatus, the white-plumed honeyeater, is a favoured host of pallid cuckoos in the east of Australia, and is also one of the most widespread of favoured hosts (Brooker & Brooker 1989; Pizzey & Knight 1997). They lay spotted white, pink or buff eggs (Slater et al. 2003). Melithreptus affinis, the black-headed honeyeater, is the favoured host in Tasmania, where it is endemic (Brooker & Brooker 1989; Higgins 1999), and lays spotted, pinkish eggs (Slater et al. 2003). Lichenostomus melanops, the yellow-tufted honeyeater, occurs in southeastern Australia and is one of the two species whose eggs are thought to be mimicked by pallid cuckoos (Brooker & Brooker 1989). Their eggs are described as spotted and blotched pinkish-buff (Slater et al. 2003). Lichenostomus chrysops, the yellow-faced honeyeater, is a common host in the east of Australia with spotted and blotched pinkish to reddish-buff eggs (Slater et al. 2003). All Lichenostomus species sampled are sympatric, but L. chrysops has a range extending farther north than the other two species and L. penicillatus has a broader range than the other two, covering the inland areas and also much of the west of Australia (Slater et al. 2003). To confirm that all clutches that were classified as parasitized actually contained a cuckoo egg, we measured the length and width of alleged cuckoo eggs. In all the cases, the dimensions of the cuckoo eggs fell within the reported range of pallid cuckoo eggs (Brooker & Brooker 1989), which are larger than host eggs.
Following the procedure of Langmore et al. (2003), we removed one randomly selected host egg and the cuckoo egg from each parasitized clutch and measured the spectral reflectance at the tip, middle and base of each egg. We used a narrow-ended UV–VIS unidirectional reflectance probe, attached to an S2000 spectrophotometer and PX-1 Xenon strobe (Ocean Optics Inc.). The probe illuminated areas approximately 1.5 mm in diameter. Reflectance was calculated relative to a reflectance standard (WS-1) between 300 and 700 nm. The probe was held at a constant 45° angle to the egg surface by a small aluminium sleeve with a bevelled edge. The three measurements from each egg were averaged to give a mean spectrum for each egg, which was later used in the statistical analysis.
(b) Statistical analysis
Each mean spectrum was summarized as 200 data points (reflectance at every 2 nm from 300 to 700 nm). We used principal components analysis (PCA), which has been recommended for analysis of reflectance spectra in animal coloration (e.g. Cuthill et al. 1999), to identify the important sources of variation between host and parasitic eggs. We used restricted maximum-likelihood models (REML) to test whether a significant amount of variation in the data could be explained by (i) the host species and (ii) whether the egg belonged to the host or the parasite. Eggs in museum collections have usually been handled to some degree, and natural skin oils can block UV reflectance (J. Endler 2005, personal communication). Museum collections also differ considerably in age, with unknown effects on colour and brightness. To account for any possible pseudoreplication caused by two eggs (i.e. host and parasite) being used from the same nest, we included the nest identifier as a random term in each statistical model. We also included the year in which the eggs were collected as a continuous variable to determine whether the age of the egg had any influence on colour. This model was repeated for each of the first three principal components (PC1, PC2, PC3). Each model was run including all terms and interactions, and then least significant terms were dropped sequentially until only significant terms remained. Normality of the data was confirmed by examining the standardized residuals and fitted values in each model. All statistics were carried out in GenStat v. 8.1.
3. Results
The spectrograms revealed that the cuckoo eggs mimicked their hosts' eggs closely in both spectral shape and brightness (figure 1). However, host eggs exhibited a small peak in the ultraviolet that was not mimicked by the cuckoo eggs (figure 1).
(a) Principal components analysis
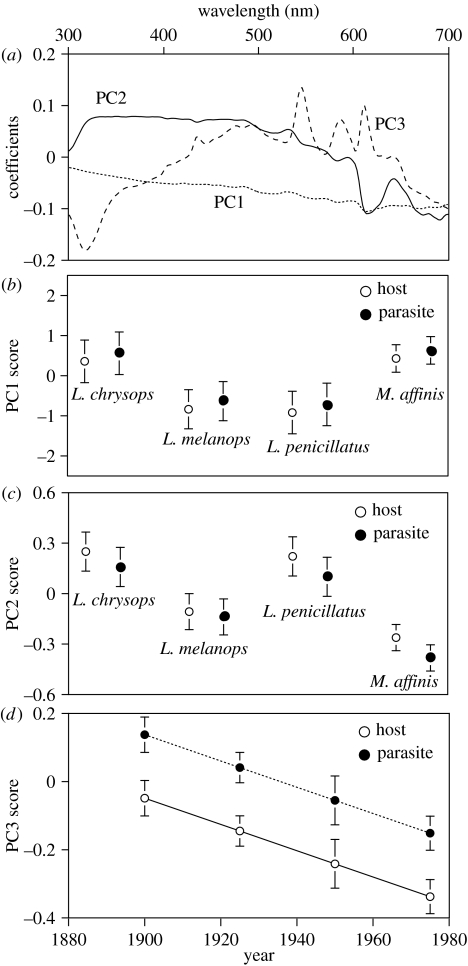
The first three principal components explained 88.8, 6.7 and 2.6% of the variation in spectrum shape, respectively (total=98.1%, figure 2). Graphs of the principal component coefficients against wavelength revealed that PC1 was mostly flat along the spectrum with a gradual decrease from lower to higher wavelengths. Thus, it appears to primarily represent achromatic brightness. PC2 varied most in the higher (‘reddish’) wavelengths. PC3 varied both at low wavelengths (less than 350 nm), indicating that it represented most of the variation in UV reflectance, and also at higher wavelengths (530–630 nm, figure 2a).
Figure 2.
(a) The coefficients of PC1, PC2 and PC3 plotted against wavelength to indicate the information summarized by each principal component. (b) The mean (±s.e.) principal component score for host species and the cuckoos that parasitized them, for PC1 and (c) PC2. (d) Mean (±s.e.) PC3 scores for host species and cuckoos in relation to the year in which the egg was laid.
For PC1, there was a significant effect of which host species' nest the eggs were from (Wald statistic, , p=0.02), but no significant effect of whether the egg was laid by the host or parasite (, p=0.34). There was no significant effect of the age of the egg (, p=0.26) and there were no significant interactions between any of the terms (). The nest identifier had a significant effect as a random term (change in deviance, , p<0.02) and showed there was a high within nest correlation for PC1 (r=0.40).
Similarly, PC2 showed a significant effect of the host species (, p<0.001), but no significant effect of whether it was laid by the host or the parasite (, p=0.19). Again there was no significant effect of the age of the egg (, p=0.099) and no significant interactions between any of the terms (). The nest identifier did not have a significant effect as a random term (change in deviance, , within nest correlation=0.09).
PC3 differed from PC1 and PC2 by showing no significant effect of host species (, p=0.12). However, PC3 did show a significant effect of both the age of the egg (, p=0.03) and whether it was laid by the host or the parasite (, p<0.001). There were no significant interactions between any of the terms (). The nest identifier did not have a significant effect as a random term (change in deviance, , within nest correlation=0).
4. Discussion
Pallid cuckoo eggs showed striking mimicry of host eggs. Most of the variation between host species occurred in the brightness of the eggs and in the higher (reddish) and lower (ultraviolet) wavelengths. Cuckoo eggs matched their respective hosts' eggs closely in brightness and the higher wavelengths, which accounted for 88.8 and 6.7% of the variation, respectively. However, pallid cuckoos did not share the small peak in UV reflectance observed in host eggs. These findings suggest that pallid cuckoos have more than one egg type. Further, the host-specificity of egg types provides a strong indication of the existence of gentes. This is the first time to our knowledge that host-specific egg types have been revealed using reflectance spectrophotometry.
Although the host eggs were superficially similar, our results showed that their reflectance spectra differed significantly from one another in the variation described by PC1 and PC2. Lichenostomus chrysops and M. affinis scored higher for PC1 than the other two hosts, whereas M. affinis eggs scored highest for PC2, followed by L. melanops. The pallid cuckoo eggs from the four different hosts' nests matched their respective hosts closely in these features. These results indicate that selection has led to differentiation between cuckoo eggs, rather than a ‘compromise’ egg type, even when hosts have similar egg types.
PC1 and PC2 accounted for most of the variation in reflectance spectra (over 95%), but a small amount of variation (2.6%) was explained by PC3, which included variation at ultraviolet wavelengths. Pallid cuckoo eggs did not share the small UV peak shown by hosts. There are three possible explanations for this result. First, mimicry of UV wavelengths may be more difficult than mimicry at other wavelengths in some way, constraining the accuracy of mimicry by cuckoos. Second, it is possible that these host species do not use UV reflectance in egg discrimination, rendering mimicry at UV wavelengths unnecessary. Cherry & Bennett (2001) found mimicry of host eggs by the red-chested cuckoo at some wavelengths but not others, and suggested that egg discrimination by hosts may be performed only at certain combinations of wavelengths depending on the spectral sensitivities of the particular host. Third, the pigments in cuckoo and host eggs may differ (Davies 2000) with the result that age has a greater effect on the reflectance in the UV on pallid cuckoo eggs compared to host eggs. If so, it is possible that freshly laid pallid cuckoo eggs will mimic their hosts in the ultraviolet as well as at other wavelengths. It is also possible that the novelty of cuckoo eggs has prompted greater handling. However, this is unlikely because the difference in UV reflectance was consistent between cuckoo eggs and host eggs, and was constant through all years sampled.
Egg mimicry by cuckoos has been shown to evolve in response to egg rejection by hosts (Davies 2000). The accuracy of egg mimicry by pallid cuckoos suggests that many of their hosts are likely to be egg rejectors. This has been confirmed for two pallid cuckoo hosts, willie wagtails and rufous whistlers Pachycephala rufiventris (Langmore et al. 2005). It is likely that many honeyeater species are also egg rejectors because they possess the typical characteristics of egg rejector species; they are favoured hosts and they build cup-shaped nests that provide good visibility for egg discrimination (Langmore et al. 2005). This is yet to be tested experimentally.
Museum collections provide a potentially biased source of parasitized clutches (Moksnes & Røskaft 1995; Aviles & Møller 2004). If egg mimicry is very accurate, egg collectors may detect parasitism only when the cuckoo egg is a relatively poor match. This effect is counteracted if hosts reject non-mimetic eggs, because egg collectors are then more likely to find clutches containing cuckoo eggs that are a good match. The former possibility can be largely discounted here because pallid cuckoo eggs are larger than host eggs, so they are detectable regardless of the degree of colour mimicry.
The second possibility, that clutches containing the most mimetic eggs are over-represented in museum collections, is of more concern because it would give the same correlation between host and cuckoo egg colour as is predicted by the gentes hypothesis. However, we consider this an unlikely explanation for our results. If the mimetic cuckoo eggs in this study are not the product of gentes, there are two alternative, but less adaptive, explanations. First, individual female cuckoos could parasitize multiple hosts and produce multiple egg types. However, studies of egg-laying behaviour in the congeneric European cuckoo suggest that females are host-specific (Nakamura & Miyazawa 1997; Honza et al. 2002; Avilés & Møller 2004). Moreover, avian egg coloration appears to be controlled genetically (Collias 1993; Gosler et al. 2000), constraining females to production of a single egg type. Finally, such a system would be less adaptive than gentes, because the probability that the cuckoo would lay the appropriate egg type for a particular host simply by chance would be substantially lower than when a cuckoo gens targets its appropriate host. Second, there could be multiple egg types in the pallid cuckoo population, but individual females produce a constant egg type that is laid randomly in the nest of any host species. Our results could then be explained by host rejection of any cuckoo eggs that do not match their own egg type. However, this strategy would be very wasteful from the cuckoo's point of view (Davies 2000) and would rapidly lead to the evolution of gentes, because a cuckoo's reproductive success would be strongly biased towards the host with the matching egg type. Most eggs laid in other host nests would be rejected, selecting against parasitism of those hosts. The existence of gentes in the pallid cuckoo could be confirmed in future studies using genetic analysis of mtDNA, as demonstrated by Gibbs et al. (2000) for the European cuckoo.
Two results show that egg coloration may be affected by handling, suggesting that it is necessary to control for both the age and origin of museum clutches in studies of parasitism. First, PC3 decreased with the age of the egg. This may indicate a decline in UV reflectance caused by handling (J. Endler 2005, personal communication), because older eggs are more likely to have been handled frequently than younger eggs. Second, we found a significant effect of the random term ‘nest ID’ for PC1, indicating a within nest correlation. This could be explained by each nest being subjected to slightly different treatment in the form of handling and storage, reflecting the need for care in selecting collections for analysis. Alternatively, the effect could indicate regional variation in host eggs. For example, L. penicillatus is reported to lay white, pink or buff eggs (Slater et al. 2003). If pallid cuckoos mimic such regional variation in host eggs, this could explain the within nest correlation. A larger sample size of parasitized clutches would be needed to explore this possibility further.
The findings in this study emphasize the inadequacies of human comparisons when assessing brood parasite mimicry of host eggs. It seems plausible that other examples of cryptic gentes await discovery, and that there may even be cryptic gentes in the European cuckoo that have not yet been detected. For example, European cuckoos reportedly have a general Acrocephalus egg that is similar to many warbler eggs, but matches none very well (Edvardsen et al. 2001). Spectrophotometry may reveal subtle, but significant differences within this Acrocephalus egg type to suggest matching is better than it appears.
Acknowledgments
We thank J. Endler for technical advice, I. Mason and R. Palmer, CSIRO and W. Boles, Australian Museum for making egg collections available, and R. Kilner and two anonymous referees for helpful comments on the manuscript. N.E.L. was supported by an A.R.C. research fellowship.
References
- Arias-de-Reyna L. Coevolution of the great spotted cuckoo and its hosts. In: Rothstein S.I, Robinson S.K, editors. Parasitic birds and their hosts. Oxford University Press; Oxford, UK: 1998. pp. 129–142. [Google Scholar]
- Avilés J.M, Møller A.P. How is host egg mimicry maintained in the cuckoo (Cuculus canorus)? Biol. J. Linn. Soc. 2004;82:57–68. 10.1111/j.1095-8312.2004.00311.x [Google Scholar]
- Baker E.C.S. Cuckoo eggs and evolution. Proc. Zool. Soc. 1923;1923:277–294. [Google Scholar]
- Beruldsen G. G. Beruldsen; Kenmore Hills, Queensland: 2003. Australian birds, their nests and eggs. [Google Scholar]
- Brooke M.D, Davies N.B. Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature. 1988;335:630–632. 10.1038/335630a0 [Google Scholar]
- Brooker M, Brooker L. Cuckoo hosts in Australia. Aust. Zool. Rev. 1989;2:1–67. [Google Scholar]
- Cherry M.I, Bennett A.T.D. Egg colour matching in an African cuckoo, as revealed by ultraviolet–visible reflectance spectrophotometry. Proc. R. Soc. B. 2001;268:565–571. doi: 10.1098/rspb.2000.1414. 10.1098/rspb.2000.1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collias E.C. Inheritance of egg-colour polymorphism in the village weaver (Ploceus cucullatus) Auk. 1993;110:683–692. [Google Scholar]
- Cuthill I.C, Bennett A.T.D, Partridge J.C, Maier E.J. Plumage reflectance and the objective assessment of avian dichromatism. Am. Nat. 1999;153:183–200. doi: 10.1086/303160. 10.1086/303160 [DOI] [PubMed] [Google Scholar]
- Davies N.B. T & AD Poyser; London, UK: 2000. Cuckoos, cowbirds and other cheats. [Google Scholar]
- Davies N.B, Brooke M.D. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 1988;36:262–284. [Google Scholar]
- Edvardsen E, Moksnes A, Røskaft E, Øien I.J, Honza M. Egg mimicry in cuckoos parasitizing four sympatric species of Acrocephalus warblers. Condor. 2001;103:829–837. [Google Scholar]
- Gibbs H.L, Sorenson M.D, Marchetti K, Brooke M.D, Davies N.B, Nakamura H. Genetic evidence for female host-specific races of the common cuckoo. Nature. 2000;407:183–186. doi: 10.1038/35025058. 10.1038/35025058 [DOI] [PubMed] [Google Scholar]
- Gosler A.G, Barnett P.R, Reynolds S.J. Inheritance and variation in eggshell patterning in the great tit Parus major. Proc. R. Soc. B. 2000;267:2469–2473. doi: 10.1098/rspb.2000.1307. 10.1098/rspb.2000.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins P.J. Parrots to dollarbird. vol. 4. Oxford University Press; Oxford, UK: 1999. Handbook of Australian, New Zealand and Antarctic birds. [Google Scholar]
- Higuchi H. Host use and egg color of Japanese cuckoos. In: Rothstein S.J, Robinson S.K, editors. Parasitic birds and their hosts. Oxford University Press; Oxford, UK: 1998. pp. 80–93. [Google Scholar]
- Higuchi H. Responses of the bush warbler Cettia diphone to artificial eggs of Cuculus cuckoos in Japan. Ibis. 1989;131:94–98. [Google Scholar]
- Higuchi H, Sato S. An example of character release in host selection and egg colour of cuckoos Cuculus spp. in Japan. Ibis. 1984;126:398–404. [Google Scholar]
- Honza M, Taborsky B, Taborsky M, Teuschl Y, Vogl W, Moksnes A, Røskaft E. Behaviour of female common cuckoos, Cuculus canorus, in the vicinity of host nests before and during egg laying: a radiotelemetry study. Anim. Behav. 2002;64:861–868. 10.1006/anbe.2002.1969 [Google Scholar]
- Hosoi S.A, Rothstein S.I. Nest desertion and cowbird parasitism: evidence for evolved responses and evolutionary lag. Anim. Behav. 2000;59:823–840. doi: 10.1006/anbe.1999.1370. 10.1006/anbe.1999.1370 [DOI] [PubMed] [Google Scholar]
- Langmore N.E, Hunt S, Kilner R.M. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature. 2003;422:157–160. doi: 10.1038/nature01460. 10.1038/nature01460 [DOI] [PubMed] [Google Scholar]
- Langmore N.E, et al. The evolution of egg rejection by cuckoo hosts in Australia and Europe. Behav. Ecol. 2005;16:686–692. 10.1093/beheco/ari041 [Google Scholar]
- Lawes M.J, Kirkman S. Egg recognition and interspecific brood parasitism rates in red bishops. Anim. Behav. 1996;52:553–563. 10.1006/anbe.1996.0197 [Google Scholar]
- Moksnes A, Røskaft E. Egg-morphs and host preference in the common cuckoo (Cuculus canorus): an analysis of cuckoo and host eggs from European museum collections. J. Zool. Lond. 1995;236:625–648. [Google Scholar]
- Nakamura H, Miyazawa Y. Movements, space and social organization of radio-tracked common cuckoos during the breeding season in Japan. Jpn. J. Ornithol. 1997;46:23–54. [Google Scholar]
- Nakamura H, Kubota S, Suzuki R. Coevolution between the common cuckoo and its major hosts in Japan: stable versus dynamic specialization on hosts. In: Rothstein S.J, Robinson S.K, editors. Parasitic birds and their hosts. Oxford University Press; Oxford, UK: 1998. pp. 94–112. [Google Scholar]
- Pizzey G, Knight F. Harper Collins Publishers Australia; Sydney, Australia: 1997. The Graham Pizzey & Frank Knight field guide to the birds of Australia. [Google Scholar]
- Reed R.A. Diederik cuckoos Chrysococcyx caprius in the Transvaal. Ibis. 1968;110:321–331. [Google Scholar]
- Rothstein S.I, Robinson S.K. Oxford University Press; Oxford, UK: 1998. Parasitic birds and their hosts. [Google Scholar]
- Slater P, Slater P, Slater R. Reed New Holland; Sydney, Australia: 2003. The Slater field guide to Australian birds. [Google Scholar]
- Soler J.J, Aviles J.M, Soler M, Møller A.P. Evolution of host egg mimicry in a brood parasite, the great spotted cuckoo. Biol. J. Linn. Soc. 2003;79:551–563. 10.1046/j.1095-8312.2003.00209.x [Google Scholar]
- Tarboton W. Struik Publishers; Cape Town, South Africa: 2001. A guide to the nests and eggs of southern African birds. [Google Scholar]