Abstract
Parasite driven time-lagged negative frequency-dependent selection of hosts has been studied in natural populations by following changes in host genotype frequencies over time. However, such dynamics have not been considered at higher taxonomic levels, for example, between parental species and their hybrids. In a field study on a Daphnia hybrid system, we observed that one Daphnia taxon first was relatively under-infected, but became over-infected after a strong increase in frequency. This finding is consistent with the idea of parasite evolution towards the most frequent host taxon. In two experiments, we investigated whether the assumptions made by a model of negative frequency-dependent selection apply to our host taxa system. First, we showed that the parasite can change the outcome of taxa competition and secondly, we confirmed that the over-infection of one host taxon observed in the field has a genetic basis. Our results indicate that the incorporation of host–parasite interactions at the species level may allow us to gain a more complete picture of forces driving dynamic taxa coexistence in Daphnia hybrid systems. More generally, we suggest that if hybrids coexist in sympatry with parental taxa, the infection patterns as observed under natural conditions may be rather temporal and unstable.
Keywords: species competition, hybridization, frequency-dependent selection, parasite evolution, Daphnia
1. Introduction
There is a growing interest in finding evidence for parasite driven time-lagged negative frequency-dependent selection in natural host populations (see Little 2002). If a particular host genotype becomes more frequent, it is expected that it will become over-infected and, consequently, will decrease in frequency (Hamilton 1980). This hypothesis has been tested in some natural systems, where the frequencies of single host genotypes were followed during the course of a study (e.g. Henter & Via 1995; Dybdahl & Lively 1998; Little & Ebert 1999) but direct evidence of a continual cycling of host and parasite genotype frequencies is very limited (discussed in Little 2002).
Since shared parasites can alter competition among coexisting species (reviewed in Hudson & Greenman 1998; Prenter et al. 2004), which fulfils an important assumption usually made in coevolutionary models (e.g. Hamilton 1980), it is reasonable to expect that not only single genotypes but also species may be subject to parasite-driven host frequency-dependent selection. However, the host–parasite associations at the species level are viewed in fixed categories of ‘clear winners and losers’ (Hudson & Greenman 1998). Particularly, in hybridizing systems, differences in infection level between parental taxa and hybrids are thought to be stable (see review by Moulia 1999). In many of these systems, however, the barrier for parasite adaptation to a new host might be spatial separation between the two hybridizing taxa, either geographical (Heaney & Timm 1985; Sage et al. 1986) or, on a finer scale, by different habitat types within a hybrid zone (Le Brun et al. 1992). In sympatric hybrid systems, parasites have the potential to access both parentals as well as their hybrids and we may, therefore, expect a dynamic coevolution. Such taxa are usually seen to be either permanently susceptible or resistant, often based on the data from only one field season (e.g. Mason & Clark 1990; Coustau et al. 1991; El Gharbi et al. 1992; Tinsley & Jackson 1998). The study of a dynamic association between taxon frequency and its infection level may add power to the interpretation of field data.
A sympatric Daphnia galeata/Daphnia hyalina complex (Schwenk & Spaak 1997) has recently been investigated in order to compare prevalence of the common and virulent parasite, Caullerya mesnili, between parental and hybrid taxa. During a summer–autumn epidemic in 2001, D. galeata×D. hyalina hybrids were frequently infected whereas D. galeata was not parasitized (Wolinska et al. 2004). The relative frequencies of Daphnia taxa in the studied lake changed thereafter, from almost complete hybrid dominance (1998–2001) to a six month long peak of D. galeata (maximum frequency 70%, May 2002; Keller & Spaak 2004). Here, we present a field study testing if 2 and 3 years after the previous investigation, D. galeata was still under-infected, despite its strong increase in abundance. If resistance of this species disappeared, this would indicate parasite evolution towards the most common host species. In addition to the field survey we conducted two laboratory experiments to test assumptions underlying the idea of frequency-dependent selection on host taxa. Since previous studies have demonstrated that considerable variation in several life-history traits exists among genotypes within these Daphnia taxa (e.g. Weider 1993; Löffler et al. 2004), we used several genotypes to partition the within- and between-taxa variance before making conclusions at the taxon level. First, we conducted an experiment where two Daphnia species were kept together in the presence and absence of the parasite, to see if the parasite was able to alter the outcome of competition (as was shown, e.g. in a classic laboratory experiment by Park 1948). Second, to examine if over-infection of one taxon as observed in the field has a genetic background, we tested whether the same infection pattern was seen under controlled laboratory conditions where other environmental forces were excluded or were kept constant (as in Moulia et al. 1993).
2. Material and methods
(a) The host–parasite system
Daphnia galeata and D. hyalina dominate Daphnia populations of many permanent European lakes. The two taxa often produce hybrids (reviewed in Schwenk & Spaak 1997). Daphnia galeata×D. hyalina populations investigated in this study originate from two lakes: Greifensee (Switzerland), a eutrophic, prealpine lake of medium size (8.5 km2), and Lake Constance (border Germany–Switzerland–Austria), an oligotrophic, large prealpine lake (476 km2).
The protozoan gut parasite, Caullerya mesnili Chatton is horizontally transmitted and infects Daphnia by free-floating spore stages (Bittner et al. 2002). C. mesnili is regularly found in natural Daphnia populations (Green 1974; Stirnadel & Ebert 1997; Wolinska et al. 2005) with reported prevalence up to 43% for Daphnia pulex (Little & Ebert 1999) or even 50% for D. galeata×D. hyalina populations (Bittner 2001).
(b) Genetic markers for the host
Daphnia were assayed for four polymorphic allozyme marker loci: phosphoglucose isomerase (PGI, enzyme commission number (EC 5.3.1.9), phosphoglucomutase (PGM, EC 5.4.2.1), aldehyde oxidase (AO, EC 1.2.3.1) and aspartate amino transferase (AAT, EC 2.6.1.1.). AO and AAT are diagnostic markers for D. galeata and D. hyalina (Wolf & Mort 1986; Gießler 1997). Specimens were differentiated into six genealogical classes (taxa): ‘pure’ parentals, both hybrid generations, and first generation backcrosses (see table 1 in Keller & Spaak 2004). The two other loci (PGI and PGM) were used to distinguish between different multi-locus genotypes (hereafter referred to as clones) within each tested taxon.
(c) Field survey of Greifensee
Weekly zooplankton samples (every other week in winter) were collected by vertical hauls of a 250 μm plankton net through the whole water column (30 m max depth), at three different locations in the Greifensee (June 2002–May 2005). Randomly chosen (n∼100) adult Daphnia females were screened for presence or absence of C. mesnili spore clusters (hereafter referred to as infected and non-infected). During C. mesnili epidemics, a subsample of infected females was additionally selected (n∼60, five sampling dates in 2003 and 15 in 2004). All individuals were frozen for subsequent electrophoretic analysis. For a more detailed description of sampling and parasite screening procedures see Wolinska et al. (2004).
(d) Competition experiment
To test whether the C. mesnili parasite is able to alter the outcome of competition between as well as within two host taxa (D. galeata and D. hyalina), we randomly selected three different multi-locus genotypes per taxon from a clone collection established from Lake Constance in February 1998. We chose this lake because in contrast to the Greifensee, both pure parental taxa are present (Jankowski & Straile 2004). The C. mesnili strain was isolated at the same time by placing females from one of the isolated D. galeata clones (C.gal.1) in medium containing a cocktail of spores from several field-infected daphnids. The parasite was cultured subsequently for 26 months in the same host clone by adding newborns from the uninfected stock cultures at approximately three week intervals. The C. mesnili strain may not represent single genotypes as multiple infections of individual hosts may occur (Ebert & Mangin 1997). However, the parasite's genetic diversity is assumed to be low, as we expected that a genetic bottleneck occurred during isolation and subsequent culturing in a monoclonal host population (see Capaul & Ebert 2003; Haag & Ebert 2004).
Fifty Daphnia of each clone (1–8 days old) were put together in a 4 l aquarium and kept under standardized experimental condition (0.45 μm filtered Lake Constance water, 20° C, 24 h dim light, fed daily with 1 mg C l−1 of Scenedesmus obliquus) for 10 weeks. There were eight infected and eight non-infected aquaria, each stocked with a total of 300 Daphnia. A plastic tube with 10 infected (or non-infected) individuals from clone C.gal.1 was placed into each aquarium. The bottom of the tube was closed with 100 μm mesh that allowed parasite spores but not Daphnia to float between compartments. We removed the tubes after one week. The medium was replaced weekly, by filtering the whole contents of the aquarium through a 100 μm mesh, and all the animals were put back into the new medium. After week 2, each replicate was checked for external signs of infection (60 daphnids). After weeks 2, 6 and 10, samples were taken and 40–60 randomly chosen females per replicate were frozen for subsequent electrophoretic analysis, whereas the rest were put back into the aquaria. After the experiment was terminated, population densities were calculated.
(e) Infection experiment
With this experiment, we aimed to check if the observed over-infection of one taxon in the Greifensee could be confirmed under controlled, laboratory conditions. Experimental clones were randomly chosen from a clone collection established from Greifensee (sampled in June and October 2002, March and May 2003); six different genotypes per taxon (D. galeata, D. hyalina and first generation of hybrids) were used. Two of the 18 clones had carried an infection at the time of their isolation, but juveniles were separated immediately after birth from their infected mothers and thus lineages were cultured without infections. Since pure D. hyalina is rarely present in Greifensee and the few apparently pure D. hyalina may be backcrossed hybrids (Keller & Spaak 2004), four backcrossed D. hyalina clones were included into the D. hyalina experimental group.
Clones were kept under standardized conditions for three generations prior to the experiment (0.45 μm filtered Greifensee water, 20° C, 16 h light–8 h dark; every other day the medium was refreshed and daphnids were fed with 1 mg C l−1 of S. obliquus). Following acclimatization, newborns (less than 12 h) were distributed between two infection treatments (‘exposure short’ and ‘exposure long’) and across three controls (‘placebo short’, ‘placebo long’ and ‘blank’); each experimental unit (50 ml medium) was stocked with 8–10 neonates from single clones. There were 3–4 replicates per infection treatment (due to limited numbers of neonates, four clones were tested under only exposure short or exposure long conditions). After 24 h the spore/placebo suspension was added. Two hundred and twenty heavily infected daphnids, freshly sampled from Greifensee (26 August 2003), were homogenized and the spore suspension was distributed; each experimental unit received spores from two donors, on average. The placebo suspension contained the macerated Daphnia tissue from uninfected, laboratory clones (60 daphnids, two donors per experimental unit, on average). At day 4 or 7 (treatments: ‘short’, ‘long’, respectively) daphnids were transferred to 200 ml of fresh medium. Between days 10 and 14, daphnids from both infection treatments/controls were again exposed to freshly prepared spore/placebo suspensions, to increase the likelihood of successful infection. During the exposure period, the water was stirred daily to ensure homogeneous spore suspensions and to maximize encounter rate. Offspring were removed and dead females were dissected and checked for presence of the parasite spores every other day. At day 11 and 18, three randomly chosen animals per experimental unit were checked for external signs of infection in order to assess the time needed for disease development. At day 22, 23 and 24, one-third of the individuals were measured and dissected and fecundity was documented.
(f) Statistical analyses
In all ANOVA tests, clone and sampling date were treated as random factors, and proportional data were arc-sine-square-root transformed (Sokal & Rohlf 1995).
(i) Field survey of Greifensee
To check if during the 2003 and 2004 epidemic years D. galeata remained relatively under-infected (i.e. less abundant in the infected than in the non-infected part of the population), the proportions of D. galeata in infected and non-infected subsamples (hereafter referred to as infection status) were analysed with an ANOVA, where infection status, epidemic year and sampling date (nested within epidemic year) were the main effects. Since the obtained pattern differed from our earlier results, we wanted to know if this shift in D. galeata infection level was significant. Therefore, an additional analysis was run with the 2001 data (Wolinska et al. 2004) included. The 2002 data were not included, because it was not possible to define an infection level of D. galeata due to the low frequency of this taxon. We were especially interested in the interaction between infection status and epidemic year; furthermore, we used a contrast test for the following effect: proportions of D. galeata in the 2001 epidemic year contrasted with proportions in the 2003 and the 2004 epidemic years (Lindman 1992).
(ii) Competition experiment
From the taxa frequencies on each sampling date, we calculated the area under the frequency curve (AUC) of each taxon over the whole experimental period. This measure summarizes the data over the entire study period into one value per taxon per replicate, thereby avoiding problems of repeated measures (Crowder & Hand 1990). We chose this method because results are not affected by assumptions on the length of the experiment (for a discussion see Capaul & Ebert 2003; Haag & Ebert 2004). To test whether an outcome of taxa competition differed among treatments, we used a t-test with AUC of taxon as the dependent variable and treatment as a main effect. Additionally, to test for a treatment effect on within-taxon clonal competition, the relative (within-taxon) AUC was calculated per clone and a t-test between treatments was conducted separately for each clone. Because it involved three comparisons per taxon, levels of significance were Bonferroni-corrected for multiple simultaneous tests.
(iii) Infection experiment
Females that died prior to day 18 were excluded from all analyses to avoid the risk of overlooking early infection stages (at day 11, infection was recorded only for one clone, whereas at day 18, more clones showed infections). The proportions of infected individuals per experimental unit were analysed with ANOVA where taxon, clone (nested within-taxon) and exposure period were used as main effects. Four clones, which were only tested under one exposure treatment, were excluded from this analysis. The residuals did not deviate from normality. Although the assumption of homogeneity of variances was not met, there was no correlation between means and variances (for a discussion see Lindman 1992; StatSoft Inc. 2006). Since the original goal was to test if D. galeata was the most heavily over-infected taxon, a contrast test was applied: infection level of D. galeata contrasted with infection levels of two other taxa.
To estimate parasite-induced fitness reduction, data from all replicates and both infection treatments were pooled and individuals were divided into two groups: infected and remaining non-infected individuals. First, we compared the proportions of gravid females between infected and non-infected daphnids, measured at the end of the experiment (R×C test of independence). For other traits, we tested if the degree of host fitness reduction differed among the six most heavily infected clones. Clonal variation in parasite-induced mortality (mortality of infected females corrected for mortality of non-infected ones, measured after day 18) was determined with a R×C test and a further R×C test was undertaken (Sokal & Rohlf 1995) to identify which clones suffer significantly more than others. To test for body size differences measured at the end of the experiment, an ANOVA was used where infection status and clone were main effects.
3. Results
(a) Field survey of Greifensee
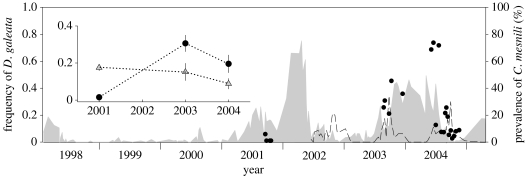
We observed that the earlier reported sudden increase in D. galeata frequencies (September 2001–February 2002 peak in Keller & Spaak 2004) persisted for at least a further year (September 2003–September 2004, figure 1). Caullerya mesnili epidemics appeared annually and lasted 6–7 months. Daphnia galeata was over-infected during both 2003 and 2004 epidemic years (infection status: F1,18=18.12, p<0.001; infection×epidemic year interaction: F1,18=0.18, p=0.67). When the 2001 epidemic year data were also included in the analysis, the interaction between infection status and epidemic year was highly significant (F2,21=17.07, p<0.0001; figure 1); D. galeata was under-infected during the 2001 epidemic and over-infected thereafter (contrast test: F1,21=31.0 p<0.0001).
Figure 1.
Changes in the frequencies of D. galeata (grey area) in the Daphnia population of Greifensee from January 1998 to May 2005. The black dots show the frequencies of D. galeata in infected subsamples during the 2001, 2003 and 2004 epidemics. The prevalence of C. mesnili in the entire Daphnia population (data available since June 2002) is indicated with a dashed line. In the sub-figure, the annual mean frequencies (± s.e.) of D. galeata in infected (black dots) and non-infected (grey triangles) subsamples are shown. The significant interaction is visible. Data before July 2002 have been published in different form by Keller & Spaak (2004) and Wolinska et al. (2004).
(b) Competition experiment
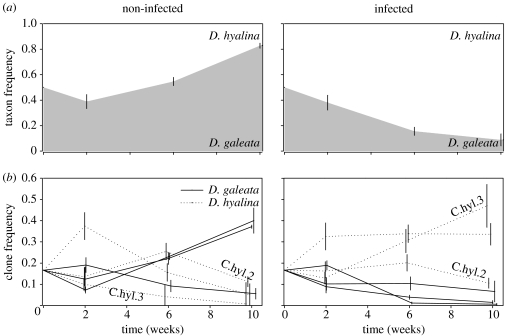
In the competition experiment, initially equally represented taxa (clones) competed against each other in the presence and absence of the C. mesnili parasite. The presence of C. mesnili infection was confirmed in all infected replicates on the first sampling date (no other parasite species were detected). One infected population went extinct after week 6 and another one was lost due to handling error. The final density did not differ significantly between the non-infected and infected treatments (on average 212 and 184 Daphnia l−1, respectively; t=0.41, d.f.=13, p=0.68). We found that the taxa success (AUC) differed significantly between treatments (table 1). D. galeata dominated all non-infected replicates (AUC: 0.56±0.06) but was outcompeted in the infected treatments (AUC: 0.24±0.08, figure 2a). We found no evidence of parasites altering clonal competition within D. galeata: the relative AUC of three D. galeata clones did not differ between treatments (table 1). In contrast, the outcome of competition was altered among D. hyalina clones: C.hyl.2 dominated the non-infected treatments (AUC: 0.47±0.11) but had the lowest abundance when C. mesnili was present (AUC: 0.24±0.09), whereas the pattern was reversed for clone C.hyl.3 (AUC: 0.13±0.04 and 0.36±0.07, respectively, figure 2b).
Table 1.
Summary of t-tests for effects between treatments on taxa (and clones within taxon) success (measured as area under the frequency curve, AUC) in the competition experiment. (A significant t-test indicates that this taxon or clone had a different representation in the two treatments. All significant values (in bold) remain significant after Bonferroni correction (uncorrected p-values are listed).)
| analysed group | t | p | |
|---|---|---|---|
| taxa | 6.24 | <0.0001 | |
| clones (D. galeata) | C.gal.1 | 1.87 | 0.09 |
| C.gal.2 | 1.07 | 0.31 | |
| C.gal.3 | 0.56 | 0.59 | |
| clones (D. hyalina) | C.hyl.1 | 0.02 | 0.99 |
| C.hyl.2 | 4.00 | 0.0017 | |
| C.hyl.3 | 7.80 | <0.0001 |
Figure 2.
Changes in (a) taxa and (b) clone frequencies in the non-infected (left) and infected (right) treatment of the competition experiment. Mean taxon/clone frequencies (±s.e.) are shown (grey area and solid lines, D. galeata; white area and dotted lines, D. hyalina). Clonal identities are given for those clones with relative frequencies (within-taxon) that differed significantly between treatments (see table 1).
(c) Infection experiment
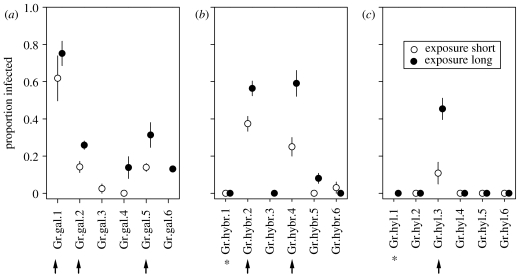
In the infection experiment, six clones from each of three taxa were exposed separately to the C. mesnili parasite. All six D. galeata, four hybrids and only one D. hyalina clone became infected by C. mesnili. No other parasite species were detected. The average infection level per taxon was 18.5 and 31.9% among D. galeata, 13.1 and 20.6% among hybrids and 2.2 and 7.6% among D. hyalina, in the exposure short and exposure long treatments, respectively (figure 3). Although in the exposure long treatment the infection level was higher (F1,11=9.76, p<0.01), some clones, including the two with initial infection, remained non-infected, which caused a significant interaction between clone (nested within taxon) and exposure period (F11,66=4.15, p<0.0002). Owing to a high variation in the infection level among clones (F11,11=12.97, p<0.0001), taxa differences were not significant (F2,11=2.08, p=0.17). The contrast test revealed, however, that D. galeata had a higher infection level than the two other taxa (F1,66=153.75, p<0.0001). Absence of infection in controls was confirmed by dissecting all females.
Figure 3.
Proportion (±s.e.) of infected individuals, (a) D. galeata, (b) hybrids and (c) D. hyalina, in the infection experiment, in relation to taxon, clone and exposure time to parasite spores. Parasites from the 2003 epidemic were used for this experiment. Clones that had carried an infection at the time of their isolation in 2002 are labelled with asterisks. The arrows indicate clones which were tested for a variation in their fitness reduction (see text).
The parasite reduced fecundity of all infected individuals: only 3% of infected females had eggs compared to 98.5% in the non-infected group (G1=370.2, p<0.0001). For other traits, only the six heaviest infected clones (see figure 3) were tested. The parasite-induced mortality differed among clones (G5=28.5, p<0.002); 31% of clone Gr.hybr.2 and 29% of clone Gr.hyl.3 died because of the parasite, whereas mortality of others was lower than 7% (G3=4.8, p=n.s.). The degree of body size reduction did not differ among clones (infection status×clone interaction: F5,316=0.86, p=n.s.), all infected females were smaller than non-infected ones (F1,5=56.85, p<0.001).
4. Discussion
In our field study, we observed a significant shift from relative under-infection of D. galeata in the year 2001 to the relative over-infection of this taxon during the 2003 and 2004 epidemics (figure 1). At the same time the pattern was reversed for the other taxa. This observation is consistent with the idea of parasite evolution towards the frequent taxon: D. galeata had been rare before 2001, but it strongly increased in frequency afterwards. Decaestecker et al. (2002) proposed that observed differences in the infection patterns among Daphnia magna clones might be caused by differences in their vertical distributions. If sediment is a source for parasite spores, the infection risk is higher for clones, which perform deeper migrations (known as the ‘deep trouble hypothesis’). At the time of the year when C. mesnili infects the Daphnia population (figure 1) vertical migrations are, however, strongly restricted by low oxygen concentrations (see Johnson et al. 1992) and therefore there is no vertical separation between Daphnia taxa in Greifensee. Our infection experiment revealed nevertheless that the over-infection of D. galeata (detected in the field) is not likely to be confounded by other traits than genetic variation for resistance. In a cyprinid hybrid system, in contrast, the observed differences in infection level were caused by habitat variation between the two hybridizing taxa (Le Brun et al. 1992).
We confirmed earlier reports that C. mesnili imposes high fitness costs on its host, in particular that infected daphnids have very low fecundity (Bittner et al. 2002; Lass & Bittner 2002; Wolinska et al. 2004). A high cost of infection, together with variation in host resistance (figure 3) could make C. mesnili a powerful selective force among taxa. The results of our competition experiment, where the parasite altered the competitive ability of two taxa (figure 2a), demonstrated that this can be the case. In this experiment, genetic drift can be regarded as unimportant because host population sizes were large and the duration of the experiment was relatively short (approx. 10 host generations). Moreover, in contrast to the expectations of drift, frequency changes were largely consistent across replicates. The parasite led to changes in the host species composition within only 10 weeks (figure 2a), whereas in the populations from which hosts and parasites were obtained, C. mesnili epidemics last 6–7 months per year, with prevalence peaks higher than 20% (figure 1 and Bittner 2001). All this implies that C. mesnili has the potential to act as a selective factor in natural host populations as well and sheds light on a cause of a commonly observed dynamic taxa coexistence within natural Daphnia hybrid populations (e.g. Spaak & Hoekstra 1997; Jankowski & Straile 2004). To explain dynamic changes in taxon frequencies, Spaak & Hoekstra (1995) proposed the ‘temporal superiority hypothesis’ which essentially says that relative taxon fitness varies with time, due to fluctuating environmental conditions. According to this hypothesis, the same environment is thought to always favour one taxon over the others, e.g. at high temperature, parental taxa perform better, whereas at low temperature D. galeata×D. hyalina hybrids are favoured (Wolf & Weider 1991). Further, variations in predation pressure (e.g. Spaak & Hoekstra 1997), food quantity (e.g. Weider 1993) and food quality (Von Elert 2004) have been implicated as possible factors that promote different taxa under different conditions. Our study indicates that parasites may also alter competition among taxa within such mixed populations. In contrast to the selective forces mentioned above, which were considered to be static in their effect on certain Daphnia taxa, parasites may evolve rapidly and therefore are a dynamic factor. Protozoan parasites, for example, can adapt to the new host within only a few transfers from one host to another (serial passage experiments, reviewed in Ebert 1998; Schmid-Hempel 2001). Since parasites have the potential to track common host genotypes, the advantage (resistance) of one taxon might quickly disappear (see figure 1), as has been shown for a clonal snail population where under-infected and common snail clones increased in the infected part of the population and became less frequent afterwards (Dybdahl & Lively 1998). Taking into account that numerous parasites are known from this Daphnia hybrid system (Bittner 2001; Wolinska et al. 2005), we think that parasites cannot be ignored in a discussion of taxa coexistence.
Consistent with studies in D. magna (Capaul & Ebert 2003; Haag & Ebert 2004) we found that a parasite can alter clonal competition within taxa (figure 2b). Members of one taxon here, as well as in other systems (e.g. Lively 1989; Henter & Via 1995), strongly differed in their susceptibility (figure 3). Since the degree to which parasites harmed their host also varied among clones, it suggests that a parasite could change the outcome of clonal competition even if all competing individuals were infected. Considering that clones from one taxon are not identical in their reaction to parasites, a next step towards better understanding the role of parasites in natural Daphnia hybrid populations may be to investigate parasite-driven host frequency changes, at the genotypic level.
In hybridizing host systems, interactions with parasites have been often explored in a very narrow time frame (reviewed in Fritz et al. 1999; Moulia 1999). The selective pressure exerted by a parasite was said to have two different consequences (after Coustau et al. 1991). Either it maintains a ‘tension zone’ (Barton & Hewitt 1989), if hybrids are more infected than their parental species (e.g. mice hybrid system in Sage et al. 1986), or it may help to extend hybrid distributions to their physiological limits, if hybrids are found to be less infected (e.g. anurans hybrid system in Jackson & Tinsley 2003). We think that in systems where parental species and hybrids coexist in sympatry, a further option can be the maintenance of multiple taxa through negative frequency-dependent selection. Hybridization is a common phenomenon in a wide variety of both plant and animal taxa (reviewed in Burke & Arnold 2001). We think that the study of host–parasite interactions in such systems can add important information to general theories explaining dynamic species coexistence. This gives a great possibility to enter a new face of research on host–parasite interactions operating on high taxonomical levels.
Acknowledgments
We thank Barbara Keller and Esther Keller for support in the field, Piotr Madej for advice and help during the infection experiment, Larry Weider for helpful discussion and linguistic help and two anonymous reviewers for comments. This work was supported by grant 31-65003.01 from the Swiss National Science Foundation to P.S. and D.E. was supported by the Swiss National Fonds.
References
- Barton N.H, Hewitt G.M. Adaptation, speciation and hybrid zones. Nature. 1989;341:497–503. doi: 10.1038/341497a0. 10.1038/341497a0 [DOI] [PubMed] [Google Scholar]
- Bittner, K. 2001 Parasitismus bei Daphnia im Bodensee. Ph.D. thesis, Universität Konstanz, Konstanz, Germany.
- Bittner K, Rothhaupt K.O, Ebert D. Ecological interactions of the microparasite Caullerya mesnili and its host Daphnia galeata. Limnol. Oceanogr. 2002;47:300–305. [Google Scholar]
- Burke J.M, Arnold M.L. Genetics and the fitness of hybrids. Annu. Rev. Gen. 2001;35:31–52. doi: 10.1146/annurev.genet.35.102401.085719. 10.1146/annurev.genet.35.102401.085719 [DOI] [PubMed] [Google Scholar]
- Capaul M, Ebert D. Parasite-mediated selection in experimental Daphnia magna populations. Evolution. 2003;57:249–260. doi: 10.1111/j.0014-3820.2003.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Coustau C, Renaud F, Maillard C, Pasteur N, Delay B. Differential susceptibility to a trematode parasite among genotypes of the mytilus-edulis-galloprovincialis complex. Genet. Res. 1991;57:207–212. doi: 10.1017/s0016672300029359. [DOI] [PubMed] [Google Scholar]
- Crowder M.J, Hand D.J. Chapman & Hall; London: 1990. Analysis of repeated measures. [Google Scholar]
- Decaestecker E, De Meester L, Ebert D. In deep trouble: habitat selection constrained by multiple enemies in zooplankton. Proc. Natl Acad. Sci. USA. 2002;99:5481–5485. doi: 10.1073/pnas.082543099. 10.1073/pnas.082543099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybdahl M.F, Lively C.M. Host–parasite coevolution: evidence for rare advantage and time-lagged selection in a natural population. Evolution. 1998;52:1057–1066. doi: 10.1111/j.1558-5646.1998.tb01833.x. [DOI] [PubMed] [Google Scholar]
- Ebert D. Experimental evolution of parasites. Science. 1998;282:1432–1435. doi: 10.1126/science.282.5393.1432. 10.1126/science.282.5393.1432 [DOI] [PubMed] [Google Scholar]
- Ebert D, Mangin K.L. The influence of host demography on the evolution of virulence of a microsporidian gut parasite. Evolution. 1997;51:1828–1837. doi: 10.1111/j.1558-5646.1997.tb05106.x. [DOI] [PubMed] [Google Scholar]
- El Gharbi S, Renaud F, Lambert A. Dactylogirids (Plathyhelminthes: Monogenea) of Barbus spp. (Teleostei, Cyprinidae) from the Iberian Peninsula. Res. Rev. Parasitol. 1992;52:103–116. [Google Scholar]
- Fritz R.S, Moulia C, Newcombe G. Resistance of hybrid plants and animals to herbivores, pathogens, and parasites. Annu. Rev. Ecol. Syst. 1999;30:565–591. 10.1146/annurev.ecolsys.30.1.565 [Google Scholar]
- Gießler S. Analysis of reticulate relationships within the Daphnia longispina species complex. Allozyme phenotype and morphology. J. Evol. Biol. 1997;10:87–105. 10.1007/s000360050011 [Google Scholar]
- Green J. Parasites and epibionts of Cladoceran. Trans. Zool. Soc. Lond. 1974;32:417–515. [Google Scholar]
- Haag C.R, Ebert D. Parasite-mediated selection in experimental metapopulations of Daphnia magna. Proc. R. Soc. B. 2004;271:2149–2155. doi: 10.1098/rspb.2004.2841. 10.1098/rspb.2004.2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W.D. Sex versus non-sex versus parasite. Oikos. 1980;35:282–290. [Google Scholar]
- Heaney L.R, Timm R.M. Morphology, genetics, and ecology of pocket gophers (genus Geomys) in a narrow hybrid zone. Bio. J. Linn. Soc. 1985;25:301–317. [Google Scholar]
- Henter H.J, Via S. The potential for coevolution in a host–parasitoid system. I. Genetic variation within an aphid population in susceptibility to a parasitic wasp. Evolution. 1995;49:427–438. doi: 10.1111/j.1558-5646.1995.tb02275.x. [DOI] [PubMed] [Google Scholar]
- Hudson P, Greenman J. Competition mediated by parasites: biological and theoretical progress. Trends Ecol. Evol. 1998;13:387–390. doi: 10.1016/s0169-5347(98)01475-x. 10.1016/S0169-5347(98)01475-X [DOI] [PubMed] [Google Scholar]
- Jackson J.A, Tinsley R.C. Parasite infectivity to hybridising host species: a link between hybrid resistance and allopolyploid speciation? Int. J. Parasitol. 2003;33:137–144. doi: 10.1016/s0020-7519(02)00255-2. 10.1016/S0020-7519(02)00255-2 [DOI] [PubMed] [Google Scholar]
- Jankowski T, Straile D. Allochronic differentiation among Daphnia species, hybrids and backcrosses: the importance of sexual reproduction for population dynamics and genetic architecture. J. Evol. Biol. 2004;17:312–321. 10.1046/j.1420-9101.2003.00666.x [PubMed] [Google Scholar]
- Johnson C.A, Sigg L, Lindauer U. The chromium cycle in a seasonally anoxic lake. Limnol. Oceanogr. 1992;37:315–321. [Google Scholar]
- Keller B, Spaak P. Non-random sexual reproduction and diapausing egg production in a Daphnia hybrid species complex. Limnol. Oceanogr. 2004;49:1393–1400. [Google Scholar]
- Lass S, Bittner K. Facing multiple enemies: parasitised hosts respond to predator kairomones. Oecologia. 2002;132:344–349. doi: 10.1007/s00442-002-0982-9. 10.1007/s00442-002-0982-9 [DOI] [PubMed] [Google Scholar]
- Le Brun N, Renaud F, Berrebi P, Lambert A. Hybrid zones and host–parasite relationships—effect on the evolution of parasitic specificity. Evolution. 1992;46:56–61. doi: 10.1111/j.1558-5646.1992.tb01984.x. [DOI] [PubMed] [Google Scholar]
- Lindman H.R. Springer; New York, NY: 1992. Analysis of variance in complex experimental design. [Google Scholar]
- Little T.J. The evolutionary significance of parasitism: do parasite-driven genetic dynamics occur ex silico? J. Evol. Biol. 2002;15:1–9. 10.1046/j.1420-9101.2002.00366.x [Google Scholar]
- Little T.J, Ebert D. Associations between parasitism and host genotype in natural populations of Daphnia (Crustacea: Cladocera) J. Anim. Ecol. 1999;68:134–149. 10.1046/j.1365-2656.1999.00271.x [Google Scholar]
- Lively C.M. Adaptation by a parasitic trematode to local populations of its snail host. Evolution. 1989;43:1663–1671. doi: 10.1111/j.1558-5646.1989.tb02616.x. [DOI] [PubMed] [Google Scholar]
- Löffler A, Wolinska J, Keller B, Rothhaupt K.O, Spaak P. Life history patterns of parental and hybrid Daphnia differ between lakes. Freshw. Biol. 2004;48:1372–1380. 10.1111/j.1365-2427.2004.01272.x [Google Scholar]
- Mason J.R, Clark L. Sarcosporidiosis observed more frequently in hybrids of mallards and American black ducks. Wilson Bull. 1990;102:160–162. [Google Scholar]
- Moulia C. Parasitism of plant and animal hybrids: are facts and fates the same? Ecology. 1999;80:392–406. [Google Scholar]
- Moulia C, Lebrun N, Dallas J, Orth A, Renaud F. Experimental-evidence of genetic determinism in high susceptibility to intestinal pinworm infection in mice: a hybrid zone model. Parasitology. 1993;106:387–393. doi: 10.1017/s0031182000067135. [DOI] [PubMed] [Google Scholar]
- Park T. Experimental studies of interspecific competition I. Competition between populations of the flour beetles, Trinolium confusum and Tribolium castaneum. Ecol. Monogr. 1948;18:267–307. [Google Scholar]
- Prenter J, MacNeil C, Dick J.T.A, Dunn A.M. Roles of parasites in animal invasions. Trends Ecol. Evol. 2004;19:385–390. doi: 10.1016/j.tree.2004.05.002. 10.1016/j.tree.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Sage R.D, Heyneman D, Lim K.C, Wilson A.C. Wormy mice in a hybrid zone. Nature. 1986;324:60–63. doi: 10.1038/324060a0. 10.1038/324060a0 [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P. On the evolutionary ecology of host–parasite interactions: addressing the question with regard to bumblebees and their parasites. Naturwiss. 2001;88:147–158. doi: 10.1007/s001140100222. 10.1007/s001140100222 [DOI] [PubMed] [Google Scholar]
- Schwenk K, Spaak P. Ecology and genetics of interspecific hybridization in Daphnia. In: Streit B, Städler T, Lively C.M, editors. Evolutionary ecology of freshwater animals. Birkhäuser Verlag; Basel: 1997. pp. 199–229. [Google Scholar]
- Sokal R.R, Rohlf F.J. W.H. Freeman and Co.; San Francisco, CA, USA: 1995. Biometry. [Google Scholar]
- Spaak P, Hoekstra J.R. Life history variation and the coexistence of a Daphnia hybrid with its parental species. Ecology. 1995;76:553–564. [Google Scholar]
- Spaak P, Hoekstra J.R. Fish predation on a Daphnia hybrid species complex: a factor explaining species coexistence? Limnol. Oceanogr. 1997;42:753–762. [Google Scholar]
- StatSoft Inc. 2006 Electronic statistics textbook from http://www.statsoft.com/textbook/stathome.html
- Stirnadel H.A, Ebert D. Prevalence, host specificity and impact on host fecundity of microparasites and epibionts in three sympatric Daphnia species. J. Anim. Ecol. 1997;66:212–222. [Google Scholar]
- Tinsley R.C, Jackson J.A. Correlation of parasite speciation and specificity with host evolutionary relationships. Int. J. Parasitol. 1998;28:1573–1582. doi: 10.1016/s0020-7519(98)00085-x. 10.1016/S0020-7519(98)00085-X [DOI] [PubMed] [Google Scholar]
- Von Elert E. Food quality constraints in Daphnia: interspecific differences in the response to the absence of a long chain polyunsaturated fatty acid in the food source. Hydrobiologia. 2004;526:187–196. 10.1023/B:HYDR.0000041604.01529.00 [Google Scholar]
- Weider L.J. Niche breadth and life history variation in a hybrid Daphnia complex. Ecology. 1993;74:935–943. [Google Scholar]
- Wolf H.G, Mort M.A. Interspecific hybridization underlies phenotypic variability in Daphnia populations. Oecologia. 1986;68:507–511. doi: 10.1007/BF00378763. 10.1007/BF00378763 [DOI] [PubMed] [Google Scholar]
- Wolf H.G, Weider L.J. Do life-history parameters of Daphnia as determined in the laboratory correctly predict species successions in the field? Verh. Internat. Verein. Limnol. 1991;24:2799–2801. [Google Scholar]
- Wolinska J, Keller B, Bittner K, Lass S, Spaak P. Do parasites lower Daphnia hybrid fitness? Limnol. Oceanogr. 2004;49:1401–1407. [Google Scholar]
- Wolinska J, Keller B, Spaak P. Parasite–host preference in hybrid Daphnia populations. Verh. Internat. Verein. Limnol. 2005;29:340–341. [Google Scholar]