Abstract
Choice of genetically unrelated mates is widely documented, yet it is not known how self-referential mate choice can co-occur with commonly observed directional selection on sexual displays. Across 10 breeding seasons in a wild bird population, we found strong fitness benefits of matings between genetically unrelated partners and show that self-referential choice of genetically unrelated mates alternates with sexual selection on elaborate plumage. Seasonal cycles of diminishing variation in ornamentation, caused by early pairing of the most elaborated males, and influx of increasingly genetically unrelated available mates caused by female-biased dispersal, lead to temporal fluctuations in the target of mate choice and enabled coexistence of directional selection for ornament elaboration with adaptive pairing of genetically unrelated partners.
Keywords: dispersal, extra-pair copulations, genetic relatedness, sexual ornamentation, sexual selection
1. Introduction
Preference for genetically unrelated mates is favoured by natural selection when it minimizes homozygosity of deleterious recessive alleles in progeny (Amos et al. 2001; Keller & Waller 2002; Keller et al. 2002; Foerster et al. 2003), results in heterosis (Turelli & Ginzburg 1983), or enhances immunocompetence (Brown 1998; Johnsen et al. 2000; Wegner et al. 2003). Yet, crucial aspects of mate choice based on self-referential genetic characteristics are still unresolved. Not only are the mechanisms by which individuals can identify genetically complementary mates unknown (Johnsen et al. 2000; Roberts & Gosling 2003), but it is also unclear how these mechanisms can evolve (Colegrave et al. 2002; Shuster & Wade 2003). More generally, the evolution of elaborate ornaments requires that the most elaborate males are disproportionally successful in attracting mates, whereas mate choice based on genetic relatedness implies that the best mate for one individual may not be the best for another (Andersson 1994). Moreover, theory suggests that the strength of self-referential mate selection should become progressively weaker with greater genetic complementarity among potential partners (Shuster & Wade 2003), yet empirical studies commonly document continuous preference for unrelated mates (Bensch et al. 1994; Amos et al. 2001).
A proposed, but empirically untested resolution of this paradox states that directional selection for sexual displays that indicate mate quality and preference for genetically unrelated mates can coexist when they have independent genetic benefits (Neff & Pitcher 2005) or when changes in temporal and spatial distribution of potential mates result in fluctuations in the opportunity for selection on sexual ornamentation or genetic complementarity (Colegrave et al. 2002; Shuster & Wade 2003). For example, in many temperate birds, strong selection for early breeding favours rapid pairing, often based on sexual signals indicating mate quality, followed by a subsequent adjustment of the initial mate choice through extrapair fertilizations (Johnsen et al. 2000; Blomqvist et al. 2002; Foerster et al. 2003) or additional matings (Freeman-Gallant 1996). When the genetic composition of a population of potential mates changes predictably across space and time, such additional mate choice allows for the selection of genetically unrelated mates in reference to social mates (Slagsvold et al. 2001; Blomqvist et al. 2002; Schmoll et al. 2005) or breeding locations (Foerster et al. 2003), as well as reduces the risk of inbreeding associated with strong directional selection on sexual ornamentation (Shuster & Wade 2003).
We hypothesized that temporal changes in the variability of sexual displays and in genetic relatedness among available mates, and corresponding changes in the targets of female mate choice, should result in the temporal alternation of directional sexual selection on ornamentation and adaptive genetic complementarity in mate choice. To test this hypothesis, we examined seasonal changes in genetic relatedness of available mates in relation to social and extrapair mate selection, genetic parentage, offspring survival, and variation in sexual ornamentation among newly mated birds in a resident population of individually marked house finches (Carpodacus mexicanus) breeding in Montana, northwestern United States, from 1995 to 2004.
2. Material and methods
(a) Study organism
From 1995 to 2004, at the onset of the breeding season in each year, all birds in the study population were captured, individually marked, and pair affiliations and nesting were monitored continuously (Badyaev & Martin 2000a,b). Once finches breed at the study site in their first year, most continue to breed there for the rest of their lives and reside within the study site throughout the year (Badyaev & Duckworth 2003). Dispersing individuals joining the population for their first breeding attempt were captured within 2 days of arrival and individually marked (Badyaev et al. 2001b). We included only newly pairing birds in the analyses of offspring survival in order to account for the effects of previous mate familiarity. To assess temporal changes in the pool of individuals available for mating, all adults were categorized in relation to residency or arrival at the study site. Individuals were categorized as ‘local’ if they were resident in the population in the winter preceding the breeding season, or ‘non-local’ if they were not present in the population during the winter. Non-local individuals were further categorized with respect to median arrival date for all other immigrants of the same sex within each year, with ‘early’ indicating an arrival before and ‘late’ indicating arrival after median arrival date.
All surviving individually marked juveniles were recaptured weekly until 80–90 days old and their survival to that age was calculated on a per nest basis (sexes combined). No significant juvenile dispersal takes place before finches are 80 days old (Badyaev et al. 2001b). We used multiple regression and general linear models to estimate the effects of internal relatedness, nest initiation date, and the interaction among these factors on proportion of survived juveniles of the brood and report standardized regression coefficients (bST, in s.d.m.) and associated statistics. The sexually selected carotenoid breast patch (Hill 1990) was photographed for each male, under standard settings, at the onset of each breeding season (Badyaev & Duckworth 2003). We used SigmaScan Pro 5.0 (SPSS, Inc.) to measure pigment hue elaboration of the patch, which varies from dull yellow to deep purple. A 10×10 pixel grid was overlaid on each image and one pixel was sampled in each square of the grid. To standardize the colour circle scores to a linear scale, we subtracted 256° from scores and inverted the scale (Badyaev et al. 2001a).
(b) Microsatellite genotyping and paternity analysis
We collected 40 μl of blood from each individual by brachial venipuncture. All adults and offspring were genotyped at 16 highly polymorphic species-specific microsatellite loci (Hofi53, HofiACAG07, HofiACAG25, Hofi16, Hofi29, Hofi10, Hofi70, HofiACAG01, Hofi30, Hofi39, Hofi19, Hofi35, Hofi69, HofiACAG15, Hofi07, Hofi26). PCR was carried out using fluorescent-labelled primers (Applied Biosystems, USA) and analysed by capillary electrophoresis in an ABI Prism 3730 DNA analyzer. Discrete microsatellite allele sizes were determined using Genotyper software (Applied Biosystems, USA). Genotypes were analysed using Cervus v. 2.0 (Marshall et al. 1998) to calculate expected and observed heterozygosities and estimate null allele frequencies, while exact tests for departures from Hardy–Weinberg equilibrium (HWE) were performed using Genepop v. 3.4 (see electronic supplementary material; Raymond & Rousset 1995; Jamieson & Taylor 1997). Two markers were excluded from further analysis due to evidence of null alleles: significant (p<0.05) deviation from HWE (Hofi30) and multiple occurrences of mother–offspring homozygote mismatches (Hofi69).
Paternity analysis was carried out using the likelihood approach implemented in Cervus (Marshall et al. 1998). This method assigns paternity based on the sum of log-likelihood ratios at each locus (LOD score) for each candidate parent, with the highest LOD score indicating the most likely sire of a particular offspring when the maternal identity is known. Statistical confidence in paternity assignments was assessed by calculating the difference between LOD scores among the two most likely candidate parents (Δ). Critical values of Δ for a 95% confidence level were obtained from simulated parentage tests based on observed allele frequencies in each year. Simulation parameters were selected as follows: the number of candidate sires was defined as the total number of males genotyped in a particular year; the proportion of males sampled was estimated from population censuses conducted systematically throughout the breeding season; the mean proportion of loci typed was calculated directly from observed genotypes; and error rate (i.e. typing errors, null alleles, and mutations) was estimated by frequency of mismatch between maternal and offspring genotypes (0.0103). Simulations were reiterated for 10 000 cycles. Paternity was assigned to a putative father only when the Δ criterion associated with a 95% confidence level was achieved. If none of the candidate males met this criterion, offspring were considered to have been sired by an unsampled individual (e.g. non-resident ‘floater’ males). We analysed paternity for 200 offspring from 57 broods using Cervus and assigned paternity at a 95% confidence level for 196 offspring (98%). Of these, nine (4.6%) offspring from seven broods were identified as having been sired by a male other than the social mate. The remaining four offspring all showed matching alleles with putative mothers, but could not be assigned to the social mate or any other sampled male with 95% confidence. Thus, we concluded that these four were also extrapair offspring that were sired by or non-local dispersing males that did not stay within the study site.
(c) Pairwise genetic relatedness and genetic diversity
Pairwise estimates of relatedness were calculated for all adults using a method of moments estimator of MER software (Wang 2002). Allele frequencies were calculated separately for each year and standard errors were from bootstrapping over loci (30 000 iterations). Genetic diversity within individuals was calculated as internal relatedness (IR; Amos et al. 2001), a maximum likelihood estimator derived from a method proposed by Queller & Goodnight (1989). While similar to multilocus heterozygosity, IR has the advantage of being weighted by the frequency of individual alleles within a population. Thus, an individual that is homozygous for a very common allele is weighted differently from an individual homozygous for two very rare alleles. This is particularly valuable for this study, because we are interested in using the markers as indicators of genome-wide diversity rather than the diversity at the individual marker loci per se. Other researchers have proposed d2—a microsatellite-specific measurement of distance among alleles—as an alternative measure of individual genetic diversity (Coulson et al. 1998). However, this measure relies on the assumption of a stepwise mutation process (Ellegren 2002) and specific population inbreeding histories (Tsitrone et al. 2001).
(d) Patterns of mate choice
The relatedness of social and extrapair partners was compared with the average relatedness of a pool of potential partners at the time of pairing or fertilization, correspondingly. For each female, we defined a potential social pairing partner as any unpaired resident male that was present in the population from the date of the female's arrival (or from 1 February for overwintering females) to the date of pair formation. A potential extrapair partner was any resident male that was present in the population during 10 days preceding the onset of ovulation of the focal female (i.e. onset of rapid yolk deposition stage in oogenesis; Young & Badyaev 2004). To examine how the genetic composition of the mating pool changed across the season, we calculated the mean pairwise relatedness between an arriving (non-local) individual and all unpaired individuals of the opposite sex present at the study site on the specific date of arrival. Because of the influx of increasingly unrelated partners into the population, the average relatedness among ‘social’ pairings late in the season is considerably lower than early in the season (i.e. social mates late in the season were more unrelated than social mates early in the season). Similarly, because sex-biased dispersal, a commonly observed pattern in birds, is likely to alter the genetic composition of the mating pool across a season, we also calculated average pairwise relatedness of each arriving individual to members of the same sex that were present at the study site at the time of their arrival (Knight et al. 1999; Gardner et al. 2001). If males and females differ in either propensity for or distance of dispersal, than we expect higher average relatedness among individuals of one sex compared to the other (Prugnolle & de Meeus 2002).
Complete genotypes of an entire population (i.e. mating females and all available social and extrapair males) were available for 45 female mate selection episodes (n=21 mating episodes early in the season and n=24 episodes late in the season). Average pairwise relatedness between arriving (non-local) individuals and individuals of the same sex present at the study site at the time of arrival was calculated for all n=155 non-local males and n=30 non-local females arriving over the course of study. Sexual ornamentation was measured for all males (local and non-local, n=930). Average pairwise female relatedness for available, social and extrapair males was calculated for each female separately as least-squared means with year as a covariate.
3. Results
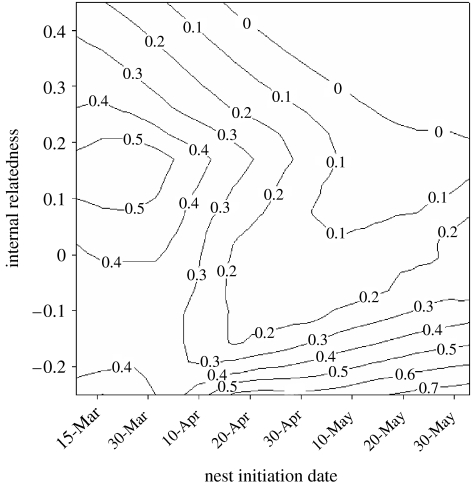
Breeding date and genetic relatedness of parents were important determinants of post-fledging survival of offspring (figure 1; FYEAR=2.76, p=0.13; FINITIATION DATE=8.91, p=0.02; FIR=12.4, p<0.01; FIR×INITIATION DATE=18.1, p<0.01). Offspring of more genetically related parents were less genetically diverse than offspring of less related parents (Spearman r=0.74, n=46 nests, p<0.01). Within families, more genetically diverse offspring had greater post-fledging survival than their less genetically diverse full-siblings (F1,117=4.29, p=0.04), and among families, offspring that hatched earlier in the season and those from genetically unrelated matings had higher post-fledging survival (figure 1; IR: bST=−0.39, t=−3.06, p=0.003; initiation date: bST=−0.20, t=−1.53, p=0.013).
Figure 1.
Fitness consequences of offspring genetic diversity in house finches in 1995–2004. The proportion of nestlings per nest (n=117) that survived to the onset of dispersal is greater in nests with higher genetic diversity and earlier-initiated nests. Larger IR values indicate lesser genetic diversity.
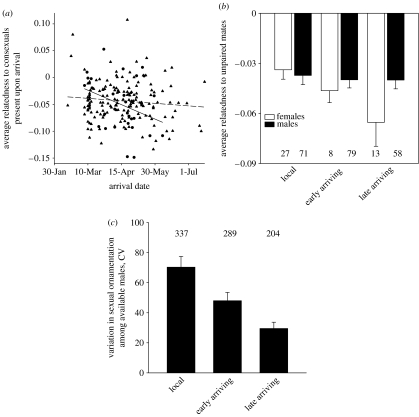
In all years, average pairwise relatedness of available mates in the breeding population changed predictably throughout the season because of the difference in breeding dispersal between the sexes. In each breeding season, later-arriving males were not genetically distinct from either local or early-arriving males and females (figure 2a,b; males: bST=−0.07, t=−0.90, p=0.37; females: bST=−0.38, t=−2.20, p=0.03; slopes are significantly different: F1,184=3.75, p=0.05, year was not a significant covariate) and were similar in the range of sexual ornamentation to unmated males already in the breeding population (figure 2b; males: F2,205=0.09, p=0.92, females, F2,45=3.45, p=0.04). On the contrary, later arriving females were progressively more genetically distinct from earlier arriving females and males (figure 2a,b). Average pairwise relatedness of breeding females to males present at the time of pairing was −0.02±0.014 (s.e.m.) early in the season, and −0.045±0.008 late in the season.
Figure 2.
Seasonal changes in genetic composition and sexual ornamentation in the breeding population. (a) Average pairwise relatedness of arriving females (filled circles, solid line) and males (filled triangles, dashed line) in relation to individuals of the same sex that were present in the population at the time of arrival (females: n=30, males: n=155). (b) Average pairwise relatedness of local resident, early-arriving non-local and late-arriving non-local males (filled bars) and females (open bars) in relation to unpaired mates in the population at the time of arrival. (c) Variation in sexual ornamentation of local males, early-arriving non-local and late-arriving non-local males (10 years, shown is the mean of coefficient of variation for each year+s.e.m). Numbers above the bars indicate the sample sizes.
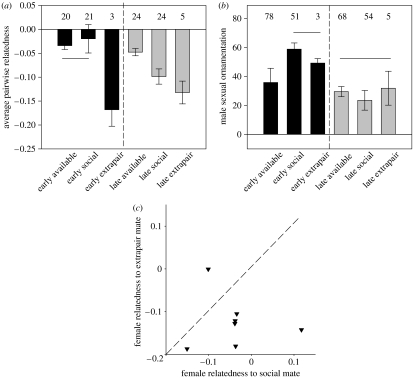
Female preference for earlier breeding with more ornamented males documented in this populations (Badyaev & Hill 2002; see also figure 3b) was associated with gradually diminishing variation in sexual ornamentation in the pool of males still available for pairing (figure 2c; F2,27=29.10, p<0.001, all groups differ, Waller–Duncan K-ratio t=2.36, p<0.05). In turn, seasonally decreasing variation in sexual ornamentation was accompanied by increasingly unrelated individuals in the pool of available mates (figure 2a) and corresponding temporal changes in patterns of mate selection (figure 3a,b). Early in the season, pairwise relatedness did not differ between potential partners at the time of pairing and chosen social partners (F1,41=2.54, p=0.18), whereas chosen extrapair males were less related to female than available males or chosen social males (Waller–Duncan K-ratio t=2.81, p<0.05; figure 3a). Late in the breeding season, more genetically dissimilar social and extrapair mates were selected compared to all available mates (all groups differ, F2,53=4.86, p=0.018; Waller–Duncan K-ratio test for social versus available groups: t=2.29, p=0.04; for social versus extrapair mates: t=2.01, p=0.05; figure 3a). Early in the season, there was no significant selection based on genetic complementarity, but consistent selection of males with the most elaborated sexual ornamentation (figure 3a,b: F3,133=7.32, p<0.01, only available and social groups differ, t=2.97, p<0.05; FSEASON (EARLY/LATE)=6.94, p<0.01, FGROUP (SOCIAL/AVAILABLE)=3.01, p=0.05, FSEASON×GROUP=14.1, p<0.01; see also Badyaev & Hill 2002). Late in the season, females chose the most genetically unrelated mates compared to all available mates at the time of pairing, but showed no mating discrimination based on colour (figure 3a,b; F2,122=1.06, p=0.68, all groups are similar).
Figure 3.
Mate selection based on genetic relatedness and sexual ornamentation across the breeding season. (a) No difference in pairwise relatedness between potential partners at the time of pairing and chosen social partners early in the breeding season. Chosen extrapair males are less related to female than available males or chosen social males. Late in the breeding season, more genetically dissimilar social and extrapair mates were selected compared to all available mates. (b) Elaboration of sexual ornamentation in males available for pairing and males selected as social and extrapair mates early and late in the breeding season. Numbers above the bars indicate the sample sizes and solid lines connect groups that did not differ statistically. (c) Comparison of relatedness of individual females to their social and extrapair mates. Points below the dashed line of equal relatedness indicate that the female's extrapair male is less related to female than the female's social male.
Throughout the season, extrapair males were more genetically unrelated to social females than were all potential extrapair sires that were available during the females' fertile period (figure 3a), and in nests with extrapair young, extrapair fathers were less related to social females than were social fathers (figure 3c; tpaired=2.03, n=7 nests, p=0.04). Apparent discrimination against males with prior residency and selection of non-local (i.e. arriving later than the female) males in extrapair matings were evident in a greater proportion of extrapair fertilizations in nests where both partners were local residents (0.35; n=23 nests) compared to nests where at least one partner was non-local (0.08; n=24 nests, Fisher's exact test for occurrence of extrapair young, p=0.03).
4. Discussion
These results have two important implications. First, we show how directional selection for a more elaborated sexual ornamentation can coexist with self-referential selection on genetic relatedness (Colegrave et al. 2002; Marshall et al. 2003). Early in the season, when availability of unrelated mates in the pool of potential mates is low, but diversity in sexual ornamentation is high (figure 2), mate selection in this population is based on elaboration of sexual ornamentation (figure 3b), and the fitness of such matings is most strongly affected by parental provisioning which varies with male ornamentation in this population (Badyaev & Hill 2002; Duckworth et al. 2003) and early nesting (see fig. 1 in Badyaev et al. 2003). Later in the season, the arrival of progressively more unrelated females and the substantially greater fitness of more genetically diverse offspring (fig. 1 in Lindstedt et al. 2006) increases the strength of and opportunity for selection for genetic complementarity (figure 3a), while the opportunity for sexual selection on ornamentation might become weaker because of its low variation among available mates (figure 2c).
Proximately, the changes in the patterns and targets of mate selection documented in this study can result from several behavioural processes, from an active change in mate choice and less effective mate guarding during an influx of new extrapair partners to differential allocation to display and mating by individuals throughout mating season (Lindstedt et al. 2006). Regardless, the documented coexistence of both directional selection on sexual ornamentation and balancing selection for genetic complementarity is especially important for the evolution and maintenance of variability in condition-dependent sexual displays that indicate resource provisioning, such as the carotenoid-based ornamentation of house finches in this study, because consistent directional selection on such traits is expected to limit their genetic and phenotypic variation. Moreover, in small populations, consistent directional selection on sexual ornamentation would increase the risk of inbreeding (Shuster & Wade 2003), whereas the temporal change in the targets of mate choice documented in this study mitigates this risk (Neff & Pitcher 2005). In addition, temporal change in targets of female choice might contribute to coexistence of distinct paternal strategies in this population (Badyaev & Hill 2002; Badyaev & Duckworth 2005).
Second, a combination of three processes often documented in birds—greater fitness of earlier breeding (Sheldon et al. 2003), preference for non-local mates (Foerster et al. 2003; Masters et al. 2003), and sex differences in natal dispersal (Greenwood & Harvery 1982)—enables the persistence of matings between unrelated individuals and may provide a general mechanism behind genetically complementary mate choice in many bird species (Johnsen et al. 2000; Blomqvist et al. 2002; Foerster et al. 2003). Thus, the greater fitness of genetically unrelated pairs (figure 1) may generate directional selection on natal and breeding dispersal (figure 2a) and individual recognition instead of discrimination based on genetic relatedness per se. Whereas this study is the first documentation of adaptive genetic complementarity in relation to seasonal changes in availability and genetic composition of mating pool, the importance of sex-biased natal dispersal as the mechanism behind matings between unrelated individuals has been documented in a number of species (Sinervo & Clobert 2003) and some studies have found that sex differences in dispersal increase as the population becomes more inbred (Perrin & Mazalov 2000; Stow & Sunnucks 2004). Reduced fitness of matings between close relatives (e.g. Keller et al. 2002) is thought to facilitate the evolution of mating preference for unfamiliar partners; females often prefer males that are non-local or mates that are distinct from the female's social mates (Peacock & Smith 1997; Masters et al. 2003; Kupper et al. 2004). In house finches, strong individual mate recognition is well documented (Lindstedt et al. 2006) and is facilitated by high breeding site fidelity, a semi-colonial social system and pairing in common resident flocks. Moreover, house finches experienced several severe bottleneck events as they spread across North America over the past 60 years (Hawley et al. 2006), and thus, inbreeding avoidance might be an important strategy in many small and recently established populations of this species. Indeed, in this population offspring of genetically dissimilar mating were more resistant to a novel pathogen than offspring of mating between genetically similar partners (Lindstedt et al. 2006).
Overall, the variable and context-dependent criteria for mate selection (Freeman-Gallant et al. 2003; Roberts & Gosling 2003; Schmoll et al. 2005) documented in this study, in combination with ecological and social determinants of mate availability, maintains diversity in the targets of mate choice and, ultimately, might enable coexistence of strong directional selection on sexual ornamentation and self-referential preference for genetically unrelated mates (Shuster & Wade 2003; Neff & Pitcher 2005).
Acknowledgments
We thank D. Acevedo Seaman, R. Duckworth, R. Ferriere, L. Landeen, K. Lessels, M. Wade, R. Young and three anonymous reviewers for comments on the manuscript, many field assistants for help in the field, and US National Science Foundation (DEB-0075388, IBN-0218313, DEB-0077804) for funding.
Supplementary Material
References
- Amos W, Wilmer J.W, Fullard K, Burg T.M, Croxall J.P, Bloch D, Coulson T. The influence of parental relatedness on reproductive success. Proc. R. Soc. B. 2001;268:2021–2027. doi: 10.1098/rspb.2001.1751. 10.1098/rspb.2001.1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Badyaev A.V, Duckworth R.A. Context-dependent sexual advertisement: plasticity in development of sexual ornamentation throughout the lifetime of a passerine bird. J. Evol. Biol. 2003;16:1065–1076. doi: 10.1046/j.1420-9101.2003.00628.x. 10.1046/j.1420-9101.2003.00628.x [DOI] [PubMed] [Google Scholar]
- Badyaev A.V, Duckworth R.A. Evolution of plasticity in hormonally-integrated parental tactics. In: Dawson A, Sharp P.J, editors. Functional avian endocrinology. Narosa Publishing House; New Delhi, India: 2005. pp. 375–386. [Google Scholar]
- Badyaev A.V, Hill G.E. Parental care as a conditional strategy: distinct reproductive tactics associated with elaboration of plumage ornamentation in the house finch. Behav. Ecol. 2002;13:591–597. 10.1093/beheco/13.5.591 [Google Scholar]
- Badyaev A.V, Martin T.E. Individual variation in growth trajectories: phenotypic and genetic correlations in ontogeny of the house finch (Carpodacus mexicanus) J. Evol. Biol. 2000a;13:290–301. 10.1046/j.1420-9101.2000.00172.x [Google Scholar]
- Badyaev A.V, Martin T.E. Sexual dimorphism in relation to current selection in the house finch. Evolution. 2000b;54:987–997. doi: 10.1111/j.0014-3820.2000.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Badyaev A.V, Hill G.E, Dunn P.O, Glen J.C. Plumage color as a composite trait: developmental and functional integration of sexual ornamentation. Am. Nat. 2001a;158:221–235. doi: 10.1086/321325. 10.1086/321325 [DOI] [PubMed] [Google Scholar]
- Badyaev A.V, Whittingham L.A, Hill G.E. The evolution of sexual size dimorphism in the house finch. III. Developmental basis. Evolution. 2001b;55:176–189. doi: 10.1111/j.0014-3820.2001.tb01282.x. [DOI] [PubMed] [Google Scholar]
- Badyaev A.V, Beck M.L, Hill G.E, Whittingham L.A. The evolution of sexual size dimorphism in the house finch. V. Maternal effects. Evolution. 2003;57:384–396. doi: 10.1111/j.0014-3820.2003.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Bensch S, Hasselquist D, von Schantz T. Genetic similarity between parents predicts hatching failure—nonincestuous inbreeding in the great reed warbler. Evolution. 1994;48:317–326. doi: 10.1111/j.1558-5646.1994.tb01314.x. [DOI] [PubMed] [Google Scholar]
- Blomqvist D, et al. Genetic similarity between mates and extra-pair parentage in three species of shorebirds. Nature. 2002;419:613–615. doi: 10.1038/nature01104. 10.1038/nature01104 [DOI] [PubMed] [Google Scholar]
- Brown J.L. The new heterozygosity theory of mate choice and the MHC. Genetica. 1998;104:215–221. doi: 10.1023/a:1026409220292. 10.1023/A:1026409220292 [DOI] [PubMed] [Google Scholar]
- Colegrave N, Kotiaho J.S, Tomkins J.L. Mate choice or polyandry: reconciling genetic compatibility and good genes sexual selection. Evol. Ecol. Res. 2002;4:911–917. [Google Scholar]
- Coulson T.M, Pemberton J.M, Albon S.D, Beaumont M, Marshall T.C, Slate J, Guinness F.E, Clutton-Brock T.H. Microsatellites reveal heterosis in red deer. Proc. R. Soc. B. 1998;265:489–495. doi: 10.1098/rspb.1998.0321. 10.1098/rspb.1998.0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth R.A, Badyaev A, Parlow A.F. Males with more elaborated sexual ornaments avoid costly parental care in a passerine bird. Behav. Ecol. Sociobiol. 2003;55:176–183. 10.1007/s00265-003-0671-7 [Google Scholar]
- Ellegren H. Microsatellite mutations in the germline: implications for evolutionary inference. Trends Genet. 2002;16:551–558. doi: 10.1016/s0168-9525(00)02139-9. 10.1016/S0168-9525(00)02139-9 [DOI] [PubMed] [Google Scholar]
- Foerster K, Delhey K, Johnsen A, Lifjeld J.T, Kempenaers B. Females increase offspring heterozygosity and fitness through extra-pair matings. Nature. 2003;425:714–717. doi: 10.1038/nature01969. 10.1038/nature01969 [DOI] [PubMed] [Google Scholar]
- Freeman-Gallant C.R. DNA fingerprinting reveals female preference for male parental care in Savannah sparrows. Proc. R. Soc. B. 1996;263:157–160. doi: 10.1098/rspb.1996.0025. [DOI] [PubMed] [Google Scholar]
- Freeman-Gallant C.R, Meguerdichian M, Wheelwright N.T, Sollecito S.V. Social pairing and female mating fidelity predicted by restriction fragment length polymorphism similarity at the major histocompatibility complex in a songbird. Mol. Ecol. 2003;12:3077–3083. doi: 10.1046/j.1365-294x.2003.01968.x. 10.1046/j.1365-294X.2003.01968.x [DOI] [PubMed] [Google Scholar]
- Gardner M.G, Bull C.M, Cooper S.J.B, Duffield G.A. Genetic evidence for a family structure in stable social aggregations of the Australian lizard Egernia stokesii. Mol. Ecol. 2001;10:175–183. doi: 10.1046/j.1365-294x.2001.01171.x. 10.1046/j.1365-294X.2001.01171.x [DOI] [PubMed] [Google Scholar]
- Greenwood P.J, Harvery P.H. The natal and breeding dispersal of birds. Ann. Rev. Ecol. Syst. 1982;13:1–21. 10.1146/annurev.es.13.110182.000245 [Google Scholar]
- Hawley D.M, Hanley D, Dhondt A.A, Lovette I.J. Molecular evidence for a founder effect in invasive house finch (Caprodacus mexicanus) populations experiencing an emergent disease epidemic. Mol. Ecol. 2006;15:263–275. doi: 10.1111/j.1365-294X.2005.02767.x. 10.1111/j.1365-294X.2005.02767.x [DOI] [PubMed] [Google Scholar]
- Hill G.E. Female house finches prefer colourful males: sexual selection for a condition-dependent trait. Anim. Behav. 1990;40:563–572. [Google Scholar]
- Jamieson A, Taylor S.C.S. Comparisons of three probability formulae for parentage exclusion. Anim. Genet. 1997;28:397–400. doi: 10.1111/j.1365-2052.1997.00186.x. 10.1111/j.1365-2052.1997.00186.x [DOI] [PubMed] [Google Scholar]
- Johnsen A, Andersen V, Sunding C, Lifjeld J.T. Female bluethroats enhance offspring immunocompetence through extra-pair copulations. Nature. 2000;406:296–299. doi: 10.1038/35018556. 10.1038/35018556 [DOI] [PubMed] [Google Scholar]
- Keller L.F, Waller D.M. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002;17:230–241. 10.1016/S0169-5347(02)02489-8 [Google Scholar]
- Keller L.F, Grant P.R, Grant B.R, Petren K. Environmental conditions affect the magnitude of inbreeding depression in survival of Darwin's finches. Evolution. 2002;56:1229–1239. doi: 10.1111/j.0014-3820.2002.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Knight M.E, van Oppen M.J.H, Smith H.L, Rico C, Hewitt G.M, Turner G.F. Evidence for male-biased dispersal in Lake Malawi cichlids from microsatellites. Mol. Ecol. 1999;8:1521–1527. doi: 10.1046/j.1365-294x.1999.00740.x. 10.1046/j.1365-294x.1999.00740.x [DOI] [PubMed] [Google Scholar]
- Kupper C, Kis J, Kosztolanyi A, Szekely T, Cuthill I.C, Blomqvist D. Genetic mating system and timing of extra-pair fertilizations in the Kentish plover. Behav. Ecol. Sociobiol. 2004;57:32–39. [Google Scholar]
- Lindstedt, E., Oh, K. P. & Badyaev, A. V. 2006 Ecological, social, and genetic contingency of extrapair behavior in a socially monogamous bird. J. Avian Biol (In press.)
- Marshall T.C, Slate J, Kruuk L.E.B, Pemberton J.M. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 1998;7:639–655. doi: 10.1046/j.1365-294x.1998.00374.x. 10.1046/j.1365-294x.1998.00374.x [DOI] [PubMed] [Google Scholar]
- Marshall R.C, Buchanan K.L, Catchpole C.K. Sexual selection and individual genetic diversity in a songbird. Proc. R. Soc. B. 2003;270(Suppl. 2):S248–S250. doi: 10.1098/rsbl.2003.0081. 10.1098/rsbl.2003.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters B.S, Hicks B.G, Johnson L.S, Erb L.A. Genotype and extra-pair paternity in the house wren: a rare-male effect? Proc. R. Soc. B. 2003;270:1393–1397. doi: 10.1098/rspb.2003.2380. 10.1098/rspb.2003.2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff B.D, Pitcher T.E. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 2005;14:19–38. doi: 10.1111/j.1365-294X.2004.02395.x. 10.1111/j.1365-294X.2004.02395.x [DOI] [PubMed] [Google Scholar]
- Peacock M.M, Smith A.T. Nonrandom mating in pikas Ochotona princeprs: evidence for inbreeding between individuals of intermediate relatedness. Mol. Ecol. 1997;6:801–811. 10.1046/j.1365-294X.1997.00249.x [PubMed] [Google Scholar]
- Perrin N, Mazalov V. Local competition, inbreeding, and the evolution of sex-biased dispersal. Am. Nat. 2000;155:116–127. doi: 10.1086/303296. 10.1086/303296 [DOI] [PubMed] [Google Scholar]
- Prugnolle F, de Meeus T. Inferring sex-biased dispersal from population genetic tools: a review. Heredity. 2002;88:161–165. doi: 10.1038/sj.hdy.6800060. 10.1038/sj.hdy.6800060 [DOI] [PubMed] [Google Scholar]
- Queller D.C, Goodnight K.F. Estimating relatedness using genetic markers. Evolution. 1989;43:258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x. [DOI] [PubMed] [Google Scholar]
- Raymond M, Rousset F. GENEPOP (Version 1.2): population genetics software for exact tests and ecumenicism. J. Hered. 1995;86:248–249. [Google Scholar]
- Roberts S.C, Gosling L.M. Genetic similarity and quality interact in mate choice decisions by female mice. Nat. Genet. 2003;35:103–106. doi: 10.1038/ng1231. 10.1038/ng1231 [DOI] [PubMed] [Google Scholar]
- Schmoll T, Dietrich V, Winkel W, Epplen J.T, Schurr F, Lubjuhn T. Paternal genetic effects on offspring fitness are context dependent within the extrapair mating system of a socially monogamous passerine. Evolution. 2005;59:645–657. [PubMed] [Google Scholar]
- Sheldon B.C, Kruuk L.E.B, Merila J. Natural selection and inheritance of breeding time and clutch size in the collared flycatcher. Evolution. 2003;57:406–420. doi: 10.1111/j.0014-3820.2003.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Shuster S.M, Wade M.J. Princeton University Press; Princeton, NJ: 2003. Mating systems and strategies. [Google Scholar]
- Sinervo B, Clobert J. Morphs, dispersal behavior, genetic similarity, and the evolution of cooperation. Science. 2003;300:1949–1951. doi: 10.1126/science.1083109. 10.1126/science.1083109 [DOI] [PubMed] [Google Scholar]
- Slagsvold T, Johnsen A, Lampe H, Lifjeld J.T. Do female pied flycathers seek extrapair copulations with familiar males? A test of the incomplete knowledge hypothesis. Behav. Ecol. 2001;12:412–418. 10.1093/beheco/12.4.412 [Google Scholar]
- Stow A.J, Sunnucks P. Inbreeding avoidance in Cunningham's skinks (Egernia cunningami) in natural and fragmented habitat. Mol. Ecol. 2004;13:443–447. doi: 10.1046/j.1365-294x.2003.02060.x. 10.1046/j.1365-294X.2003.02060.x [DOI] [PubMed] [Google Scholar]
- Tsitrone A, Rousset F, David P. Heterosis, marker mutational processes and population inbreeding history. Genetics. 2001;159:1845–1859. doi: 10.1093/genetics/159.4.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M, Ginzburg L.R. Should individual fitness increase with heterozygosity? Genetics. 1983;104:191–209. doi: 10.1093/genetics/104.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. An estimator for pairwise relatedness using molecular markers. Genetics. 2002;160:1203–1215. doi: 10.1093/genetics/160.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner K.M, Kalbe M, Kurtz J, Reusch T.B.H, Milinski M. Parasite selection for immunogenetic optimality. Science. 2003;301:1343. doi: 10.1126/science.1088293. 10.1126/science.1088293 [DOI] [PubMed] [Google Scholar]
- Young R.L, Badyaev A.V. Evolution of sex-biased maternal effects in birds. I. Sex-specific resource allocation among simultaneously maturing follicles. J. Evol. Biol. 2004;17:1355–1366. doi: 10.1111/j.1420-9101.2004.00762.x. 10.1111/j.1420-9101.2004.00762.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.